Corresponding author: Eva Rachmi E mail: [email protected] Eva Rachmi, Yudanti Riastiti
Laboratory of Anatomy and Histology, Faculty of Medicine, Universitas Mulawarman
: Kemampuan hati mengatasi stres oksidatif dapat ditingkatkan dengan konsumsi antioksidan eksogen yang berasal dari alam. Penelitian ini ditujukan untuk mempelajari kemampuan hepatoprotektif dari ekstrak metanol daun Fagraea racemosa, dengan menggunakan CCL4 sebagai model sumber radikal bebas.
: Tiga kelompok perlakuan tikus Wistar (enam ekor per kelompok), masing*masing diberi dosis ekstrak berturut*turut 50, 100, 200 mg/kg bb per oral, sekali perhari selama 30 hari. CCl4 diinjeksikan intraperitoneal kepada ketiga kelompok , dua kali per minggu (1,5 ml/kg bb). Sebagai pembanding, digunakan dua kelompok kontrol, yaitu kontrol normal dan kontrol CCl4. Pada hari ke*30, tikus dibunuh dan hati diwarnai dengan hematoksilin*eosin. Perubahan histopatologi ditentukan berdasar derajat steatosis, degenerasi hidropik, dan inflamasi. Data dianalisis dengan Anova dan uji post hoc LSD (p≤0.05) menggunakan SPSS versi 13.0
Hasil menunjukkan perbaikan derajat degenerasi hidropik dan inflamasi (p≤0,05) pada ketiga kelompok perlakuan bila dibanding dengan kelompok kontrol CCl4. Tetapi, derajat steatosis meningkat pada kelompok perlakuan dosis 50 dan 100 mg/kg bb, dan kemudian menurun secara bermakna pada perlakuan 200 mg/kg bb.
Ekstrak methanol daun Fagraea racemosa mampu melindungi hati dari radikal bebas yang dihasilkan dari CCl4. Hasil ini mengindikasikan bahwa Fagraea racemosa menjanjikan untuk dikembangkan sebagai suplemen antioksidan. ! "#$% &
ekstrak methanol, Fagraea racemosa, hepatoprotektif
: The ability of the liver in dealing with oxidative stress can be enhanced by consumption of exogenous antioxidants derived from nature. This study aimed to explore the hepatoprotective ability of Fagraea racemosa leaves methanolic extract against CCl4 exposure as a model of free radicals source.
: Three different doses (50, 100, 200 mg/kg bw) were administered orally to three treatment groups of Wistar rats (six Wistar rats each), respectively, once per day for 30 days. CCl4 injected intraperitoneally to those
three groups, twice a week (1,5 ml/kg bw). Two control groups were provided that were one normal control group and one CCl4 control group. On the 30th day, the rats were killed and its liver examined with
Haematoxyllin eosin staining. Histopathological changes were graded based on the degree of steatosis, hydropic degeneration, and inflammation. Data were analyzed with ANOVA and LSD post hoc (p≤0.05) using SPSS version 13.0
The results showed improvement between the three treatment groups and the CCl4 control group about
the degree of hydropic degeneration and inflammation (P ≤ 0.05). However, there were significant increased of steatosis 50 and 100 mg/kg bw treatment groups, before its significantly decrease at 200 mg/kg bw treatment group (2.5 g/kg).
: Fagraea racemosa leaves methanolic extract could protect liver from free radicals generated by CCl4. The result indicated that Fagraea racemosa has promising quality to be explored as antioxidant
supplement. ! "#$% &
Liver as the main organ of metabolism and detoxification have greater risk of oxidative stress than others. Various sources of free radicals in the liver produced by mitochondria in the oxidation phosphorylation process, inflammatory process, or xenobiotics meta bolism (drugs and chemical substances).1 In the state of excessive free radicals, endogenous antioxidants are not able to cope with oxidative stress, thus needs exogenous antioxidants from the diet.2
Borneo forest stores thousands of medicinal plants that have not been optimally utilized as an alternative medicine because of scientific evidence about the benefits and safety is still lacking.3One of the interesting plants studied is Fagraea racemosaJack ex Wall (F. racemosa). Borneo forest is overgrown by 42 Fragaea species, especially in secondary forest with a lot of sunlight. F. racemosa can be grown in different soil types both marshy and dry, at an altitude of 0000 2000 m asl.4 Some tribes use it as medicine. Dayak tribe use stems bark and root for heat lowering medications and pain relievers. A community around Mentoko Bontang forest in using this plant shoots as ulcer treatment. Banjar tribe use the leaves for muscles relaxant on stroke patients.5
Phytochemical and pharmacological studies on F. racemosa have not been much done. Biological activity tests of these extracts showed the ability of analgesics and relaxation of arteries through norepinephrin antagonisme.6 Another study by Purwatiningsih on chloroform and methanol extracts from roots, root barks, stems, stem barks, and leaves of F. racemosa by measuring DPPH reduction (2,2 diphenyl 1 picrylhydrazil), shows highest antioxidant ability of leaf extracts (74%) compared to other parts of the plant extracts. Toxicity tests of methanol leaves extracts on shrimp larvae showed lowest toxicity compared to other parts of the plant extracts.7
Antioxidant ability of F. racemosa leaf extracts in vitro need to be tested in vivo using liver as target organ and carbon tetrachloride (CCl4) as
free radical generator model. CCl4 is activated
in endoplasmik reticulum by hepatic cytoch rome P450 to form trichloromethyl free radicals
(CCl3 and / or CCl3OO ). 8,9
Covalent bond between trichloromethyl with cell protein is the first step of the chain reactions that cause lipid peroxidation and ultimately cause cell necrosis followed by inflammation process.10 In addition to acute toxicity which causes tissue damage, CCl4 also caused apoptosis when administered
at low or moderate doses.11 Damage on cell membran induce hydropic degeneration on hepatocyte. Lipid peroxidation induce the damage to mitochondria, decreased ATP prod uction, and damage to the sodium pump, thereby increasing the intracellular osmotic pressure.12 Effective antioxidant capacity of F. racemosa leaves extract in vivo will inhibit development of liver damage which indicated by milder necrosis, inflamation, dan hydropic degeneration.
This study aimed to explore the hepato protective ability of F. racemosa leaves metha nolic extract against CCl4 exposure as a model
of free radicals source.
!"#$%&
An experimental study was conducted on female Wistar rats (average age 2 months, body weight 150 200 g) during January–May 2008.
Leaves of F. racemosa was collected from forest around Balikpapan town, part of East Kalimantan during February 2008, The taxo nomic identification of plant materials was determined by an ethnobotany expert from Wanariset, Balikpapan. The F. racemosa leaves were dried in shade at room temperature and then ground to a fine powder with mechanic grinder. Then the powdered plant materials soaked with methanol 80% (PA from Merck) overnight, which is repeated several times until the solvent was clear. After the filtration of the solvent, the organic phases were independently concentrated under vacuum evaporator at 80o C.
Wistar rats were obtained from Biology Laboratory of Universitas Brawijaya, which were acclimatized for 14 days in a 12 h light/dark cycle at a constant room temperature with free access to standard pellet food and tap water. All experiments were carried out according to the guidelines for the care and use of experimental animals. 13 The protocol was approved by Pharmacology Laboratory of Universitas Brawijaya
The animals were randomly divided into 5 groups of six Wistar rats each. Group I was maintained as normal control received distil led aqua 5 ml/kg bw orally. All the animals of group II to V received CCl4 (Merck, diluted 1:1 with corn oil) at dose of 1.5 ml/kg intra peritoneally for twice a week. Group II animals were maintained as CCl4 control without any
extract treatment. Group III, IV and V anim als were treated with methanolic extract 50, 100 and 200 mg/kg body weight, respectively by oral route. The methanolic extract was were maintained under normal diet and water ad libitum.
The animals of all the groups were sacrific ed by ketamine anesthesia (1 mg/kg BW on 30th day.14 Livers were removed and preserved in 10% formalin solution for histopathological studies.
Liver tissues collected were used for the preparation of histopathological slides by using microtome and were suitably stained and observed under microscope for architec tural changes. Liver tissue was processed by the paraffin slice technique and sections were stained with hematoxylin and eosin according to the commonly used van Gieson's method.
The stained slices of liver tissue were subjected to the evaluation of steatosis, hydropic degeneration, and inflammation. Steatosis (fatty degeneration) was evaluated based on the number of cells containing fat droplets (foam cell) per 500 cells in one lobule, at 400x magnification. Hydropic degeneration was det ermined by the number of cells contained
hydropic vacuole per 500 cells in one lobule, 400 x magnifications. Both were modification from steatohepatitis grading by Brunt.15 The level of inflammation was evaluated based on the number of lobules infiltrated by dense leukocytes using 100 x magnification, which was modification of Knodell criteria.16 Calcu variance (ANOVA), followed by Least Signi ficance Different (LSD) test. SPSS version 13 was used as statistical software.
!&'("&
During the study, no experimental animals were died. The average increase in animal’s body weight was between 10 20 grams per week. Weight gain was followed by adjusting the dose of extract and CCl4.
The liver of rats in first group had normal architecture, cords of hepatocyte well preserved, cytoplasm not vacuolated, sinusoid well demarcated, no fatty change, no fatty degene ration and no area of infiltration by inflam matory cells (Figure 1a). Figure 1b demonstra ted that CCl4 (compared to normal control
group) induces fat droplet, hydropic vacuolation of cytoplasm, distended hepatocytes, compres sion of sinusoids, and nuclear pyknosis.
Administration of methanolic F. racemosa leaves extract to the test groups (Fig. 1c, 1d and 1e) generally showed improvement when compared with CCl4 treated control group. The
biggest extract dose in these experiment (200 ml/kg) showed minimal damage after induced by CCl4.
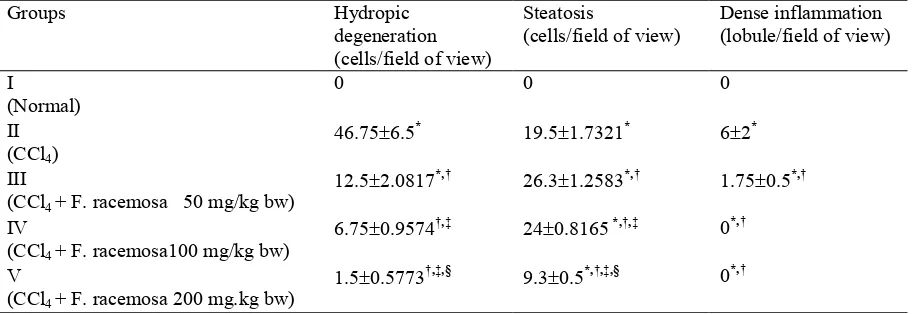
At the end of the 30th day treatment, liver tissue of CCl4 treated control group showed significant
decreased of hydropic degeneration and inflam mation as compared to the CCl4 treated group
(p<0.05). The maximum protection was shown by methanolic extract at the dose of 200 mg/kg bw (Table 1).
F. racemosa extract treatment groups at dose 50 and 100 mg/kgBW induced higher steatosis (35% and 23%, respectively) than CCl4 treated
control group, eventhough hydropic degene
ration and inflamation degree decreased. But at the dose 200 mg/kgBW, F racemosa extract reduced steatosis matched with the decrease of hydropic degeneration and inflam mation. Dose 200 mg/kg bw was also proven to reduce hydropic degeneration and inflammation optimally almost in the level of normal control group, only steatosis level remained signifi cantly different from the normal control group (p<0.05).
) *+ #
(A) normal control group; (B) CCl4 control group; Next picture are (C) 50 mg/kgBW; (D) 100
mg/kgBW, and 200 mg/kgBW (E) F. racemosa extract treated groups
White arrow: steatotic cell, Yellow arrow: hydropic degenerate cell, Blue arrow: inflammatory cell
Table 1. Interplay between treatment of methanolic extract F. racemosa leaves with liver histological change
Groups Hydropic
degeneration (cells/field of view)
Steatosis
(cells/field of view)
Dense inflammation (lobule/field of view)
I
(Normal)
0 0 0
II (CCl4)
46.75 6.5, 19.5 1.7321, 6 2,
III
(CCl4 + F. racemosa 50 mg/kg bw)
12.5 2.0817,-. 26.3 1.2583,-. 1.75 0.5,-.
IV
(CCl4 + F. racemosa100 mg/kg bw)
6.75 0.9574.-/ 24 0.8165,-.-/ 0,-.
V
(CCl4 + F. racemosa 200 mg.kg bw)
1.5 0.5773.-/-0 9.3 0.5,-.-/-0 0,-.
LSD statistical analitics (p<0,05) which significantly different result from:
%1& '&&1$2
Wistar rats were selected as experimental animals because it widely recognized as the most important model for human diseases and disorders. They comprise the majority of all experimental mammals and tend to be the model of choice used for research into many diseases/disorders.17 Rat variations were limited by selected the same gender, and also more or less the same age and body weight.
F. racemosa extract administration five days prior to treatment was intended to reach optimal and stable F. racemosa extract’s blood level given CCl4 (on the fifth day of the study). This
method will clarify the preventive effects of F. racemosa extracts. The research was only tried out 3 doses so the effects of extracts of F. racemosa at higher doses were not analyzed.
Exposure to CCl4 in group II showed clear
intracellular vacuoles which might contain lipids. These vacuoles appear clear because of the embedding process (before haematoxyllin eosin staining) used solvents that dissolve lipids. Accumulation of intracellular lipids occurs because the reactive metabolites of CCl4
(CCL3, CCl3O2) caused lipid peroxidation of
rough endoplasmik reticulum (RER) membrane that resulted on released poliribosom attached to it, separated into single ribosomes. Structured damage of poliribosom disrupts the production apoprotein which make lipid cannot be trans ported out of hepatocytes. In the early stages of exposure, lipids accumulate in the smooth endoplasmik reticulums (SER), which appear as small vacuoles in the cytoplasm. If the process continues, the vacuole will enlarge and pushed the nucleus to the periphery.18
Histologic change in the form of liver cells swelling with clear cytoplasm (hydropic degeneration) at the transition area between healthy and necrotic area, also occurs in CCl4
exposure.19 Hydropic degeneration caused by increased membrane permeability due to lipid peroxidation, resulting in electrolyte imbalance and drive intercellular fluid into intracellular.20 Importation of water causes the cells to swell three times normal size, and depressed nucleus at the center of the cell and become pyknotic. In addition, inflammatory cells mainly
lymphocytes and neutrophil infiltrate perisentral and periportal. Research by Venukumar in rats exposed to CCl4 for 13 weeks, causes changes
such as steatosis, necrosis sentrilobular, hydropic degeneration (ballooning), and fibr osis.21
The degree of steatosis was significantly increased in treatment groups of F. racemosa leaf extract dose 50 and 100 mg/kg bw, eventhough hydropic degeneration and inflammation decreased. The degree of steatosis seen increases may be caused by inhibition of hydropic degeneration so that the cells contain ed lipid droplet can be seen clearly without covered by hydropic vacuoles in it. These result was because the F. racemosa leaf extract can catch metabolite trichloromethyl so it’s not damaging the plasma membrane that prevent hydropic degeneration, but still could damaging endoplasmik reticulum membrane immediately after formed from CCl4 biotrans formation
process that induce steatosis. Its indicate that at dose 50 and 100 mg/kg bw, F. racemosa leaf extract only sufficient to block free radical that emerge at cytoplasm.
At dose of 200 mg/ kg bw, there was inhibition of lipid peroxidation in both plasma membrane and poliribosom, showed by fewer foam cells and hydropic degeneration. A clearer marker of antioxidant potential of F. racemosa leaf extracts was 100 % inhibition of dense inflam mation cells formation at doses of 50 and 100 mg/kg bw compared to CCl4 control group. The
absence of dense inflamation cells could be interpreted as the absence of necrosis.
In conclusion, methanolic extract of F. racemosa leaf can inhibit liver damage induced by CCl4. Mechanism of liver damage caused by
CCl4 is through the formation of free radicals,
which allegedly scavenge by methanolic extract of F. racemosa leaf working as scavenger of free radicals.
!)! !2 !&
1. Halliwell B, Gutteridge J. Free radical in biology and medicine, 3th ed. Oxford: New York; 1998.
2. Gey KF. Vitamins E plus C and interacting conutrients required for optimal health. A critical and constructive review of epidemiology and supplementation data regarding cardio vascular disease and cancer. Biofactors. 1987;1–2:113–74.
3. Hargono D. The development of policy,
guidance, and control of Traditional Medicine. Presented at the Scientific Meeting of Plant
Medicine II, 28 September 1994. Jakarta,
Indonesian.
4. Keßler PJA, Sidiyasa K. Trees of the
Balikpapan Samarinda area, East Kalimantan, Indonesia: a manual to 280 selected species. Wageningen: The Tropenbos Foundation; 1994. Indonesian.
5. Kulip JA. Preliminary survey of traditional med icinal plants in the west coast and interior of Sa bah. 2000. [cited 2008 July 03]. Available from http://www.borneofocus.com/vaic/R&D/articles. html
6. Okuyama E, Suzumura K, Yamazaki M. Pharma
cological Active components of Todo pon Puok (Fagraea racemosa), a Medicinal Plant from Borneo, ChemPharmBull. 1995; 43:2200 4. 7. Purwatiningsih S. Studi Activity and chemical
content of Fagraea racemosa Jack ex Wall:
Laboratory experimental research [thesis]. Surabaya. Universitas Airlangga. 2003. Indo nesian.
8. Jeong HG, You HJ, Park SJ. Hepatoprotective effects of 18β Glycyrrhetinic acid on Carbon tetrachloride induced liver injury: inhibition of Ctytochrome P450 2E1 expression. Pharma cological Research. 2002;46:221 7.
9. Ulicna O, Greksak SJ, Vancova O. Hepato
protective effect of Rooibos tea (Aspalathus linearis) on CCl4 induced liver damage in rats. Physiol. Res. 2003;52:461 6.Andrews LS, Snyder R, Casarett et al. Toxicology: the basic
science of poison, 4th ed. New York; Pergamon Press: 1991.
10. Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of Carbon tetrachloride, Amer ican J Pathology. 1998. 153;2:514 25.
11. Cabana EM. Cellular degeneration and infiltr ation. College of Veterinary Science and Med icine. 2003.Nueva Ecija, Philippines. [Cited 2008 December 12]. Available from http://www .mozcom.com/~emcdvm/path01.html
12. CPCSEA Guidelines for laboratory animal facility. Indian J Pharmacology. 2003;35:257 74.
13. Minister of Agriculture Prevention of Cruelty to Animal. Code of practice for the housing and care of laboratory mice, rats, guinea pigs and
rabbit. Victorian Government Gazette, 16
December 2004. Department of Primary Industries, The State of Victoria.
14. Brunt EM. Nonalcoholic steatohepatitis:
Definition and pathology. Semin Liv Dis. 2001;21:3 16.
15. Knodell RG, Ishak KG, Black WC. Formulation
and application of a numerical scoring system for assessing histological activity in asymp tomatic chronic active hepatitis. Hepatology. 1981;1:431 5.
16. Hedrich HJ, Bullock GR. The laboratory mouse : The handbook of experimental animals. San Diego (Ca)+ Academic Press. 2004.
17. Schiff Alpers DH, Sabesin SM, White HM. Fatty liver: biochemical and clinical aspects. In: Schiff L, Schiff ER, editors. Diseases of the liv er. Philadelphia: JB Lippincott; 1993. p. 825–5. 18. Lauria P, Sharma VN, Vanjani S, et al.. The
Effect of Luffa Echinata in Liver Injury, Indian
J Pharmacology.1976;8:129 33.
19. Cotran RS, Kumar V, Collins T. Robbins Pathologic basis of disease. Philadelphia: WB Saunders. 2000.