Spatial orientation of atherosclerotic plaque in non-branching
coronary artery segments
A. Jeremias, H. Huegel, D.P. Lee, A. Hassan, A. Wolf, A.C. Yeung, P.G. Yock,
P.J. Fitzgerald *
Room H3554,Di6ision of Cardio6ascular Medicine,Center for Research in Cardio6ascular Inter6entions,Stanford Uni6ersity Medical Center, Stanford Uni6ersity School of Medicine,300 Pasteur Dri6e,Stanford,CA 94305-5246, USA
Received 2 March 1999; received in revised form 13 September 1999; accepted 3 November 1999
Abstract
It has been postulated that atherosclerotic plaque deposition is spatially related to regions of low shear in non-branching vessel segments. Intravascular ultrasound (IVUS) allows precise spatial orientation of coronary artery plaque formation in humans. The objective of this study was to test the hypothesis that coronary plaques have a higher prevalence on the myocardial side in regions that encounter low surface shear stress. IVUS allows the determination of the inner versus the outer curve of the vessel based on vascular and perivascular landmarks. We studied 30 consecutive patients pre-intervention using IVUS and measured vessel area, lumen area and plaque area (vessel-lumen area) during a motorized pullback at 1 mm intervals. Vessel segments near a side branch (within two times the diameter of the vessel) were excluded from analysis because of flow disturbances. All plaques were classified as concentric or eccentric and all eccentric plaques were further divided with respect to their spatial orientation in the vessel into quadrants: myocardial (inner curve, lower shear stress), epicardial (outer curve, higher shear stress) and lateral (two quadrants intermediate). A total of 613 cross-sections were analyzed in 14 left anterior descending, six left circumflex, and ten right coronary arteries. Plaque distribution was found to be concentric in 321 (52.4%) and eccentric in 292 (47.6%) cross sections. Of all eccentric plaques, 184 cross sections were oriented toward the myocardial side (62.6%) compared to only 54 toward the epicardial side (17.3%) and 54 in the 2 lateral quadrants (19.5%,PB0.001). No difference in plaque area (6.7592.70 vs. 6.7692.60 mm2), vessel
area (15.2894.73 vs. 15.3594.40 mm2), or plaque thickness (1.2690.37 vs. 1.2590.43 mm) was noted between myocardial or
epicardial plaques. These results suggest that atherosclerotic plaques develop more frequently on the myocardial side of the vessel wall, which may relate to lower shear stress. However, plaque size is similar on the epicardial and myocardial side. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Coronary artery disease; Intravascular ultrasound; Shear stress
www.elsevier.com/locate/atherosclerosis
1. Introduction
Atherosclerotic plaques in coronary arteries have been shown to be unevenly distributed within the coro-nary tree [1]. Lesions progress at nearly independent rates within the same patient exposed to the same risk factors and systemic hemodynamics [2]. Right coronary artery plaques progress at a more rapid rate than do lesions in the other coronary arteries [3]. These observa-tions have led to the hypothesis that different flow patterns may determine the relative deposition and
orientation of localized coronary artery disease. Differ-ences in wall shear stress [4,5], flow separation [6], variations in blood flow patterns [7] and turbulence [8] have been proposed as possible factors promoting atherosclerosis. Originally proposed by Caro et al. it has been suggested that low wall shear stress may retard the mass transport of atherogenic substances away from the wall, resulting in increased intimal accu-mulation of lipids [9]. Studies of plaque localization in the human carotid bifurcation have shown that regions of low flow velocity and wall shear stress are prone to develop atherosclerotic lesions, whereas areas of high shear stress are spared [5]. It is now recognized that arterial branching points are particularly susceptible to
* Corresponding author. Tel.+1-650-498-6034; fax+ 1-650-498-6035.
plaque formation depending on the variation of local shear forces [10]. In a postmortem preparation of trans-parent coronary arteries, atherosclerotic plaques were localized almost exclusively on the outer wall at major bifurcations (the ‘hips’) and along the inner wall of curved segments (the ‘inseam’) [1]. A pathological study in carotid arteries [11] and an angiographic study in femoral arteries [12] confirmed the predilection of atherosclerosis for the inner curvature of the vessel at areas of low shear stress. Sabbah et al. have shown that the clearing rate of contrast material is slower along the inner wall, bordering the myocardium, suggesting that velocity is lower at that site compared to the outer wall [13].
Intravascular ultrasound (IVUS) imaging technology allows direct visualization of the distribution and mor-phology of atherosclerotic plaques in vivo and has greatly contributed to our understanding of plaque characteristics, especially in coronary arteries [14 – 16]. In two recent IVUS studies the distribution and the morphology of atheroma in the proximal left anterior descending coronary artery were described [17,18] and found to be in accordance with previous pathological data supporting the role of fluid dynamic factors in the development of atheroma opposite to branching points. However, in vivo data describing the distribution of atherosclerotic plaques in non-branching coronary artery segments is still lacking. The aim of the present study was, therefore, to examine the distribution of
coronary atherosclerosis apart from major side
branches in a systematic fashion using IVUS to test the hypothesis that atherosclerotic lesions are more fre-quently found along the inner walls of curved arterial segments. The principle of orientation within the vessel using IVUS has been described previously and is based on the visualization of various periadventitial structures (veins, pericardium) as well as congruent branching vessels [19]. These landmarks, unique in each coronary segment, provide accurate spatial orientation during the entire length of an IVUS imaging sequence for a partic-ular vessel.
2. Material and methods
2.1. Patient population
Intravascular ultrasound was performed in 30 pa-tients (28 men and two women aged 60.6910.5 years). All patients underwent IVUS imaging before any catheter based intervention and none of the patients had undergone prior intracoronary intervention in the target vessel. All lesions were located in native vessels; vein graft lesions were excluded from analysis. Intra-coronary nitroglycerin (100 – 200 mg) was given during
all IVUS studies before imaging. Informed consent was
obtained from all patients and the institutional review board approved the study.
2.2. Intra6ascular ultrasound image acquisition and analysis
A 3.2F (Microview, CVIS/Boston Scientific) IVUS imaging catheter with a single element transducer at the tip was used. The transducer was mechanically rotated within a protective sheath at 1800 rpm to provide cross-sectional images via an ultrasound diagnostic imaging console (ClearView, CVIS/Boston Scientific). The IVUS-catheter was tracked over a 0.014-in. guide wire up to a position distal to the diseased segment. Ultrasound time gain compensation settings was ad-justed to allow maximal gray scale differentiation. In every case a continuous motorized pullback of the ultrasound transducer was performed at a constant speed of 0.5 mm/second. All IVUS studies were recorded on 1/2-in high-resolution super VHS tapes for off-line analysis.
Quantitative (cross-sectional) analysis of the IVUS images was performed using a commercially available software program (TapeMeasure, Indec). Validation of qualitative and quantitative IVUS analysis has been reported previously [14,15]. Plaque plus media cross-sectional area was used as a measurement of plaque area, since media thickness can not be measured di-rectly [20]. Maximal and minimal wall thickness
mea-surements also included plaque plus media.
End-systolic frames were selected at two-s intervals of the automated pullback; thus IVUS cross-sections were analyzed at 1 mm increments. All cross-sections located near a side branch (within two times the diameter of the vessel) were excluded from analysis to minimize con-founding by flow turbulence. For each cross-section the following measurements were made:
1. vessel area (assumed to be equal to external elastic membrane area, since adventitial thickness can not be precisely determined),
2. lumen area,
3. plaque area (vessel area minus lumen area), 4. percent cross-sectional luminal stenosis (plaque area
divided by vessel area),
5. maximal and minimal plaque thickness, and (6) plaque angle (measured from the geometric center of the lumen) as the circumferential span of plaque within the cross-section.
the lesion. A three-layered appearance with an intimal thickening of B0.2 mm was considered the upper limit of a ‘normal’ arterial wall [21]. Cross-sections with excessive calcification (calcium arc \90°) were ex-cluded from analysis because of acoustic shadowing of deeper structures, precluding accurate measurement of the vessel area. Lesions with B90° of calcium arc were measured by extrapolation assuming that the vessel circumference was circular and by axial movement of the transducer to identify the vessel area of adjacent non-calcified segments as described previously [22].
All cross-sections with eccentric plaque distribution were classified based on their plaque orientation being
centered on the pericardial, myocardial or either lateral side of the vessel. For vessel orientation, perivascular IVUS landmarks were used [19], including:
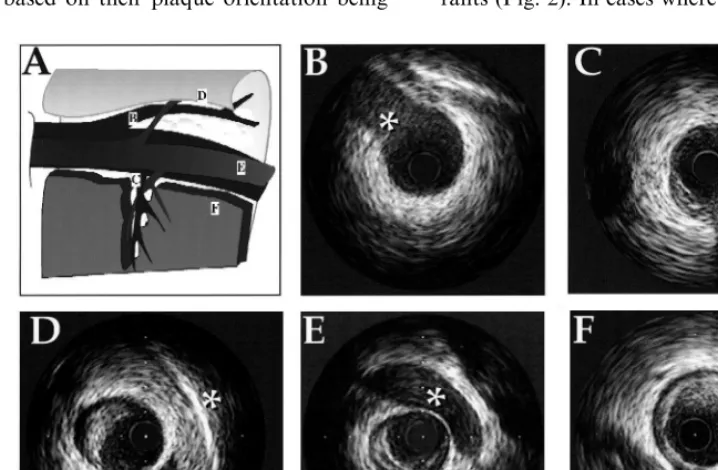
1. coronary veins, 2. pericardium, 3. myocardium, and 4. side branches (Fig. 1).
Based on these landmarks, the vessel was divided into four quadrants: myocardial quadrant (inner curve of the vessel), epicardial quadrant (outer curve of the vessel), and two lateral quadrants (intermediate). Each cross-section was grouped into one of the four quad-rants (Fig. 2). In cases where the plaque angle exceeded
Fig. 1. Perivascular and branching landmarks seen by intravascular ultrasound (IVUS) for spatial orientation in the coronary artery. The illustration in panel (A) represents the anatomical relationship between veins, pericardium and branching points in the left anterior descending artery. (B): Diagonal branch (asterix), (C): Septal branch (asterix), (D): pericardium (asterix), (E): vein (asterix) and (F): myocardium (asterix).
Table 1
Baseline characteristics of the study population
Characteristics
Cardio6ascular risk factors
18 (60)
aMean9standard deviation. bNumber of patients.
3.2. Distribution of atherosclerotic plaque
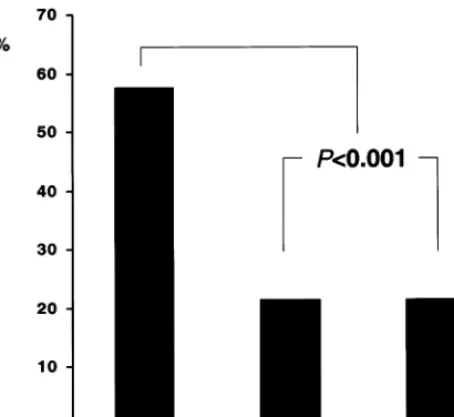
In all cases the visualization of the pericardium, one or more accompanying veins and side branches allowed a precise spatial orientation. Pericardium was identified in 29% of the cross-sections, veins were visualized in 41% of the cases and the myocardium was seen in 13%. Of the 292 eccentric plaques, 184 cross-sections were oriented toward the myocardial side as compared to only 54 cross-sections oriented toward the epicardial side and 54 toward the two lateral quadrants. Individ-ual data for each patient are displayed in Table 2. When comparing the percentage of the plaque distribu-tion derived from each individual patient, plaque was oriented significantly more often toward the myocardial side (62.6%) as compared to the epicardial (17.3%) or lateral (19.5%) side (PB0.001, Fig. 3). Comparing plaques oriented toward the myocardial and the peri-cardial quadrants, there was no difference in vessel area (15.2894.73 vs. 15.3594.40 mm2), plaque area
(6.7592.70 vs. 6.7692.60 mm2, Fig. 4), plaque
thick-ness (1.2690.37 vs. 1.2590.43 mm) or plaque-angle (125934 vs. 133948°).
4. Discussion
The results of the present study suggest that atherosclerotic plaques are more likely to develop at the inner side of curved non-branching coronary segments (myocardial side) in areas of lower shear stress. These areas are prone to flow separation and/or reversal, flow turbulence and eddying. However, no difference in plaque size and vessel size was observed between plaques oriented toward the myocardial versus the epi-cardial side.
4.1. Comparison to pre6ious studies
Wall shear stress in the arterial system is the tangen-tial drag force produced by blood moving across the endothelial surface [23]. It is a function of the velocity gradient of blood near the endothelial surface and is directly proportional to blood flow and blood viscosity. Many studies have focused on fluid dynamic forces to explain the localized nature of coronary artery disease. Initially, high shear stress was thought to potentiate plaque formation by producing endothelial injury and disruption [24]. However, it is now recognized that regions of low shear stress are more susceptible to atheroma formation and many studies have demon-strated an association between low shear stress and atherosclerotic deposits [5,9,10]. In a pathological study of human carotid bifurcations, intimal plaques were found to form in the low stress region of the carotid sinus opposite to the flow divider and not in the high 90° or the plaque was distributed in between two
quadrants, the quadrant with the maximal plaque thickness was chosen for grouping.
2.3. Statistical analysis
All data is presented as the mean9standard devia-tion. The comparison of the plaque distribution among the groups was performed by one-way ANOVA testing of the mean percentage of the distribution for each individual patient. Thus for each patient the percentage of individual plaque distribution was calculated and the mean myocardial, epicardial and lateral plaque orienta-tion of the total group was derived form the individual percentage distribution. Data between the groups was compared using an unpaired Student’s t-test; data not normally distributed between the groups was compared by the Mann – Whitney rank sum test. A P value of
B0.05 was considered statistically significant.
3. Results
Of the 30 vessels undergoing IVUS morphometric analysis, 14 were the left anterior descending, six the left circumflex and ten the right coronary arteries. A total of 613 cross-sections were analyzed, 234 (38.2%) in the left anterior descending artery, 259 (42.2%) in the right coronary artery, and 120 (19.6%) in the left cir-cumflex artery. Baseline characteristics of the patient population are presented in Table 1.
3.1. Eccentric 6ersus concentric plaque
Plaque distribution was found to be concentric in 321 (52.4%) and eccentric in 292 (47.6%) cross-sections. Vessel area (15.6694.41 vs. 15.2895.37 mm2), plaque
area (6.9792.86 vs. 7.6693.19 mm2
shear stress region along the inner wall [5]. The pattern of plaque localization in human coronary arteries is similar to that observed at the carotid bifurcation, as atherosclerosis develops almost exclusively at the outer wall of major bifurcations [1]. The increased tendency of plaque formation at coronary bifurcations has also been confirmed in vivo using IVUS [17,18,25,26].
Several studies have attempted to relate the pattern of atheroma distribution in the coronary arteries to estimated shear stress. In the Harvard Atherosclerosis Reversibility Project pilot study, 20 patients were en-rolled and followed over a period of 3 years to study the relation of wall shear stress to atherosclerosis pro-gression [27]. A finite-difference model of the Navier – Stokes’ equation was used to assess vessel wall shear stress at numerous points along the vessel and plaque progression was monitored by quantitative coronary angiography. In 15 of the 20 arterial segments there was
a significant correlation between low shear stress and an increased rate of atherosclerosis progression. Krams et al. evaluated endothelial shear stress and 3-dimen-sional geometry as factors determining the development of atherosclerosis in a human right coronary artery in vivo [28]. By combining a 3-dimensional reconstruction of the vessel from angiography and IVUS with compu-tational fluid dynamics, it was possible to correlate endothelial shear stress to atherosclerotic plaque forma-tion. Wall thickness was significantly larger and shear stress was significantly less at the inner curve of the vessel. There was an inverse relationship between wall thickness and shear stress for each velocity level under study.
Using IVUS imaging in the catheterization labora-tory permits an optimal technique to investigate the distribution of coronary atheroma as a function of vessel curvature using direct visualization of plaque
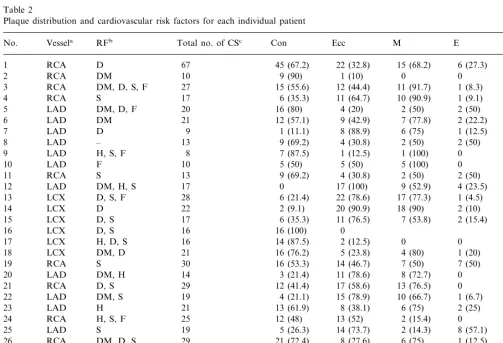
Table 2
Plaque distribution and cardiovascular risk factors for each individual patient
Ecc
aVessel (RCA, right coronary artery; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery).
bCardiovascular risk factors (RF: D, dyslipidemia; DM, diabetes mellitus; S, smoking; F, family history; H, hypertension) and plaque distribution for each patient.
Fig. 3. Distribution (%) of the IVUS cross-sections in the four quadrants (myocardial, epicardial, and two lateral).
evaluated and the vessel area was divided into two semicircles with the same area by a line that was parallel to the pericardium. This method of characteri-zation is substantially different from our approach in which each cross-section was measured and classified into one quadrant depending on the orientation of the maximal plaque thickness. Furthermore, in the present study the complete vessel was evaluated at 1 mm incre-ments excluding only branching points. Thus areas of high, medium and low plaque burden were included in the analysis to yield a broad insight into plaque distri-bution over the entire length of the vessel. An addi-tional difference is that Tsutsui et al. examined coronary sites with mild disease patterns and did not exclude the effects of branch vessels.
4.2. Limitations
In the present study, no conclusion can be drawn regarding the relation of the degree of the vessel curva-ture to the plaque location or plaque thickness. Plaque distribution can only be related to inner or outer curve. It may be of interest to analyze the relationship be-tween plaque accumulation and degree of vessel curva-ture but this is currently confined by technical limitations of the IVUS system.
5. Conclusions
This study provides evidence that human atheroscle-rotic lesions form more frequently along the inner walls (myocardial side) of coronary arteries away from arte-rial branching points. These sites are prone to low shear stress, in which flow separation, flow reversal, turbu-lence, and eddying occur.
Acknowledgements
Dr Jeremias was supported by a grant from the German Academic Exchange Service (DAAD, Bonn, Germany).
References
[1] Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res 1990;66:1045 – 66.
[2] Gibson CM, Sandor T, Stone PH, Pasternak RC, Rosner B, Sacks FM. Quantitative angiographic and statistical methods to assess serial changes in coronary luminal diameter and implica-tions for atherosclerosis regression trials. Am J Cardiol 1992;69:1286 – 90.
[3] Bruschke AV, Wijers TS, Kolsters W, Landmann J. The anatomic evolution of coronary artery disease demonstrated by Fig. 4. Vessel area (VA) and plaque area (PA) in lesions oriented
toward the myocardial and the epicardial side. Results are not significant.
coronary arteriography in 256 nonoperated patients. Circulation 1981;63:527 – 36.
[4] Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg 1987;5:413 – 20.
[5] Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 1983;53:502 – 14.
[6] Grottum P, Svindland A, Walloe L. Localization of atheroscle-rotic lesions in the bifurcation of the main left coronary artery. Atherosclerosis 1983;47:55 – 62.
[7] Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation positive correla-tion between plaque locacorrela-tion and low oscillating shear stress. Arteriosclerosis 1985;5:293 – 302.
[8] Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Gimbrone MA Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 1986;83:2114 – 7. [9] Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and aretrial wall shear, observations, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond (Biol) 1971;177:109 – 59.
[10] Friedman MH, Bargeron CB, Deters OJ, Hutchins GM, Mark FF. Correlation between wall shear and intimal thickness at a coronary artery branch. Atherosclerosis 1987;68:27 – 33. [11] Sakata N, Takebayashi S. Localization of atherosclerotic lesions
in the curving sites of human internal carotid arteries. Biorheol-ogy 1988;25:567 – 78.
[12] Smedby O, Johansson J, Molgaard J, Olsson AG, Walldius G, Erikson U. Predilection of atherosclerosis for the inner curvature in the femoral artery: a digitized angiography study. Arterioscler Thromb Vasc Biol 1995;15:912 – 7.
[13] Sabbah HN, Khaja F, Hawkins ET, et al. Relation of atheroscle-rosis to arterial wall shear in the left anterior descending coro-nary artery of man. Am Heart J 1986;112:453 – 8.
[14] Nissen SE, Gurley JC, Grines CL, et al. Intravascular ultrasound assessment of lumen size and wall morphology in normal sub-jects and patients with coronary artery disease. Circulation 1991;84:1087 – 99.
[15] Tobis JM, Mallery J, Mahon D, et al. Intravascular ultrasound imaging of human coronary arteries in vivo: analysis of tissue characterizations with comparison to in vitro histological speci-mens. Circulation 1991;83:913 – 26.
[16] Yock PG, Linker DT. Intravascular ultrasound: looking below the surface of vascular disease. Circulation 1990;81:1715 – 8.
[17] Kimura BJ, Russo RJ, Bhargava V, McDaniel MB, Peterson KL, DeMaria AN. Atheroma morphology and distribution in proximal left anterior descending coronary artery: in vivo obser-vations. J Am Coll Cardiol 1996;27:825 – 31.
[18] Watanabe H, Yoshida K, Akasaka T, Hozumi T, Yoshikawa J. Intravascular ultrasound assessment of plaque distribution in the ostium of the left anterior descending coronary artery. Am J Cardiol 1996;78:827 – 9.
[19] Fitzgerald PJ, Yock C, Yock PG. Orientation of intracoronary ultrasonography: looking beyond the artery. J Am Soc Echocar-diogr 1998;11:13 – 9.
[20] Mintz GS, Popma JJ, Pichard AD, et al. Limitations of angiog-raphy in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation 1996;93:924 – 31.
[21] Fitzgerald PJ, St Goar FG, Connolly AJ, et al. Intravascular ultrasound imaging of coronary arteries: is three layers the norm? Circulation 1992;86:154 – 8.
[22] Mintz GS, Potkin BN, Keren G, et al. Intravascular ultrasound evaluation of the effect of rotational atherectomy in obstructive atherosclerotic coronary artery disease. Circulation 1992;86:1383 – 93.
[23] Zarins CK, Taylor CA. Hemodynamic factors in atherosclerosis. In: Moore WS, editor. Vascular Surgery. A Comprehensive Review. Philadelphia: W.B. Saunders Company, 1996: 9 – 7110. [24] Ross R. The pathogenesis of atherosclerosis-an update. New
Engl J Med 1986;314:488 – 500.
[25] Kapadia SR, Nissen SE, Ziada KM, et al. Development of transplantation vasculopathy and progression of donor-transmit-ted atherosclerosis: comparison by serial intravascular ultra-sound imaging. Circulation 1998;98:2672 – 8.
[26] Tuzcu EM, Hobbs RE, Rincon G, et al. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation: insights from intravascular ultrasound. Circula-tion 1995;91:1706 – 13.
[27] Gibson CM, Diaz L, Kandarpa K, et al. Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries. Arterioscler Thromb 1993;13:310 – 5.
[28] Krams R, Wentzel JJ, Oomen JA, et al. Evaluation of endothe-lial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coro-nary arteries in vivo: combining 3D reconstruction from angiog-raphy and IVUS (ANGUS) with computational fluid dynamics. Arterioscler Thromb Vasc Biol 1997;17:2061 – 5.
[29] Tsutsui H, Yamagishi M, Uematsu M. Intravascular ultrasound evaluation of plaque distribution at curved coronary segments. Am J Cardiol 1998;81:977 – 81.