Electrical signal from root to shoot in
Sorghum bicolor
: induction
of leaf opening and evidence for fast extracellular propagation
Neeti Sanan Mishra
a, B.N. Mallick
b, Sudhir K. Sopory
a,*
aInternational Center for Genetic Engineering and Biotechnology,Aruna Asaf Ali Marg,New Delhi,110067,India bNeurobiology Laboratory,School of Life Sciences,Jawaharlal Nehru Uni6ersity,New Delhi,110067,India
Received 13 January 2000; received in revised form 29 August 2000; accepted 4 September 2000
Abstract
We have observed earlier that primary leaf opening inSorghumis a light-dependent process. We now show that giving a short photo-exposure to the roots alone also induced leaf opening over a similar time scale. However, any injury to the primary root inhibited the leaf formation. To check the propagation rate and response in this plant, the excitable properties and capability of conduction of electrical stimulus were investigated by extracellular recordings.Sorghumseedlings (5 – 7 days) were examined using non-damaging electrical stimuli. We demonstrate that seedlings when stimulated in one organ, the root region, produced a characteristic response, which could be recorded further up from the stimulating region in another organ, the shoot tissue. The minimum period of stimulation was 150ms and threshold stimulus intensity was 100mA. The general characteristic
electrophys-iological properties of the seedlings and the extracellular propagation of electrical signal suggest that S.bicolorexhibit typical excitable properties comparable to neural tissues. Moreover, electrical stimulus given to the root medium could overcome the requirement of photo-exposure to induce primary leaf formation in etiolated seedlings. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Stimulus; Threshold; Strength – duration relation; Rheobase; Chronaxie
www.elsevier.com/locate/plantsci
1. Introduction
The development and adaptation of plants de-pends on communication between the environ-ment and the cell, between cells in a tissue and between organs. In the last few years there has been increasing evidence for a novel signaling mechanism, involving root – shoot communication, for efficient regulation of plant growth and devel-opment. There is evidence that information from roots, regarding the soil water content, regulates stomatal behaviour, leaf growth, rate of respira-tion and photosynthesis [1,2]. The root – shoot in-teractions may also play an important role in other aspects of plant development. Tripathy and Brown [3] showed that light, when perceived by
roots, inhibited chlorophyll accumulation in leaves of wheat seedlings.
Although so far hormones, cytokinins and ab-scisic acid (ABA) were considered as the likely candidates for the message flowing from the roots to the leaves, evidence also supports the involve-ment of electrical signals. Stimulation of Salix
6iminalis roots by providing nutrients, hormones
or pH-changes caused changes in action potentials in the leaves [4]. These were followed by changes in the rate of photosynthesis and respiration. Sim-ilarly, it was shown that sudden osmotic stress applied to the roots of sunflower generated an electrical signal that resulted in a decrease in the stomatal conductance.
The existence of an electrical or fast regulatory mechanism has not been well established in higher plant systems [5]. The earlier electrophysiological studies in plants were limited to single cells of
* Corresponding author. Fax: +91-11-6162316.
E-mail address:[email protected] (S.K. Sopory).
characean algae [6 – 9] and leaf movements of
Mimosa pudica [10,11], Aldro6anda 6esiculosa [12]
and Dionaea muscipula [13]. Lately there is some
evidence for electrical activity in plants as a mechanism for signal propagation and for regu-lation of various physiological and biochemical responses [14,15]. Mechanically stimulated depo-larizing transients have been shown to be in-volved in regulating the elongation growth of the stem in Luffa cylindrica [16], affecting phloem transport in Mimosa pudica [17], initiating protein synthesis in tomato [18] and increasing the rate of respiration of ovary during pollina-tion in Hibiscus rosasinensis [19]. The characteris-tics of action potentials have been studied in shoots of Lupinus angustifolius [20,21], Helianthus
annuus [22] and Salix 6iminalis [23].
In this study we provide evidence for root – shoot interactions during primary leaf formation (emergence and expansion) in Sorghum bicolor. We identified the involvement of a fast (possibly electrical) signal as the basis of rapid inter-organ communication. In view of this an attempt was made to investigate whether young Sorghum seedlings exhibit excitable properties and could conduct electrical impulses from the root to the shoot.
2. Materials and methods
2.1. Plant material and growth conditions
Seeds of Sorghum bicolor var. PC-6 were ob-tained from the Indian Agricultural Research In-stitute, Regional Center, Karnal. All experiments were carried out at 2691°C in total darkness or continuous light as per requirement. The treat-ment and growth conditions are same as de-scribed earlier [24]. Seedlings (5 – 7 day old) grown in distilled water alone were used for these experimental studies [24].
2.2. Treatment
To study the effect of a short light-exposure on primary leaf formation, 5 day old etiolated seedlings of uniform height and morphology were used. Any exception to this is mentioned specifically in the text. As required, either the root or shoot region of the etiolated seedlings
was irradiated once with light, for 5 min, fol-lowed by continuous darkness. To study the ef-fect of light exposure on roots, the shoot portion was carefully covered by two layers of foil (un-der safe light) before irradiating the roots. Simi-larly the roots were covered before irradiating the entire shoot portion of etiolated seedlings.
The red light (lmax 650 nm, 500 mW cm−2)
was obtained by filtering the light from four 100 W tungsten lamps through CBS filters (Carolina Biological Supply Co., USA) and white light (2600 lux) through white fluorescent tubes. The characteristics of light sources were according to Sharma and Sopory [25].
For electrical recordings, individual seedlings were stimulated with a low intensity electrical pulse (described below). For following the effect of electrical stimulus on primary leaf formation, stimulus of low intensity (0.5 – 1mA, 5 – 10 s) were provided simultaneously to a group of 20 – 25 seedlings, by placing the electrodes in the root medium, to ensure a uniformity in treat-ment.
2.3. Measurement of response
The changes in the morphology of shoot tips were monitored after every 24 h beginning from the day of treatment up to day 5 after the treat-ment [24]. In the case of seedlings growing in the absence of light, the shoot tips were observed very carefully under dim green safe light. The stage of leaf formation and number of seedlings showing the changes in each set were recorded. Besides this, percentage leaf formation was cal-culated as:
Percentage leaf formation=
No. of seedlings showing formation of the first leaf Total no. of seedlings in the set
×100.
2.4. Extracellular recording of electrical impulse
A schematic diagram of the recording setup is shown in Fig. 2. A pair of stainless steel insulated (except at the tip), wires 1 – 1.5 mm apart served as stimulating electrodes. The lower end of seedling (2 – 3 cm from the tip of the root) was placed on the electrodes such that it touched the bare sur-face. The stimulating electrodes were connected to a Grass S44 stimulator through a Grass PSIU6 isolation unit. A stainless steel insulated (except a small portion at the tip) wire approximately 100 mm diameter was used as the recording electrode. It was made to touch the shoot region (3 – 4 cm from the seed) with the help of a micromanipula-tor. The signal was amplified (AM systems) and displayed on an oscilloscope (TDS 420, Tektronix, USA) and a hard copy of the record taken on a printer connected to the latter.
The repeatability of the signal was seen for a minimum of three times on at least three seedlings. The data presented in Fig. 3 is a representative recording of the signal.
3. Results and discussion
3.1. Root-shoot interaction in primary leaf
formation
We have observed earlier that Sorghum leaf formation (emergence from coleoptile sheath and subsequent expansion) is a light-dependent process [24]. There is no leaf formation in seedlings in the absence of light even after 10 – 11 days of growth. However, a short but saturating pulse of light given to the shoot portion of 5 day old seedlings resulted in primary leaf formation. The leaf started emerging from the coleoptile sheath within 1 h of receiving light irradiation and in approximately 24 h a partially open primary leaf was obtained.
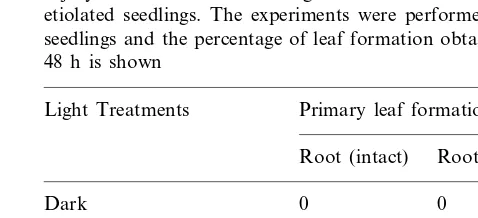
Interestingly, injury to the root inhibited light-induced leaf formation in etiolated seedlings (Table 1). A 5 min photo-exposure could induce primary leaf formation in 5 day old etiolated seedlings but could not induce primary leaf forma-tion if the root tip of the seedlings was cut/ re-moved (Fig. 1A – g). If such seedlings were placed under continuous light (for up to 48 h), the tissue became green, but the leaf remained enclosed within the coleoptile sheath (Fig. 1A – h). In
con-trol plants transferred from darkness to light, pri-mary leaf was obtained within 6 – 10 h. However, primary leaf formation was delayed by more than 48 h in Sorghum seedlings grown and germinated in continuous light if root was cut (Fig. 1A – b) and even in this case a fully expanded leaf was not obtained. This suggests the involvement of root – shoot interactions in the formation of primary leaves.
Primary leaf formation could also be induced in etiolated seedlings when the roots were exposed to a 5 min pulse of white light or red light, and the kinetics of leaf formation followed a similar tem-poral relation, as observed on giving light irradia-tion to the shoot porirradia-tion only (Fig. 1B). This indicated that the signal must be communicated rapidly to the shoot tips, suggesting the presence of a fast, possibly electrical, signaling pathway. Considering that the stimulus was given to the root tissue while the response was measured in the shoot region, the possibility exists of a pathway for rapid conduction of signals in the form of an electrical impulse as one of the viable mechanisms for communication between the two main organs of the seedlings. There is other evidence that indi-cates long-range communication between the root and shoot, facilitating regulation of normal growth and development. Earlier studies impli-cated phytohormones, cytokinins and ABA, as likely candidates for transmission of the signal [1]. However recent studies have demonstrated that in addition electrical transients may be involved [15]. The regulation would thus involve an interaction between the electrical and hormonal messages.
The photosignal has been shown to mediate changes in membrane potentials in maize [26] and oat [27] coleoptiles, expanding leaves of pea [28]
Table 1
Injury to the root inhibited light-induced leaf forination in etiolated seedlings. The experiments were performed on 5 d seedlings and the percentage of leaf formation obtained after 48 h is shown
Light Treatments Primary leaf formation (%)
Fig. 1. (A) Representative photograph to show the degree of light induced leaf formation obtained after cutting the root of light grown (a – d) and etiolated seedlings (e – h). The various panels represent (a) Light grown seedling with intact root; (b) Light grown seedling with cut root; (c) Light grown seedling transferred to darkness (for 24 h) after cutting the root; (d) Light grown seedling transferred to darkness (for 48 h) after cutting the root; (e) Etiolated seedling with intact root; (f) Etiolated seedling with cut root; (g) Etiolated seedling in which root was removed immediately after the 5 min red light exposure; (h) Etiolated seedling transferred to continuous light after cutting the root. (B) The time course of response obtained on providing a 5 min light exposure to shoot and root tissues of 5 d etiolated seedlings is compared. WL, white light; RL, red light.
in ion transport are thought to be part of a transduction chain that links a blue light receptor to inhibition of hypocotyl growth.
To look for the possibility of electrical conduc-tion between the root and shoot, the excitable properties of 5 – 7 day old Sorghumseedlings were studied. One of the properties of excitable tissues is their characteristic response to a threshold stim-ulus and their ability to propagate an impulse if stimulated by a suprathreshold stimulus. This has been routinely studied in neural tissues. The elec-trophysiological property of an excitable tissue is that it follows the all-or-none law, has a threshold and the tissue has a characteristic strength – dura-tion curve. Hence, we recorded surface potential (at the shoot), which is comparable to extracellular potential (Fig. 2), in S. bicolor.
3.2. Characteristics and modulation of the signal
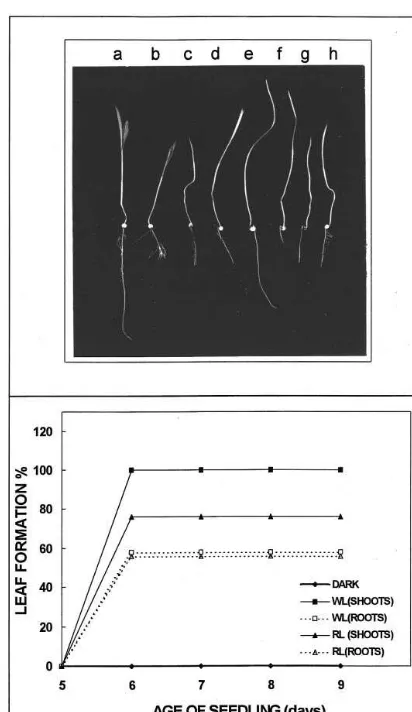
Five day old seedlings, when stimulated electri-cally either in the root or epicotyl (shoot) region, produced a characteristic response, which could be recorded further up from the stimulating region in the shoot tissue. When the tissue was stimulated with an electrical square wave pulse of low inten-sity only a stimulus artifact appeared (Fig. 3A). The artifact indicates the time of stimulation. At a
Fig. 2. A schematic diagram of the experimental set up for stimulation and recording of response in Sorghum bicolor. The Stimulator (S44) is connected through the current isola-tion unit (PSIU6) to a pair of stimulating electrodes, which are in contact with the root surface. The signal was amplified and displayed on the oscilloscope (CRO-TDS420) and hard copy taken as print out.
Fig. 3. Recordings of the response. (A) Only stimulus artifact, which marks the point of stimulation, can be seen at low intensity of stimulation. (B) The characteristic response/biological-signal was recorded (arrow) from the epicotyl portion when the root tissue was stimulated at 0.5 Hz, 300ms, 100mA. (C) 25 stimulus bound responses were overlapped in this to show the response
was consistent and reproducible. (D) On cooling the area of root tissue between the stimulating and recording electrodes the response was gradually lost. (E) The response (arrow) started reappearing after 30 min. (F) The response is lost after 10 min of ether treatment (between the root and the shoot). Five stimulus bound responses have been overlapped here to show the consistency in recording. (G) The recording from a transected seedling. Only the stimulus artifact was seen after transecting the plant in between the stimulating and recording electrodes and keeping the two cut ends in physical contact. (H) The recordings from a normal and a transected seedling are overlapped to show the loss of response.
higher intensity of stimulation the ‘response’ was recorded (Fig. 3B). The response was recorded at a latency of 400ms and the rate of propagation of the signal was calculated to be 270920 m s−1. It
is difficult to comment from this study if the signal was an action potential or a hyperpolarising po-tential. As the signal was recorded after some delay it is unlikely to be an induced current such as that of the artifact. According to standard neurophysiological knowledge it may be an action potential, since it is likely to be propagatory. Since there is as yet no definite evidence that there are propagatory action potentials in plants, we have preferred to term it as a ‘response’.
3.3. Threshold
The response was not obtained until the stimu-lation strength was increased to 100mA. Moreover
the intensity of the response did not change signifi-cantly with a further increase in the stimulation strength. The response was slow and it did not faithfully follow a stimulation frequency of more than 1 Hz. Even during a 1 Hz stimulation the first response was greater than the subsequent ones. Stimulation at 0.5 Hz was best in producing uniform reproducible responses to every stimulus, as shown in the overlap response (Fig. 3C).
3.4. Modulation of the response
(Fig. 3D). It is likely that the cooling did not allow the signal (generated at the stimulation site) to reach the recording site. The response started to gradually reappear within 20 – 30 min following withdrawal of cold treatment (Fig. 3E) and the recovery process was hastened, within 5 – 10 min, by increasing the surface temperature to 25°C by the application of warm water. This indicated that the cold induced alteration in response was not due to permanent damage to the tissues. The tissue responded to a heat shock (given by application of 55 – 60°C warm water on the tissue) in a manner similar to that for the cold shock.
A reduction in the response was also observed by the application of a few drops of organic solvent (ether) on the root surface. The response was significantly reduced within 8 – 10 min after ether application (Fig. 3F). This is likely to be due to changes in the biological parameters and this indicated that the observed response was not a physical process. Moreover, the response was not observed after transecting the plant in between the stimulating and the recording electrodes. The re-sponse was not observed even if the transected ends were kept in physical contact (Fig. 3G and H). This also suggested that the response was likely to be a biological phenomenon that was lost on severing the biological conductivity.
The signal is unlikely to be a physical process, primarily because it followed the basic characteris-tics of excitation. The rate of conduction of the response was calculated to be 270920 m s−1,
which is much slower than the speed of conduc-tion of electricity. A physical conducconduc-tion of elec-tric current would neither have latency nor have been affected by a small change in temperature nor by organic solvent. Also it should have been recorded even when the cut ends of the seedling were kept in contact with each other. These studies also indicate the existence of a physiological path-way for conduction of a fast signal in Sorghum seedlings. Since the stimulus was given to the root tissue while the response was picked up in the shoot region, it is likely that there exists a mecha-nism for communication between the two main organs of the plants. Faster conduction (2 – 8 cm s−1), possibly through conducting tissues, has
been reported in different plants [22,23]. In this study response up to 10 m was recorded and we observed faster conduction than has been earlier reported. However, there may be explanations for
such fast conduction. It is possible that in young seedlings secondary depositions are not present in the cell walls. The current may be propagating along the surface without regeneration in every cell, unlike the classical picture of the action po-tential in neurons. It is also possible that there could be some physiological factors explaining such fast propagation, as it was not seen in the older seedlings.
That the response was a biological process may be argued by the fact that it was not seen in plants that had shriveled up and dried. It was also seen that the degree of excitability of plant tissues decreased with age. Five to 7 day old seedlings exhibited high excitability while 9 – 10 day seedlings were less excitable and responded only to a very high intensity of stimulation (0.5 Hz, 300 ms, 1.5 mA). Moreover, in the latter case, the response did not show repeatability. A possible explanation is that as the seedlings grew older their response to the stimulus diminished due to changes in membrane structure or wall deposi-tions. In fact studies have shown that the velocity of transmission in the petiole of Mimosa [11] and in the apical part of the stem in Lupinus [21] decreases on maturity of the tissue. It has also been shown that amplitude and velocity of propa-gation in Helianthus were higher in the upper, younger parts of the stem [22].
3.5. Strength–duration (S–D) cur6e
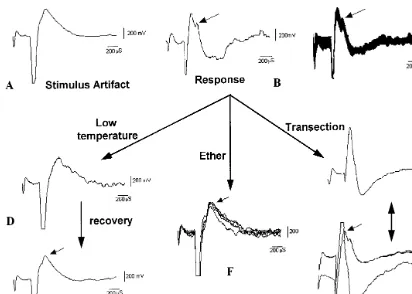
The excitable cells/tissues, including neurons and muscles follow a characteristic S–D relation-ship. This is considered an important parameter for studying excitability characteristics of excitable tissues, hence the same was studied for S. bicolor. In order to plot a S–D curve, the duration of the stimulus was kept constant and the current strength was slowly increased till the response appeared i.e. the minimum current strength re-quired to get the response was noted. The dura-tion of the stimulus pulse was gradually increased in steps of 25 ms and at each position the mini-mum current required to produce the response was noted. S.bicolorfollowed a classicalS–Dcurve as shown in Fig. 4.
Fig. 4. Strength – duration curve ofS.bicolorseedling. As seen from the plot, the response was not obtained unless a mini-mum strength of 100mA and a minimum duration of 150 ms
was delivered to the plant tissue. The plot shows mean (9 SD) of recordings from five seedlings. R — Rheobase; C — Chronaxie.
replace the requirement of light for primary leaf formation [24]. In this paper we show that in addition to chemically induced leaf formation, eti-olated leaves could be obtained in the absence of light by stimulating the 5 day dark grown seedlings with an electric pulse of low intensity (Table 2). The electric pulse was provided to a group of 20 – 25 seedlings by placing the electrodes in the root medium, such that they did not come in direct contact with the seedlings. The data obtained indicated that leaf formation induced by electrical stimulation, showed a qualitative depen-dence on the current strength, as an optimum response was obtained on providing 5 mC current (0.5 mA for 10 s or 1.0 mA for 5 s). With a further increase in current there was a decline in the percentage of leaf formation obtained. It is possi-ble that the electrical pulse directly or indirectly triggers a secondary pathway, involving a second messenger system, which in turn after a required period of 12 – 24 h, would lead to the desired response i.e. emergence of the leaf from coleoptile sheath and its expansion.
Since the experiments were conducted in a group of seedlings (not individual seedlings) and also in the dark, precise placement of the elec-trodes (stimulating electrode as well as recording electrode) was not possible. The electrical current was delivered to the medium where only part of the roots were exposed and the other parts of the seedlings were unexposed to the medium and, since the current had to pass from the medium to the root of the seedling, a much higher intensity of current (c.f. direct stimulation of individual seedling as in Fig. 3) was required to induce the response. Although this data suggests that the electrical current induced leaf opening was likely to have been mediated through the root, direct experimental evidence is needed.
unless a minimum duration of 150 ms was deliv-ered to the plant tissue. The rheobase (threshold stimulus intensity) was 100 mA and chronaxie (minimum duration to get the response at an intensity twice that of the rheobase) was 370 ms. Based on the observations in this work it can be concluded thatS. bicolortissue has excitable prop-erties. The seedlings when stimulated electrically either in the root or epicotyl region produced a characteristic response, which could be recorded further up from the stimulating region. The re-sponse produced by the electric stimulus (pulse) had a threshold, followed the all-or-none law and exhibited a characteristic S–Drelationship. More-over, it could be modulated by properties of the biological conducting tissue.
3.6. Electrical stimulus can replace the
requirement of light
We have earlier observed that exogenous addi-tion of calcium and/or other ions could partially
Table 2
Effect of an electric pulse on primary leaf formation. Etiolated leaves were obtained by stimulating the 5 d dark grown seedlings with an electric pulse. The percentage of leaf formation obtained after 48 h is shown
Duration of electric pulse Intensity of electric pulse¡
15 (s)
1 (s) 5 (s) 10 (s)
70.691.12 58.691.7
34.791.9
0.5 (mA) 56.592.7
42.392.1 61.391.53 63.692.1 51.191.3
4. Conclusion
The existence of an electrical signaling mecha-nism in higher plants has been reported by measur-ing action potentials in insectivorous plants [31] and during leaf movements in Mimosa pudica [10,11]. Experiments have been done on several higher plants using intra-cellular microelectrodes and sur-face-contact electrodes [32]. Frachisse et al. [33] have observed a correlation between electrical events and plant development by studying shoot-apex formation inBidens pilosus. Wildon et al. [18] have shown that wounding induced the propagation of electrical transients in tomato seedlings. These were followed by the production of the mRNA proteinase inhibitor protein, Pin2, within 4 h, and proteinase inhibitor activity within 24 h, in the non-wounded leaves. The characteristics of action potentials have been studied in the shoots of He
-lianthus annuus[22] and Salix6iminalis[23]. These
studies were performed on older plants (16 – 22 days), which exhibited high excitability levels (re-quiring a minimum stimulus of 2 V, 1 s). Plants of similar size, shape and age, grown under identical conditions, exhibited high variability in the degree of excitation.
In this study we observed that electrical stimulus can replace the requirement of light for primary leaf formation in S. bicolor. It was also seen that root – shoot interaction plays an important role during the early stages of growth and the establish-ment of seedlings. The rapid transmission of the message/signal seems to involve the generation and transduction of electrical impulses which, in turn, can manifest a response either directly or through a second messenger pathway. The young seedlings (5 – 7 day) were highly excitable and showed a consistency and repeatability in the response (Fig. 4), but with an increase in age the tissues became less excitable and lost the repeatability of response. The generation of a fast response due to an electric stimulus and the existence of a pathway for the conduction of this response, suggests that this signaling process could form the basis of rapid communication between different tissues and/or organs in Sorghum, in addition to the known chemical signaling mechanisms.
Acknowledgements
The work was partly supported by the University
Grants Commission, New Delhi, in the form of a research grant to SKS and a Senior Research Fellowship to NSM.
References
[1] W.J. Davies, J. Zhang, Root signals and the regulation of growth and development of plants in drying soil, Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 (1991) 55 – 76.
[2] W.J. Davies, F. Tardieu, C.L. Trejo, How do chemical signals work in plants that grow in drying soil, Plant Physiol. 104 (1994) 309 – 314.
[3] B.C. Tripathy, C.S. Brown, Root – shoot interaction in the greening of wheat seedlings grown under red light, Plant Physiol. 107 (1995) 407 – 411.
[4] J. Fromm, W. Eschrich, Electric signals released from roots of willow (Salix6iminalisL.) change transpiration
and photosynthesis, J. Plant Physiol. 141 (1993) 673 – 680.
[5] R. Wayne, The excitability of plant cells: with a special emphasis on characean internodal cells, Bot. Rev. 60 (1994) 265 – 267.
[6] H. Dziubinska, A. Paszewski, K. Trebacz, T. Zawadzki, The effect of excitation on the rate of respiration in the liverwort Conocephalum conicum, Physiol. Plant. 75 (1983) 417 – 423.
[7] G.P. Findlay, Voltage-clamp experiments with Nitella, Nature 191 (1961) 812 – 814.
[8] D. Gradmann, ‘‘Metabolic’’ action potentials inAcetab
-ularia, J. Membr. Biol. 29 (1976) 23 – 45.
[9] A.B. Hope, N.A. Walker, The physiology of giant algal cells, Cambridge University Press, Cambridge, 1975. [10] T. Sibaoka, Excitable cells in Mimosa, Science 137
(1962) 226.
[11] T. Sibaoka, Action potentials and rapid plant move-ments, in: F. Skoog (Ed.), Plant Growth Substances 1979, Springer, Berlin, 1980, pp. 462 – 469.
[12] T. Iijima, T. Sibaoka, Action potential in the trap-lobes of Aldro6anda 6esiculosa, Plant Cell Physiol. 22 (1981)
1595 – 1601.
[13] D. Hodick, A. Sievers, The action potential ofDionaea muscipulaEllis, Planta 174 (1988) 8 – 18.
[14] E. Davies, Action potentials as multifunctional signals in plants, a unifying hypothesis to explain apparently dis-parate wound responses, Plant Cell Environ. 10 (1987) 623 – 631.
[15] J.F. Thain, D.C. Wildon, Electrical signalling in plants, in: M. Smallwood, J.P. Knox, D.J. Bowles (Eds.), Mem-branes: Specialized Functions in Plants, BIOS Scientific Publ, UK, 1996, pp. 301 – 317.
[16] T. Shiina, M. Tazawa, Action potentials inLuffa cylin
-drica and its effects on elongation growth, Plant Cell Physiol. 27 (1986) 33 – 39.
[18] D.C. Wildon, J.F. Thain, P.E.H. Minchin, I.R Gubb, A.J. Reilly, Y.D. Skipper, H.M. Doherty, P.J. O’Don-nell, D.J. Bowles, Electrical signalling and systemic proteinase inhibitor induction in the wounded plant, Nature 360 (1992) 62 – 65.
[19] J. Fromm, M. Hajirezaei, I. Wilke, The biochemical response of electrical signalling in the reproductive sys-tem of Hibiscus plants, Plant Physiol. 109 (1995) 375 – 384.
[20] A Paszewski, T. Zawadzki, Action potentials inLupinus angustifolius L. shoots, J. Expt. Bot. 25 (1974) 1097 – 1103.
[21] T. Zawadzki, Action potentials inLupinus augustifolius
L. shoots. V. Spread of excitation in the stem, leaves and root, J. Expt. Bot. 31 (1980) 1371 – 1377.
[22] T. Zawadzki, E. Davies, H. Dziubinska, K. Trebacz, Characteristics of action potentials inHelianthus annuus, Physiol. Plant. 83 (1991) 601 – 604.
[23] J. Fromm, R. Spanswick, Characteristics of action po-tentials in willow (Salix 6iminalisL.), J. Expt. Bot. 44
(1993) 1119 – 1125.
[24] N. Sanan, S.K. Sopory, A role of G-proteins and cal-cium in light regulated primary leaf formation in Sor
-ghum bicolor, J. Expt. Bot. 49 (1998) 1695 – 1703. [25] A.K. Sharma, S.K. Sopory, Independent effects of
phy-tochrome and nitrate on nitrate reductase and nitrite reductase activities in maize, Photochem. Photobiol. 3 (1984) 491 – 494.
[26] R.H. Racusen, A.W. Galston, Phytochrome modifies
blue-light-induced electrical changes in corn coleoptiles, Plant Physiol. 66 (1980) 534 – 535.
[27] I.A. Newman, Rapid electric responses of oats to phy-tochrome show membrane processes unrelated to pel-letabilty, Plant Physiol. 68 (1981) 1494 – 1499.
[28] M. Staal, T.M. Elzenga, A.G. vanElk, H.B.A. Prins, E. Van-Volkenburgh, Red and blue- stimulated proton efflux by epidermal leaf cells of the argenteum mutant of
Pisum sati6um, J. Expt. Bot. 54 (1994) 1213 – 1218.
[29] E.P. Spalding, An apparatus for studying rapid electro-physiological responses to light demonstrated on Ara
-bidopsis leaves, Photochem. Photobiol. 62 (1995) 934 – 939.
[30] E.P. Spalding, D.J. Cosgrove, Large plasma-membrane depolarization precedes rapid blue-light induced growth inhibition in cucumber, Planta 178 (1989) 407 – 410. [31] S.E. Williams, B.G. Pickard, The role of action
poten-tials in the control of capture movements ofDroseraand
Dionaea, in: F. Skoog (Ed.), Plant Growth Substances 1979, Springer, Berlin, 1980, pp. 470 – 480.
[32] J.F. Thain, Electrophysiology, in: D.W. Gallbraith, H.J. Bohnert, D.P. Bourque (Eds.), Methods in Cell Biology, vol. 49, Academic Press, San Diego, 1995, pp. 259 – 274. [33] J.M. Frachisse, M.O. Desbiez, P. Champagnat, M. Thel-lier, Transmission of a traumatic signal via a wave of electric depolarization, and induction of correlations between the cotyledonary buds in Bidens pilosus, Phys-iol. Plant. 64 (1985) 48 – 52.