Precambrian Research 102 (2000) 21 – 46
A crustally contaminated komatiitic dyke – sill – lava complex,
Abitibi greenstone belt, Ontario
M.S. Stone *, W.E. Stone
Kambalda Nickel Operations,WMC Resources,Kambalda,WA6442,Australia
Received 26 May 1999; accepted 19 November 1999
Abstract
Detailed field and laboratory studies indicate the Shaw Dome area of the Abitibi greenstone belt, Ontario, contains a lower horizon of intrusive komatiitic rocks (LKH), komatiitic dykes, and an upper horizon of extrusive komatiitic rocks (UKH). The LKH is 500 – 1000 m thick and consists of undifferentiated dunite (45 – 50% MgO), laterally equivalent wehrlite (20 – 50% MgO), and differentiated wehrlite – amphibole gabbro sills. A differentiated wehrlite – amphibole gabbro dyke intrudes calc-alkalic volcanic rocks and iron-formation. Undifferentiated wehrlite dykes (17 – 25% MgO) intrude calc-alkalic volcanic rocks and sulphide iron-formations stratigraphically underlying the LKH and contain xenoliths of those rocks. The UKH is 500 – 3000 m thick and consists of up to 50 m thick, weakly differentiated komatiites that grade laterally and vertically to thinner, undifferentiated and spinifex textured komatiite (20 – 35% MgO) and komatiitic basalt (5 – 20% MgO) flows, and host minor nickel sulphide mineralisation. These vertical and lateral variations are interpreted to be primary komatiitic lithofacies variations in a single intrusive – ex-trusive stratigraphic sequence. This sequence represents a dyke – sill – lava complex and constrains facies models for komatiitic magmatism – volcanism. The vertical and lateral transition from LKH dunite to wehrlite to wehrlite – am-phibole gabbro sills represents the transition from proximal dynamic flow conduits to more static, ponded distal facies in a sub-volcanic magma chamber. The LKH is underlain by a largely hidden system of wehrlite dykes, possibly feeders to the overlying sills. The distribution of UKH undifferentiated, spinifex textured, and brecciated komatiite and komatiitic basalt flows represents the transition from proximal channel to distal sheet flow volcanic facies. Geochemical modelling utilising MELTS suggests that the LKH and UKH are related by liquid – crystal fractionation and accumulation of olivine9clinopyroxene from magma that assimilated variable amounts of calc-alkalic volcanic rocks9iron-formation. Furthermore, the modelling results suggest derivation of dunite and wehrlite and komatiite flows from uncontaminated magma and differentiated wehrlite-amphibole gabbro units and komatiitic basalt flows from contaminated magma. This contrast in degree of contamination is explained by intrusion and eruption of uncontaminated komatiite followed by assimilation of fused crustal material adjacent to magma conduits, fractional crystallisation, and eruption of the contaminated magma as komatiitic basalt. Given the generally accepted relationship of contamination and dynamic/static flow transitions to nickel sulphide mineralisation, komatiitic dyke/sill contacts should be sought and targeted in nickel sulphide exploration. © 2000 Elsevier Science B.V. All rights reserved.
www.elsevier.com/locate/precamres
* Corresponding author.
E-mail address:[email protected] (M.S. Stone)
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
22
Keywords:Geochemistry; Petrology; Komatiites; Archaean; Dykes; Sills; Facies
1. Introduction
The concept that Archaean komatiite melts flowed dynamically during ascent and emplace-ment (Nisbet 1982; Huppert et al., 1984) and assimilated crustal materials has critical implica-tions for models of Archaean mantle
composi-tions and thermal regimes (Arndt, 1986a),
magmatism and volcanism (Huppert and Sparks, 1985a), crustal evolution (e.g. Arndt and Jenner, 1986), and nickel sulphide deposit genesis (Hup-pert et al., 1984; Lesher and Campbell, 1993). A wide variety of evidence exists that some komati-ites thermally eroded crustal wall rocks adjacent to melt conduits and footwall rocks to lava chan-nels during magma ascent and lava emplacement (Arndt and Jenner, 1986; Compston et al., 1986; Frost and Groves, 1989; Chauvel et al., 1993; Lesher and Arndt, 1995; Perring et al., 1995). However, virtually all the evidence is from extru-sive rocks. Contamination of intruextru-sive komatiitic rocks has not been reported. Recognition of con-tamination in subvolcanic dykes and sills and in associated lava flows would greatly benefit models
for komatiitic magmatism – volcanism. Such
recognition could also be economically important in targeting komatiite terrains for nickel sulphide deposit potential, because of the relationship of contamination to komatiite-associated nickel de-posit genesis (Lesher, 1989).
This paper (1) documents the field relationships and textural and mineralogical characteristics of komatiitic rocks in an Archaean komatiitic dyke – sill – lava complex in the Shaw Dome, Abitibi belt, (2) documents major, minor and trace element geochemical evidence of contamination, and (3) utilises these data, in conjunction with field data and the results of MELTS program modelling (Ghiorso and Sack, 1995), to constrain models for the geochemical evolution of the complex. The study focuses on the Shaw Dome, because it is a key area for komatiitic intrusive rocks (Muir, 1979) and the intrusive and extrusive komatiitic rocks are petrologically related and form a single stratigraphic sequence, representing a dyke – sill –
lava complex (Larson, 1996). The range of incom-patible element contents in the evolved komatiitic intrusions and related komatiitic basalts is argued to indicate crustal contamination.
2. Geologic setting and background
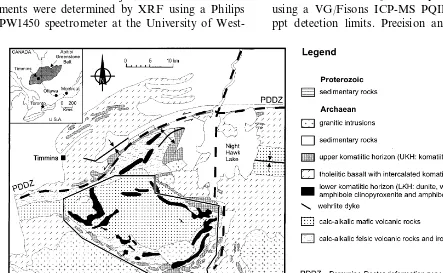
Komatiitic rocks are preserved in the margins of the Shaw Dome, a major anticline centred
:20 km southeast of Timmins, in the Abitibi
greenstone belt, Ontario (Muir, 1979; Green and Naldrett, 1981; Fig. 1). The komatiitic rocks are present in two horizons in a 4-km thick sequence of komatiitic to calc-alkalic rocks and iron-forma-tions (Fig. 1), and in dykes intruding underlying calc-alkalic rocks and iron-formations. The two horizons are referred to as the lower komatiitic horizon (LKH) and the upper komatiitic horizon (UKH). The LKH consists of intrusive komatiitic rocks, whereas the UKH consists of extrusive komatiitic rocks.
The age of the LKH is bracketed by U-Pb dates for zircons from underlying calc-alkalic felsic
vol-canic rocks (272591 Ma; Corfu and Noble,
1992) and a gabbro differentiate of a thick
wehrlite – gabbro dyke of the LKH (270793 Ma;
Corfu et al., 1989; Corfu and Noble, 1992). The age of the UKH is bracketed by a U-Pb age for zircons from overlying calc-alkalic felsic volcanic
rocks (270391.5 Ma; Corfu et al., 1989).
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 23
The metamorphic alteration caused extensive geochemical mobility, as indicated by wide scat-tering of Na, K, Rb, Cs, Sr, Ba, Eu, Ca, Cu, Zn, and S data on MgO variation diagrams (Larson, 1996). Such mobility is common (Lahaye et al., 1995; Lahaye and Arndt, 1996) and these data are not considered further. All other elements exhibit regular trends on MgO variation plots and are therefore considered immobile. The REE are mo-bile during carbonate alteration (Lahaye et al., 1995), but the REE trends described below do not appear to have been affected and these samples contain only minor to trace amounts of carbonate minerals.
3. Analytical methods
Concentrations of major, minor and trace ele-ments were determined by XRF using a Philips PW1450 spectrometer at the University of
West-ern Ontario. Precision and accuracy errors are within 1% for the major elements and 5% for the
minor and trace element values \15 ppm and
30% for values B5 ppm (Larson, 1996). Probe
polished thin sections were prepared and olivine and amphibole analysed at the University of
Ala-bama using a JEOL JXA-8600/3 electron probe
equipped with five wavelength-dispersion X-ray spectrometers and a TN-5400 energy-dispersion X-ray analysis spectrometer.
The REE were analysed by ICP-MS in the Ultratrace lab at the University of Montreal util-ising the analytical strategies of Cheatham et al. (1993) and Lahaye and Arndt (1996). Whole-rock samples, 100 mg in size, were dissolved in
high-purity HF and HClO4 under clean-room
condi-tions. The resulting fluoride and perchlorate salts were converted to high-purity nitrates and diluted
to 200 ml in 2% HNO3. All samples were analysed
using a VG/Fisons ICP-MS PQII+ with :10
ppt detection limits. Precision and accuracy
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
24
rors, as determined from replicate analyses of internal and international rock standards, are within 5%.
4. Komatiitic rock types
A wide variety of komatiitic rock types with diverse textures, geochemical compositions, grain sizes, and rock units are recognised in the LKH and UKH. They are described in detail below.
4.1. Lower komatiitic horizon and footwall dykes
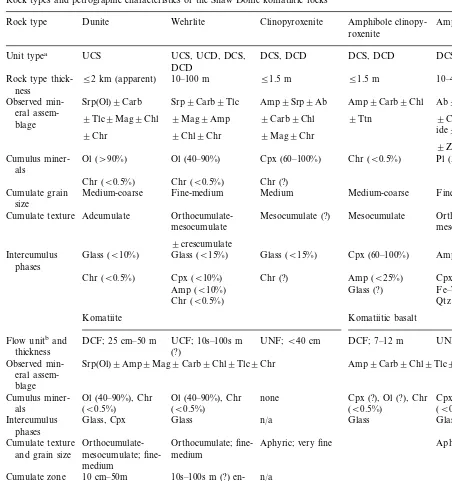
The LKH rock types are dunite, ortho- to mesocumulate wehrlite, amphibole clinopyroxen-ite, clinopyroxenite and cumulate and noncumu-late amphibole gabbro (Table 1). These rock types comprise a variety of undifferentiated and differ-entiated sills and dykes (Table 2) interlayered with iron-formation and calc-alkalic volcanic rocks. In-trusive contacts were observed in some outcrop areas, whereas others were covered.
The LKH consists of massive dunite sills that are inferred at map scale to pass laterally to
massive wehrlite and differentiated wehrlite
(base)-clinopyroxenite-amphibole gabbro (top)
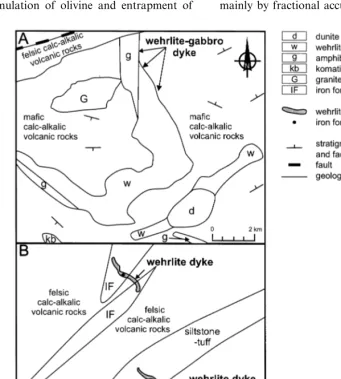
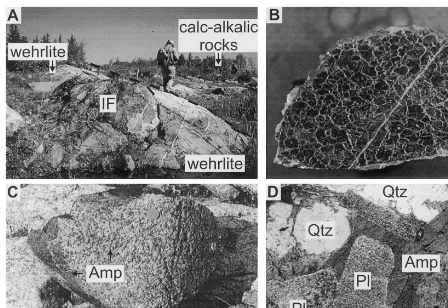
sills (Pyke, 1982; Tables 1 and 2). The amphibole gabbro zone in a single differentiated sill appears to cut the surrounding rocks and is interpreted in part to be a dyke (Fig. 2A). Wehrlite dykes have been recognised in a single area stratigraphically downsection of the LKH, which appear to cut the footwall rocks (Fyon, 1980; Fig. 1, 2B and 3A).
The dunite sills are fine to coarse grained and locally appear to embay the underlying calc-alka-lic volcanic rocks (Fig. 3B). Olivine is preserved in only a single LKH dunite sample (Table 3;
Fo92.4). The massive wehrlite sills show
mesocu-mulate to orthocumesocu-mulate textures and appear to be stratigraphically conformable. The differenti-ated wehrlite – amphibole gabbro units are mainly sills up to 1 km thick and, from stratigraphic bottom to top, consist of: wehrlite, clinopyroxen-ite, amphibole clinopyroxenclinopyroxen-ite, and amphibole gabbro zones. These rock zones are described below.
The wehrlite zones in the differentiated units are 5 – 20 m thick and are indistinguishable from those of the massive wehrlite sills (Tables 2 and 3), except for the presence of igneous amphibole. The clinopyroxenite and amphibole
clinopyroxen-ite zones are B3 m thick each, consisted of
mesocumulus clinopyroxene and rim amphibole, and appear to grade on a scale of centimetres to tens of centimetres to amphibole gabbro (Pyke, 1982; this study). The amphibole gabbro zones are up to 50 m thick, medium-grained, leuco-cratic, and contain coarse-grained to pegmatitic patches near the base (Table 2; Fig. 3C and D). Igneous amphiboles are preserved as kaersutite and titanian pargasite to hastingsite oikocrysts (Table 3). Upsection, the proportion of igneous amphibole gradually decreases relative to plagio-clase, and that of igneous quartz increases. The absence of cumulate textures and the high abun-dance of Si, alkalis, and incompatible trace
ele-ments (Table 4) suggest the quartz-bearing
amphibole gabbros are noncumulate rocks, and could represent near-liquid compositions. The contact of amphibole gabbro with the overlying calc-alkalic volcanic rock is exposed only in a single location and is sharp.
At a single locality, the lower portion of the amphibole gabbro zone, in what appears in part to be a dyke rather than a sill (Fig. 2A), contains subrounded patches of amphibole clinopyroxen-ite. The amphibole clinopyroxenite patches may represent xenoliths of partially melted roof-rock fragments. Alternatively, these patches could have been derived from the adjacent amphibole gab-bro. Evidence in support of a xenolith origin for the amphibole clinopyroxenite patches is not apparent.
Two massive wehrlite dykes intrude calc-alkalic volcanic rocks and sulphide iron-formations, which stratigraphically underlie the LKH and contain xenoliths of those rocks (Fyon, 1980; Fig. 1, 2B and 3A). These dykes are talc-carbonate altered and the degree of alteration precludes identification of igneous textures. However, the geochemistry of the dykes suggests that cumulate
olivine9clinopyroxene were originally present
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 25
Table 1
Rock types and petrographic characteristics of the Shaw Dome komatiitic rocks
Wehrlite Clinopyroxenite
Dunite Amphibole
clinopy-Rock type Amphibole gabbro
roxenite
Unit typea UCS UCS, UCD, DCS, DCS, DCD DCS, DCD DCS, DCD
DCD
52 km (apparent) 10–100 m
Rock type thick- 51.5 m 51.5 m 10–40 m
Cumulus miner- Chr (B0.5%) Pl (35–65%)
als
Chr (B0.5%) Chr (?) Chr (B0.5%)
Medium-coarse Fine-medium Medium Medium-coarse Fine-pegmatitic Cumulate grain
size
Orthocumulate-
Orthocumulate-Adcumulate
Cumulate texture Mesocumulate (?) Mesocumulate
mesocumulate mesocumulate
9crescumulate Glass (B10%)
Intercumulus Glass (B15%) Glass (B15%) Cpx (60–100%) Amp (10–20%) phases
Intercumulus Glass, Cpx Glass n/a Glass Glass
phases
Orthocumulate-Cumulate texture Orthocumulate; fine- Aphyric; very fine Aphyric; very fine and grain size mesocumulate; fine- medium
medium
and texture (platy) and fine ran- ented (acicular) and
dom fine random
Spinifex zone 30 cm–2m n/a n/a 3–3.5m n/a
thickness
aUnit type: UCS, undifferentiated cumulate sill, UCD, undifferentiated cumulate dyke, DCS, differentiated cumulate sill, DCD, differentiated cumulate dyke. Mineral abbreviations after Kretz (1983).
M
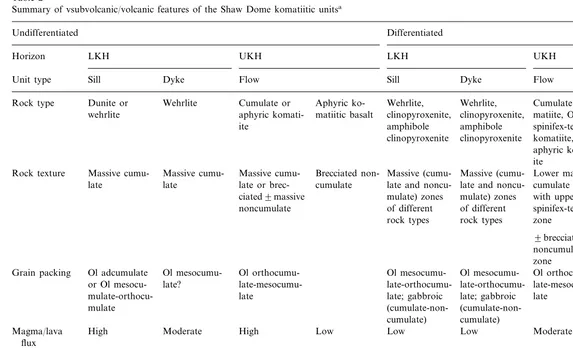
Summary of vsubvolcanic/volcanic features of the Shaw Dome komatiitic unitsa
Undifferentiated Differentiated
Horizon LKH UKH LKH UKH
Sill Dyke Flow
Unit type Sill Dyke Flow
Wehrlite Cumulate or Aphyric ko- Wehrlite,
Dunite or Cumulate ko- Cumulate
ko-Rock type Wehrlite,
matiitic basalt
wehrlite aphyric komati- clinopyroxenite, clinopyroxenite, matiite, Ol matiitic basalt, amphibole amphibole spinifex-textured
Massive cumu- Massive
cumu-Rock texture Brecciated non- Massive (cumu- Massive (cumu- Lower massive Lower massive late or
brec-late late cumulate late and noncu- late and noncu- cumulate zone cumulate zone mulate) zones
ciated9massive mulate) zones with upper with upper of different
noncumulate of different spinifex-textured spinifex-textured rock types rock types zone zone
9brecciated noncumulate zone
Ol mesocumu- Ol orthocumu- Ol
mesocumu-Grain packing Ol adcumulate Ol mesocumu- Ol orthocumu- Cpx
orthocumu-late?
or Ol mesocu- late-mesocumu- late-orthocumu- late-orthocumu- late-mesocumu- late-mesocumu-late
mulate-orthocu- late late; gabbroic late; gabbroic late?
(cumulate-non-mulate
cumulate) cumulate)
High Low Low Low Moderate Low
Magma/lava High Moderate flux
Lobe Sheet Dyke
Dyke Channel Sheet Sheet
Channel Subvolcanic/
vol-(dunite) or sheet canic facies
(wehrlite)
Central Channel, lobe Lobe Mid-distal
Central Mid-distal Distal
Location Proximal
North, east, East North, west East, south, West North, east, North, east, west
Dome flank North, south
west
south, west south
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 27
Whole-rock geochemical analyses indicate that the dunites are characterised by high Mg (44 – 51%
MgO), Mgc (\92) and Ni (2800 – 4300 ppm Ni),
and low Fe (6 – 10% FeOt), Al (B1.1% Al2O3,), Ti
(B0.1% TiO2), and REE (REE=1.1 ppm),
con-sistent with accumulation of forsteritic olivine (Pyke, 1982; Tables 3 and 4). In comparison,
wehrlites have lower Mg (\29% MgO), Mgc
(82 – 91) and Ni (\600 ppm Ni), and higher Fe
(B15% FeOt), Al (B6% Al2O3), Ti (B0.5%
TiO2), and REE (REE B4.0 ppm), consistent
with accumulation of olivine and entrapment of
residual liquid. Pyroxenites and gabbros have the
lowest Mg (4% MgO), Mgc (60) and Ni (50 ppm
Ni), and highest Fe (13% FeOt), Al (18% Al2O3),
Ti (1.3% TiO2), andREE (84.0 ppm), consistent
with accumulation of clinopyroxene9plagioclase
or entrapment of residual liquids (Table 4). The lack of spinifex textures and breccia and inferred presence of altered glass (Table 1) are considered evidence for emplacement of the ko-matiitic dykes and the LKH as subvolcanic bodies (Table 2). The komatiitic cumulate rocks formed mainly by fractional accumulation of olivine (e.g.
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
28
Fig. 3. (A) Photograph of a wehrlite dyke enclosing a large xenolith of sulphide iron-formation (IF). Person for scale is 1.8 m tall. This dyke outcrops stratigraphically below the LKH on the east flank of the Shaw Dome. (B) Photograph of very coarse-grained serpentinised dunite. Coin for scale is 1 cm in diameter. (C) Photograph of amphibole gabbro. Coin for scale is 1 cm in diameter. (D) Photomicrograph of amphibole gabbro in doubly polarised light. Igneous amphibole (Amp) occurs in the interstices between anhedral quartz grains (Qtz) and altered subhedral plagioclase (Pl). Field of view is 6 mm.
dunites) 9clinopyroxene (e.g. wehrlites; Tables 1
and 2). By analogy to Hill et al. (1995), the presence of dunite implies olivine accumulation under dynamic flow conditions, under which the cumulate pile is drained of magma in a proximal flow-through environment. The presence of
ig-neous hydroxy-amphibole suggests that the
magma from which the differentiated wehrlite – clinopyroxenite – amphibole gabbro units formed was hydrous (e.g. Stone et al., 1997). The vertical variation from dykes to sills and the lateral varia-tions from dunite to wehrlite – gabbro bodies are interpreted to represent the transition from hotter, more dynamic and channelised (proximal) to cooler, more static and sheet flow (distal) facies variations. Komatiitic intrusions and intrusive
fa-cies models are not well documented in the scien-tific literature.
4.2. Upper komatiitic horizon
The rock types of the UKH are cumulate, spinifex textured, and aphyric komatiitic rocks (Table 1). They occur in a variety of undifferenti-ated and differentiundifferenti-ated, noncumulate and cumu-late komatiite and komatiitic basalt flow units (Table 2) interlayered with and overlain by tholei-itic basalt flows.
The UKH consists of up to 50 m thick, mainly massive komatiite flows which appear at map scale to pass laterally and vertically to a series of
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 29
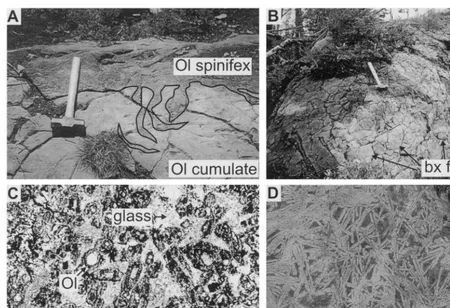
spinifex textured komatiite flows (Fig. 4A). These flows at map scale are inferred to pass laterally and vertically to massive or spinifex textured ko-mati-itic basalt flows (Fig. 4B). Locally, the 50-m thick massive flows host minor nickel sulphide minerali-sation (Green and Naldrett, 1981).
The undifferentiated UKH flows are massive and (or) brecciated (Fig. 4B). The differentiated flows exhibit significant geochemical and textural differ-entiation, and comprise at least two different zones (Fig. 4A): an upper spinifex textured zone and a lower olivine cumulate zone (Fig. 4C and D), typical of komatiites (e.g. Pyke et al., 1973; Arndt et al., 1979). These zones exhibit fine-scale phase and textural zonation. Preserved olivine cores range from Fo91.7-93.1 (Fig. 4C; Table 3).
The komatiite flows are geochemically
character-ised by high Mg (18 – 45% MgO), Mgc (80 – 91)
and Ni (up to 2700 ppm Ni), and low Fe (7 – 13% FeOt), Al (3 – 12% Al2O3), Ti (B0.5% TiO2), and
REE (4.2 – 23.6 ppm), consistent with
accumula-tion of forsteritic olivine (Table 4). The komatiitic
basalts are characterised by lower Mg (down to 5%
MgO), Mgc (70 – 80) and Ni (B1000 ppm Ni),
and higher Fe (up to 13% FeOt), Al (8 – 14% Al2O3),
Ti (up to 0.6% TiO2),REE (7.0 – 19.5 ppm), and
other olivine incompatible elements (Table 4). The asymmetric development of spinifex textures and volcanic breccia together with the presence of major amounts of inferred altered glass and altered skeletal olivine grains in the UKH are considered evidence for extrusion and rapid solidification (Arndt, 1977; Auvray et al., 1982; Stone et al., 1995). The komatiitic cumulate rocks formed by accumu-lation of olivine9clinopyroxene (Tables 1 and 2). The vertical and lateral variations in flow unit thickness (thick to thin), texture (massive to differ-entiated), and mineralogy (olivine to clinopyrox-ene) are interpreted to represent the transition from hotter, dynamic (proximal) to cooler, more static (distal) facies variations. This interpreted channel to sheet flow facies transition is similar to facies models for the Kambalda komatiites (e.g. Cowden and Roberts, 1990; Lesher and Arndt, 1995).
Table 3
Representative average (1 S.D.) electron microprobe analyses of relict igneous olivines and amphiboles from the lower and UKHs in the Shaw Dome
Phase Olivine Olivine Olivine Olivine Amphibole Amphibole
amp gabbro Wehrlite
oc komatiite oc komatiite
Rock typea Dunite oc komatiite
9 5 8
n 4 1 1
42.5 42.5
(0.45) 41.6 (0.43) 40.8 (0.15) 41.8
SiO2(wt%) 41.4 (0.41) (0.29) 6.79 (0.27) 6.64 (0.21) 8.09
FeOt 6.96 (0.21)
0.21 (0.30) 0.12 (0.01) 0.09
MnO (0.01) 0.12 (0.01) 16.0 13.8
MgO 50.7 (0.13) 51.0 (0.35) 50.5 (0.32) 50.2 (0.29) 0.11 0.10
B0.02
CaO 0.16 (0.02) 0.22 (0.01) 0.23 (0.03) 0.26 (0.03) 11.6 11.3
2.84 1.62
Totalb 99.9 (0.95) 100.4 (0.60) 98.9 (0.40) 101.1 (0.37) 97.3 97.5
92.4 93.1
aRock type: oc, orthocumulate; amp, amphibole; FeO
t, total iron as FeO; n.d., not determined. bTotal, analytical total.
cFo, forsterite content calculated by Mg/(Mg+Fe) atomic ratio. dMgcliq, equilibrium liquid composition calculated assumingK
M
Representative whole-rock geochemical analyses of Shaw Dome komatiitic rocks (volatile-free)
27586 27597 22161 22145 22469 27584 22138 22122
Sample 22401 22338 22388 22390 27589 22466 22140
LKH Dike LKH LKH LKH LKH LKH
LKH LKH
Horizon LKH LKH LKH LKH LKH LKH
Rx typea dunite wehrlite wehrlite amp gb wehrlite amp gb wehrlite dunite dunite wehrlite wehrlite wehrlite cpx amp cpx amp gb
oc ac ac oc mc
oc
ac mc
Texture oc
41.2 43.2 43.4 42.8 53.1 44.6 42.1 41.6 44.9 44.6 42.5 53.4 50.5 54.4
SiO2(wt%) 50.2
0.125
0.023 0.093 0.113 1.17 0.282 0.275 B0.010 0.013 0.189 0.09 0.083 0.189 0.203 1.08
TiO2
12.86 5.28 0.09 0.88 3.91 2.1 1.88
2.06 4.11
2.90 17.02 4.53 18.0
Al2O3 0.3 1.87
0.634
0.164 0.486 0.426 0.001 0.192 0.462 0.19 0.182 0.316 0.56 0.2 0.492 0.514 0.022
Cr2O3
6.76 11.9 6.60 6.64 10.2 11.4 10.4
FeOt 7.79 8.63 10.9 12.47 11.9 8.97 10.2 7.46
0.15 0.15 0.10 0.09 0.14 0.15 0.18
0.16 0.18
0.11 0.17 0.12
MnO 0.14 0.15 0.14
40.8
49.7 45.1 41.5 4.70 12.6 30.3 50.4 50.0 38.9 40.4 38.4 26.1 25.3 6.48
MgO
0.022 0.287 0.439 0.479 0.295 0.32 0.39 0.039
NiO 0.426 0.433 0.319 0.006 0.321 0.158 0.002
1.40 6.77 0.02 0.09 1.11 0.37 5.94
1.17 6.36
8.73 8.31 7.39
CaO 0.26 0.04 0.36
1.41 B0.01 0.04 B0.01 B0.01 0.03 0.01
B0.01 0.016 B0.01 0.008 0.182 0.088 0.075
0.184 0.14
B0.005 0.087 B0.01
S 0.056 0.118 1.12
11.8
12.9 14.2 11.2 1.3 1.00 27.4 18.7 20.3 10.3 10.7 9.9 5.1 5.8 2.8
L.O.I.
99.78
99.88 99.97 99.89 101.18 100.14 100.11 99.9 100.06 100.23 100.00 99.71 100.46 99.49 99.05
Totalb
78.7 83.5 93.8 93.7 88.3 87.5 81.8 85.2
87.2 83.1
177 2181 3455 3775 2331 2521 3076 306
Ni 3356 3412 2511 47 2526 1239 173
7 B5 B5 B5 7 B5 B5
1158 3429 2998 7 1354 3506 1338 1282 2235 3958 1413 3484 3602 154
Cr
0.125 0.376 0.292 0.533 3.05
La (ppm) 12.04
6.94
Ce 0.325 0.716 0.69 29.11 1.01
0.948 0.136
0.047
Pr 0.083 0.113 3.96
0.607
0.196 0.337 0.664 16.34 3.99
Nd
1.03
Sm 0.054 0.09 0.267 3.94 0.191
0.332 0.142
1.26
Eu 0.006 0.03 0.059
1.30
Gd 0.067 0.124 0.418 4.65 0.296
0.222 0.052
Tb 0.012 0.024 0.077 0.756
0.363
0.078 0.166 0.524 4.60 1.40
Dy
0.305 0.083
0.018
Ho 0.041 0.12 0.989
0.234
0.059 0.127 0.336 2.77 0.840
Er
Tm 0.011 0.024 0.052 0.417 0.039 0.130
0.854 0.259
0.165 0.347 2.74
Yb 0.071
0.043
0.013 0.029 0.057 0.409 0.129
Lu
1.470
REE 1.082 2.332 4.016 83.981 3.988
1.859 1.756
2.613
(La/Sm)nc 1.461 0.688 1.922
1.388
1.181 1.537 0.567 2.969 2.414
M
Table 4 (Continued)
45.9
57.7 44.0 49.5 44.2 45.1 45.5 53.7 52.5 49.5 45.7 45.2 45.3 44.6 54.2
SiO2(wt%)
0.264 0.353 0.647 0.524 0.274 0.294 0.419
TiO2 0.92 0.158 0.274 0.236 0.373 0.374 0.396 0.528
5.63 7.23 13.1 10.6 5.14 6.15 8.43
6.98 7.52
16.5 8.27 11.2
Al2O3 3.05 5.14 4.83
0.427
0.02 0.252 0.287 0.371 0.342 0.420 0.125 0.210 0.287 0.317 0.441 0.395 0.479 0.281
Cr2O3
11.2 12.0 10.4 10.3 10.5 11.2 12.7
FeOt 7.60 8.28 10.5 11.5 12.2 12.1 12.9 9.79
0.18 0.19 0.15 0.16 0.14 0.16 0.21
0.15 0.20
0.14 0.17 0.20 0.14
MnO 0.11 0.15
26.5
5.72 43.6 25.2 35.5 32.6 27.1 12.0 15.8 25.2 30.5 24.3 25.9 26.4 14.2
MgO
0.215 0.181 0.025 0.061 0.165 0.195 0.136
NiO 0.016 0.293 0.165 0.247 0.169 0.159 0.155 0.067
4.43 6.72 6.38 7.86 8.80 5.46 7.73
7.22 7.65
7.33 6.43 6.07
CaO 0.10 8.80 2.83
0.06
3.11 0.06 B0.01 B0.01 B0.01 0.21 3.3 2.0 B0.01 B0.01 0.37 0.27 0.13 3.35
Na2O
0.03 0.07 0.01 0.01 B0.01 0.03 0.01 B0.01
K2O 0.87 B0.01 B0.01 B0.01 0.01 B0.01 0.01
0.02 0.04 0.09 0.06 0.02 0.05 0.06
0.03 0.04
0.19 0.04 0.08
P2O5 B0.01 0.02 0.02
0.046
B0.01 0.045 B0.005 0.075 0.060 0.007 0.009 B0.005 B0.005 0.078 0.005 0.027 0.013 0.022
S
8.7 6.6 9.9 27.4 20.3 12.9 10.9 10.3
L.O.I. 2.3 18.7 4.9 11.0 5.8 10.7 5.1
99.73 100.10 100.18 100.58 99.36 99.62 99.56
99.59 99.54
99.97 99.65 99.85
Totalb 98.98 99.86 100.08
81.1
59.8 91.3 82.6 85.9 85.3 81.8 69.7 75.3 86.0 84.4 79.2 80.9 80.3 74.2
Mgc
1692 1426 194 484 1298 1532 1064
1325 1248
123 1215 529
Ni 2310 1298 1951
47
41 B5 27 B5 B5 34 103 69 27 B5 36 35 B5 247
Cu
53 62 69 72 36 52 74 68 55 65
Zn 88 26 36 60 64
124 169 220 178 107 139 187
141 168
107 115 165 201
V 152 66
2998
152 1778 2023 2616 2407 2963 885 1487 2023 2223 3097 2769 3367 1977
Cr
0.293 0.349 3.81 3.04
0.332
0.246 0.228 0.391
La (ppm)
1.34 0.883 1.07 9.83 8.28
0.740 0.691
Ce 0.984
0.157 0.212 1.46 1.21
Pr 0.123 0.144 0.186 0.268
1.02 1.26 6.92 5.70
1.52
Nd 0.635 0.868 1.03
0.650 0.487 0.622 2.22 1.77
0.247 0.403 0.472
Sm
0.200 0.224 0.624 0.453
Eu 0.092 0.112 0.201 0.263
0.849 1.08 3.00 2.35
1.01
Gd 0.434 0.683 0.750
0.186 0.164 0.199 0.530 0.419
0.076 0.126 0.137
Tb
1.08 1.40 3.53 2.73
Dy 0.551 0.891 0.954 1.28
0.245 0.317 0.766 0.598
0.281
Ho 0.129 0.193 0.214
0.792 0.710 0.883 2.22 1.75
0.374 0.549 0.576
Er
0.105 0.138 0.337 0.266
Tm 0.064 0.082 0.088 0.120
0.690 0.892 2.21 1.71
0.775
0.416 0.530 0.563
Yb
0.116 0.105 0.140 0.346 0.265
0.069 0.080 0.087
Lu
6.988 5.046 37.799 30.541
REE 4.196 5.580 6.574 4.852
0.378 0.353 1.083 1.078
0.379 0.443
(La/Sm)nc 0.627 0.356
0.286
(La/Yb)n 0.400 0.290 0.399 0.341 0.264 1.167 1.201
aRx type; amp gb, amphibole gabbro; cpx, clinopyroxenite. All data recalculated to 100% volatile-free (L.O.I. and S). FeO
t, total iron as FeO. bTotal=XRF+L.O.I.−O=S.
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
32
Fig. 4. (A) Discordant spinifex veins intruding the lower olivine cumulate komatiite zone of a flow. Hammer for scale is 40 cm in length. (B) Photograph of a thin auto-brecciated (bx) komatiitic basalt flow. Hammer for scale is 40 cm in length. (C) Fine-grained subhedral and coarser grained, more skeletal relict olivine grains (Fo91.7; Table 3) set in an altered glassy groundmass of an orthocumulate komatiite. Field of view is 6 mm. (D) Coarse-grained, randomly-oriented serpentine pseudomorphs of skeletal olivine in the top of a differentiated komatiite flow. Coin for scale is 1 cm in diameter.
5. Geochemical modelling
Possible indications of crustal contamination could be deviations from uncontaminated model fractionation trends, enrichments in incompatible immobile elements that are significantly enriched in the continental crust and, perhaps, the presence of igneous hydroxy-amphibole (Stone et al., 1997). In particular, deviations from uncontami-nated model fractionation trends and LREE en-richments in the LKH and UKH rocks are investigated below.
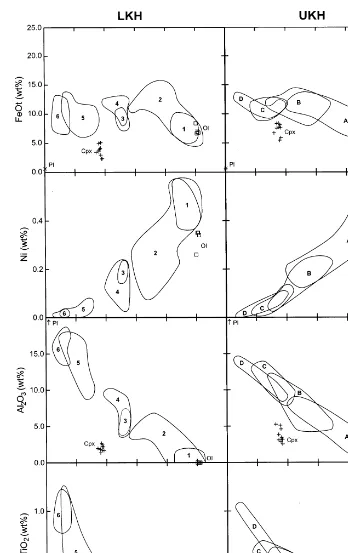
The immobile elements in MgO variation dia-grams (Fig. 5) define three distinct trends. First, as the content of MgO decreases from 50 to 20 to
B10%, FeOtincreases, then decreases, and finally
remain somewhat constant. Second, Ni contents in the same MgO interval first decrease and then remain relatively constant. Third, the contents of
Al2O3 and TiO2 increase with decreasing MgO.
The olivine incompatible elements define trends relative to increasing magnesium contents that extrapolate approximately to the compositions of olivines analysed from dunite and olivine cumu-late samples (Table 3). These incompatible
ele-ment trends extrapolate to :51% MgO at 0%
Al2O3and TiO2, and to :42% SiO2, :5% FeOt,
:0.08% MnO, :4000 ppm Ni, and :1200 ppm
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 33
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
34
liquid-crystal fractionation of olivine to clinopy-roxene to plagioclase.
The geochemical similarities of the LKH and UKH rocks (Fig. 5) and their close spatial and apparent stratigraphic associations are considered to be strong evidence for a genetic relationship (Muir, 1979; Pyke, 1982). Possible genetic rela-tionships between the different LKH and UKH rock types, and between the LKH and the UKH, have been modelled utilising the fractional crys-tallisation and assimilation – fractional crystallisa-tion program MELTS (Ghiorso and Sack, 1995). MELTS was chosen because most thermodynamic properties (except thermal conductivity) are con-sidered for individual phases and liquid under a wide variety of equilibrium conditions. However, trace element compositions are not modelled and are therefore considered separately. Prior to de-scribing the results of the MELTS modelling, an overview of the model assumptions is presented (see also Appendices A and B).
5.1. Liquid compositions
The initial komatiitic liquid composition was estimated utilising variations in whole-rock Mg numbers of the LKH. The highest Mg number
corresponds to dunite (\90% olivine, sample
c22493; Larson, 1996) and constrains the
maxi-mum Mg number of the most primitive cumulate olivine and the initial liquid. The remaining liquid components were estimated from the geochemical trends on MgO variation diagrams at 32% MgO, following Lesher (1989) (see Nisbet et al., 1993 for a revised view of such high MgO contents). Thus, the initial liquid composition is estimated to have
contained 32% MgO, 0.3% TiO2, 5.5% Al2O3,
11% FeOt, 1750 ppm Ni, 75 ppm Co, and 2700
ppm Cr. This magma would have crystallised
Fo94.9 olivine (assuming KD=0.30 and Fe
3+/
SFe=0.1; Roeder and Emslie, 1970), which is
more magnesian than the analysed olivines (Fo91.7 – 93.1; Table 3), but similar to that inferred
from the olivine adcumulate whole-rock composi-tions (Fo95; Table 4). The Na content was derived
from the :28% MgO liquid composition of
Arndt (1986b) (sample cM666) and adjusted to
corresponding values at 32% MgO. The P content
was estimated to be 0.01% P2O5. The geochemical
composition of the estimated initial liquid (Table 5) is similar to compositions of other highly
mag-nesian komatiitic liquids, except that the FeOt
content appears to be higher.
Clinopyroxenite compositions in the MgO vari-ation diagrams appear to have formed from derivative liquids with 10 – 15% MgO and the amphibole gabbros from liquids with 4 – 6% MgO. The presence of igneous amphibole rather than clinopyroxene indicates that the presence of
hy-drous residual liquids. The H2O content of these
amphibole-supersaturated liquids is assumed to be 3%, based on experimental studies of basalt sys-tems (Semet and Ernst, 1981; Gilbert et al., 1982). The H2O content of the initial liquid is therefore
estimated to be 1 – 2%, adjusting these liquid com-positions for 60 – 70% fractionation. Hence, FC models were run anhydrous and with 1 and 2 wt%
H2O added to the liquid. However, the results of
the anhydrous and hydrous modelling were not significantly different. The A-FC models were run with the anhydrous estimated initial komatiitic liquid composition and with 1% water added to the initial komatiite liquid composition.
The UKH olivine and clinopyroxene spinifex textured samples and aphyric samples from mar-ginal flow breccia zones were preferentially sam-pled and analysed to determine erupted liquid compositions. Aphyric rocks are interpreted to represent liquid compositions and spinifex tex-tured rocks represent near-liquid compositions (cf. Donaldson, 1982). These samples are charac-terised by lower MgO contents (up to 30% MgO), less cumulus olivine and clinopyroxene and a high proportion of glassy interstitial areas. The Fe/Mg ratio of the liquid is further constrained by the composition of relict olivines in three komatiite samples (Fo91.7-93.1; Table 3) which suggests
crys-tallisation from liquid containing up to 30 wt% MgO. The geochemical variation trends suggest that the komatiitic basalts formed from derivative
liquids with B10 – 15% MgO.
5.2. Contamination and other physical
assumptions
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 35
wehrlite dyke stratigraphically down section of the LKH represents tangible evidence for at least
localised assimilation. However, calc-alkalic
basalt, andesite and dacite are more abundant than iron-formation and most of the latter is stratigraphically upsection of the LKH (Pyke, 1982). Therefore, iron-formation is not considered to be a significant contaminant. Average composi-tions of calc-alkalic basalt and dacite (mafic and felsic end-members) utilised in the modelling are listed in Table 7. Geochemical modelling indi-cated no significant change in the liquid
composi-tion until assimilacomposi-tion of \10% of the mass of
the initial liquid.
If the UKH formed from LKH liquid(s), the latter may have extruded prior to or following assimilation of calc-alkalic basalt. In addition, iron-formation assimilation might possibly have been an important factor in the evolution of the UKH (Green and Naldrett, 1981). The average composition of sulphide iron-formation is listed in Table 6.
The system pressure was constrained from the apparent map-scale thickness of the calc-alkalic volcanic pile (4 km average; Pyke, 1982) intruded by the LKH and an assumed ocean water depth of 1 km. The latter depth would be that of UKH extrusion (ocean floor).
A lithostatic pressure gradient of 0.3 kb/km
and a hydrostatic pressure gradient of 0.1 kb/km
yield a pressure estimate of 1.3 kb.
5.3. Summary
One hundred and two MELTS models were run. The most likely FC and the spectrum of A-FC model liquid evolution trends for the LKH and UKH rocks are presented in Fig. 6 and discussed below.
5.4. Fractional crystallisation
All but one of the LKH FC model runs can reproduce the most primitive olivine compositions
observed (\Fo94.6). Although the liquid
evolu-Table 5
Model initial komatiitic liquid compositions
Shaw Dome Kambalda Yakabinde Alexo Dumont
Duke (1986) Arndt (1986b)
Duke and Naldrett (1978) Lesher (1983)
This study
45.6 45.6 44.9 45.4 47.5
SiO2(wt%)
MgO 32.0 32.0 27.9 27.5
0.21 0.22
NiO 0.16 0.20 0.14
0.01 0.01
0.01
CoO 0.01 0.01
4.50 5.70
CaO 5.30 6.50 6.00
Na2O 0.26 0.35 0.35 0.29 0.15
Total 99.57 99.12 99.70
20
b 6Fo, equilibrium olivine composition calculated assuming K
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
36
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 37
Table 6
Average whole-rock XRF analyses (1 standard deviation) of possible contaminants (altered) in the Shaw Dome
Calc-alkalic Calc-alkalic
Calc-alkalic Sulfide
Basalt Andesite Dacite Iron-formation
12 4
6
n 3
(2.3) 61.8 (2.6) 65.0 (0.33) 53.5 (15.5)
SiO2(wt%) 59.9
(0.141) 0.615 (0.102) 0.352
0.729 (0.095)
TiO2 0.079 (0.073)
Al2O3 15.4 (0.76) 16.3 (0.88) 17.1 (0.41) 2.1 (1.1)
NiO (0.005) 0.008 (0.002) B0.010 (0.001) 0.004 (0.002)
0.001
CoO (0.000) 0.003 (0.001) B0.010 (0.000) B0.010 (0.001)
(1.2) 4.3 (2.4)
(0.05) 0.16 (0.04) 0.12
P2O5 0.19 (0.04) 0.19 (0.12)
bL.O.I. values are assumed to represent the H2O content of the sample because they lack carbonate alteration and (except the iron-formation) are virtually devoid of sulphide minerals.
tion trends for all the FC runs are similar, no single model liquid evolution trend can reproduce the trend of all the whole-rock compositions. The liquid evolution models that most closely approxi-mate the composition of the most differentiated amphibole gabbro samples are produced by frac-tionation of hydrous komatiitic liquid. The addi-tion of H2O suppresses plagioclase crystallisation
to lower Mg contents, but does not closely
repro-duce the compositions of the noncumulate amphi-bole gabbro samples.
Titanium and Al are important elements to consider when evaluating the validity of the liquid evolution models, because of their incompatibility in olivine. Considering this, the FC liquid evolu-tion model that best approximates the whole-rock geochemical trend of the LKH compositions is an
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
38
depth). As illustrated in Fig. 6, Ti increases and Al decreases in the residual liquid at low Mg
contents (B10 wt% MgO), during plagioclase
fractionation and accumulation. However, the
model trend on the Al2O3 versus MgO variation
diagram exhibits a sharp decrease in Al prior to evolving to the most differentiated amphibole gabbro compositions (Fig. 6). This suggests that the model liquid is crystallising plagioclase too early or conversely that the samples crystallised plagioclase later than predicted. A similar trend
is evident on the FeOMgO variation diagram
(Fig. 6). Thus, the results of the FC modelling do not provide a single liquid evolution trend that closely represents the actual compositions of the noncumulate LKH rocks. Furthermore, this result suggests that, either the amphibole gabbro is not petrologically related to the asso-ciated dunite and wehrlite, or that the LKH formed by different crystallisation processes, possibly A-FC.
5.5. Assimilation-fractional crystallisation
The range of A-FC trends that fractionate olivine as magnesian, as the most magnesian olivine composition in the Shaw Dome samples, and that can produce the most differentiated
amphibole gabbro compositions, are shown
graphically in Fig. 6. These trends were gener-ated by assimilation of calc-alkalic basalt by an-hydrous komatiitic liquid and FC at 2.0 kb
pressure (:6 km crustal depth). No single
calc-alkalic basalt A-FC model can produce the
olivine compositions and amphibole gabbro
compositions required. This suggests that if A-FC was responsible, variable amounts of assimi-lation were involved in producing the LKH rocks. This is a reasonable interpretation, be-cause differential heat loss and flux, as well as different flow velocities and therefore erosive po-tential, are likely in a relatively large magmatic – volcanic system the size of that inferred for the Shaw Dome. Assimilation of dacite produces similar liquid evolution trends. In detail, assimi-lation of smaller amounts of dacite is required to produce trends with similar silica contents.
On Al2O3 and TiO2 versus MgO variation
di-agrams, the composition of the calc-alkalic as-similant (cab) plots close to the extrapolated position of olivine and clinopyroxene fractiona-tion curves (control lines). This closeness
sug-gests these rocks could have formed by
contamination of komatiites by upper crustal material.
A scenario yet to be considered is that amphi-bole gabbro is not petrologically related to wehrlite and the associated pyroxenitic rocks. Instead, the amphibole gabbro units could rep-resent melted and recrystallised wall rocks or they could be intrusions unrelated to the ko-matiitic rocks. If they are recrystallised wall
rocks, the amphibole gabbro compositions
should all plot in about the same location as the calc-alkalic basalt composition on MgO varia-tion diagrams (Fig. 6). This is not the case. The second possibility also does not appear to be reasonable (see Section 4.1).
The fractional crystallisation model that best defines the geochemical trend of the UKH whole-rock and olivine compositions involves variable amounts of calc-alkalic basalt assimila-tion (Fig. 6). The komatiitic basalt samples ap-parently crystallised from liquids significantly more crustally contaminated than the liquid(s) from which the komatiite samples crystallised
(cf. SiO2MgO variation diagram of Fig. 6).
The A-FC models that involve iron-formation produce olivine of appropriate Mg contents. However, these liquids do not reproduce the ko-matiitic basalt compositions. Therefore, if iron-formation assimilation occurred, it may have done so only locally and iron-formation contam-inated rock types were not sampled.
The petrologic modelling and the whole-rock
geochemical trends suggest that the UKH
formed mainly as a result of olivine fractiona-tion, similar to dunite and wehrlite of the LKH. The komatiitic basalt geochemical compositions are remarkably similar to those of the LKH am-phibole gabbros.
5.6. REE 6ariations
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 39
marked changes in REE profiles, particularly in LREE relative to HREE. This results from the high
LREE contents and high LREE/MREE and low
MREE/HREE and low HREE contents of most
crustal rocks. The LREE profiles of contaminated komatiites should therefore markedly differ from the parental melt. Consequently, REE geochemical compositions of the LKH and UKH rocks are utilised to further constrain the possibility of relationships by A-FC processes.
Examination of the primitive mantle-normalised REE profiles (Fig. 7) indicates marked differences between the LKH and UKH rock types. All but a
single LKH sample (sample c22388; Larson,
1996) are slightly enriched in LREE relative to
MREE and HREE [(La/Sm)n=1.461 – 2.613;
(La/Yb)n=1.388 – 2.969]. In contrast, the UKH
komatiite and olivine cumulate samples are all
depleted in LREE [(La/Sm)n=0.353 – 0.627;
(La/Yb)n=0.264 – 0.400], whereas the komatiitic
basalt samples are relatively more enriched in LREE [(La/Sm)n=0.918 – 1.083; (La/Yb)n=0.912
– 1.201] (Table 4). The fact that these LREE variations are at relatively high MgO cannot be explained by the FC models.
The marked REE compositional gap between the LKH and UKH is explained by genetic differences. However, it is not possible to conclusively distinguish the effects of source heterogeneity, melting processes, and A-FC on the basis of
geochemical compositions. Nevertheless, the
LREE variations are consistent with A-FC processes. The LKH amphibole gabbros and the UKH komatiitic basalts are considered to represent A-FC products, whereas the LKH dunite and the UKH komatiites are considered to represent depleted, primary melts (e.g. Lesher and Arndt, 1995).
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
40
5.7. Accumulation, differentiation and
contamination
As discussed, the evolution of the Shaw Dome komatiitic rocks can be attributed to variable amounts of olivine accumulation followed by liq-uid differentiation and (or) crustal contamination (Fig. 8). The massive dunite and wehrlite sills and dykes and massive komatiitic basalt flows can be explained by fractional olivine accumulation. In contrast, the differentiated wehrlite – pyroxenite – gabbro and olivine cumulate to spinifex textured to aphyric komatiite units can be explained by differentiation and (or) A-FC. Differentiation can be distinguished from A-FC on the basis of geo-chemical and mineralogical parameters as inferred from different rock types. For example, A-FC processes are represented by LREE enrichment and, perhaps, by the presence of igneous amphi-bole in the amphiamphi-bole gabbro lithology.
6. Evolution of the Shaw Dome complex
Field mapping and petrographic and geochemi-cal variation studies of the Shaw Dome komatiitic rocks indicate that the thick, undifferentiated LKH wehrlite sills and the thick, undifferentiated UKH komatiitic flows strongly resemble each other in geochemical and mineralogical composi-tion. The amphibole gabbros and the komatiitic basalts exhibit many similar geochemical charac-teristics. In consequence, the LKH and UKH are inferred to be genetically related and to constitute
a single intrusive – extrusive stratigraphic
se-quence. The sequence is interpreted to represent a contaminated dyke – sill lava complex and con-strains facies models for komatiitic magmatism – volcanism.
A model for the evolution of the Shaw Dome dyke – sill – lava complex is presented in Fig. 9. In this model, the adcumulate dunite sills represent flow differentiation texture formed by fractional accumulation of olivine in dynamic flow-through conduits. Ponding of komatiitic magma in adja-cent, more distal magma chambers, where it un-derwent olivine fractional crystallisation under more static conditions, and formed cogenetic
wehrlite sills. Eruption of the initial magma re-sulted in emplacement of cogenetic, channelised komatiite flows. Eventually, heated crustal mate-rial adjacent to the proximal magma conduits was thermally eroded and assimilated to produce con-taminated komatiite magmas. Ponding of this
magma and fractional crystallisation of olivine9
clinopyroxene produced the wehrlite – amphibole gabbro units. Eruption of the contaminated magma resulted in emplacement of the cogenetic komatiitic basalt flows. In this model, the dunite and the non-brecciated komatiite represent more ‘proximal’ channel facies. The wehrlite sills, differ-entiated wehrlite – gabbro sills, brecciated flows and komatiitic basalt flows represent more ‘distal’ sheet flow facies (Table 7).
The reason for the lack of contamination in the komatiite flows remains to be determined. Magma ascent could have been more rapid and eruption rates been greater than estimated by Huppert et al. (1984) and Huppert and Sparks (1985a,b), and (or) the crust may have been very thin. The viscosity variation in cooler boundary zones might have inhibited convection at the margins of dykes and flows, and substantially reduced erosive capability (cf. Bickle et al., 1993). Alternatively, contaminated komatiite lava might have flowed downstream away from the study area.
7. Discussion and implications
The Shaw Dome dyke – sill – lava complex
model (Fig. 9) will be valuable in interpreting komatiitic facies variation in other less well ex-posed and (or) more structurally disrupted ko-matiitic successions. The model implies that the Shaw Dome area was a major locus for komatiitic magmatism in the Abitibi greenstone belt. It is the only area in the Abitibi in which inferred subvol-canic intrusives and cogenetic extrusives are recognised.
M
.
S
.
Stone
,
W
.
E
.
Stone
/
Precambrian
Research
102
(2000)
21
–
46
41
Table 7
Subvolcanic and volcanic setting of the Shaw Dome komatiitic rocksa
NiS deposit potential Volcanic features
Subvolcanic features
Setting Subvolcanic thermal Volcanic thermal erosion
based on thermal erosion erosion
capability
High Large amounts of thermal
(Not recognised) chaotic
Feeder dykes9feeder sills Large amounts of thermal
Central
(0–1 km?) volcanic stratigraphy, py- erosion erosion
roclastic rocks, sheet flows, lava channels ex-pected
Proximal Undifferentiated cumulate Channelised sheet flows9 Significant amounts of Significant amounts of ther- High-moderate sills lava channels thermal erosion, especially mal erosion, especially
be-(1–5 km?)
neath lava channels and beneath dunite sills and
channelised sheet flows wehrlite sills
Channelised sheet flows9
Undifferentiated cumulate Thermal erosion beneath Low
Mid-distal Thermal erosion beneath
undifferentiated sills only lava ponds9lava
chan-sills9differentiated cumu- lava channels only
(5–10 km?)
nels9lava lobes late sills
Lava lobes9pillow
Differentiated cumulate Negligible Negligible Very low
Distal
(\10 km?) sills lavas9flow-front breccias
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
42
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 43
different styles of komatiitic magmatism or differ-ent preservation. The absence or presence of
in-trusive komatiitic facies may also have
significance in mineral exploration. The relatively
small proportion of komatiite flows up to ]100
m thick in the Shaw Dome complex, suggests a lower potential for komatiite-associated nickel sulphide deposits like those at Kambalda (Cow-den and Roberts, 1990).
Alternatively, the Shaw Dome may have a
higher potential for intrusion (dyke – sill) associ-ated nickel sulphide deposits (Table 7). Contami-nation in komatiitic systems is obviously not restricted to extrusive environments. Given the importance of contamination (Lesher, 1989) and
dynamic/static flow transition (Cowden and
Roberts, 1990) in komatiite-associated nickel sul-phide mineralisation, hypothetical dyke/sill con-tacts in the Shaw Dome complex and elsewhere should be sought and targeted for exploration.
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
44
Acknowledgements
This paper is based on M.Sc. Thesis research by M.S. Stone (nee Larson) supervised by C.M. Lesher and funded by grants from Outokumpu Mines (Canada), Sigma Xi and the National
Sci-ence Foundation (EAR-9405994 and
EAR-9526877). It benefited from thorough informal reviews by Steve Beresford and Yann Lahaye, and critical formal reviews by Euan Nisbet, Phil Thurston and Peter Lightfoot. We thank Charlie Wu at the University of Western Ontario for generation of the XRF data, Gilles Gauthier and Yann Lahaye at the University of Montreal for generation of the REE data, and Mike Bersch at the University of Alabama for assistance with the EMPA.
Appendix A. Temperature, pressure, and oxygen fugacity assumptions
The MELTS models were run at temperatures
between :1650 and 1100°C and pressures of 2.0
and 0.5 kb. The temperature range of crystallisa-tion was determined by the liquidus temperature (calculated by MELTS) and the approximate solidus temperature of komatiitic systems (Green, 1973; Bickle, 1978). Liquid evolution was mod-elled with temperature decreasing in intervals of 10°C.
The oxygen fugacity of the komatiitic magma was set to the fayalite – magnetite – quartz (FMQ)
buffer. MELTS calculated Fe2O3from total Fe as
FeO in the liquid according to FMQ.
Appendix B. Models
The LKH FC models were run with 0, 1 and 2
wt% H2O. The LKH A-FC models were run with
10 – 300 g assimilant per 100 g of initial liquid. The assimilant was added in 100 increments of 1 – 3 g to the liquid during fractionation. Both hydrous and anhydrous initial liquids were mod-elled during the A-FC runs.
The following UKH models were run: (1) frac-tional crystallisation of the ‘best’ LKH model
liquid, (2) fractional crystallisation of the same LKH model liquid after assimilation of 5 – 15 g of iron-formation, (3) fractional crystallisation of the anhydrous initial komatiite liquid after assimila-tion of up to 15 g calc-alkalic basalt and up to 15 g iron-formation per 100 g initial liquid, and (4) assimilation of up to 300 g calc-alkalic basalt by
the initial komatiite liquid and fractional
crystallisation.
References
Arndt, N.T., 1977. Thick, layered peridotite – gabbro lava flows in Munro Township, Ontario. Can. J. Earth Sci. 14, 2620 – 2637.
Arndt, N.T., 1986a. Komatiites: a dirty window to the Archean mantle. Terra Cogn. 6, 59 – 66.
Arndt, N.T., 1986b. Differentiation of komatiite flows. J. Petrol. 27, 279 – 303.
Arndt, N.T., Francis, D., Hynes, A.J., 1979. The field charac-teristics and petrology of Archean and proterozoic komati-ites. Can. Miner. 17, 147 – 163.
Arndt, N.T., Jenner, G.A., 1986. Crustally contaminated ko-matiites and basalts from Kambalda, Western Australia. Chem. Geol. 56, 229 – 255.
Auvray, B., Blais, S., Jahn, B.M., Piquet, D., 1982. Komatiites and komatiitic series of the Finnish greenstone belts. In: Arndt, N.T., Nisbet, E.G. (Eds.), Komatiites. Allen and Unwin, London, pp. 131 – 145.
Bickle, M.J., 1978. Heat loss from the earth: a constraint on Archean tectonics from the relation between geothermal gradients and the rate of plate production. Earth Planet. Sci. Lett. 40, 301 – 315.
Bickle, M.J., Arndt, N.T., Nisbet, E.G., Orpen, J.L., Martin, A., Keays, R.R., Renner, R., 1993. Geochemistry of the igneous rocks of the Belingwe greenstone belt: alteration, contamination and petrogenesis. In: Bickle, M.J., Nisbet, E.G. (Eds.), The Development of The Belingwe Green-stone Belt: A Study of The Construction of The Continen-tal Crust. Geological Society of Zimbabwe Special Publication 2, pp. 175 – 223.
Chauvel, C., Dupre´, B., Arndt, N.T., 1993. Pb and Nd iso-topic correlation in Belingwe komatiites and basalts. In: Bickle, M.J., Nisbet, E.G. (Eds.), The Geology of the Belingwe Greenstone Belt. Geological Society of Zim-babwe Special Publication 2, pp. 167 – 174.
Cheatham, M.M., Sangrey, W.F., White, W.M., 1993. Sources of error in external calibration ICP-MS analysis of geolog-ical samples and an improved non-linear correction proce-dure. Spectrochim. Acta 48, 487 – 506.
crust-M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46 45
below the Kambalda – Norseman greenstones. Earth Planet. Sci. Letts. 76, 299 – 311.
Corfu, F., Krogh, T.E., Kwok, Y.Y., Jensen, L.S., 1989. U-Pb zircon geochronology in the southwestern Abitibi green-stone belt, Superior Province. Can. J. Earth Sci. 26, 1747 – 1763.
Corfu, F., Noble, S.R., 1992. Genesis of the southern Abitibi greenstone belt, Superior Province, Canada: evidence from zircon Hf-isotope analyses using a single filament tech-nique. Geochim. Cosmochim. Acta 56, 2081 – 2097. Cowden, A.C., Roberts, D.E., 1990. Komatiite hosted nickel
sulphide deposits, Kambalda. In: Hughes, F.E. (Ed.), Ge-ology of the Mineral Deposits of Australia and Papua New Guinea. The Australian Institute of Mining and Metal-lurgy, Melbourne, pp. 567 – 581.
Donaldson, C.H., 1982. Spinifex-textured komatiites: a review of textures, compositions and layering. In: Arndt, N.T., Nisbet, E.G. (Eds.), Komatiites. Allen and Unwin, Lon-don, pp. 211 – 244.
Duke, J.M., 1986. Petrology and economic geology of the Dumont sill: an Archean intrusion of komatiitic affinity in northwestern Que´bec. Geol. Surv. Can., Econ. Geol. Re-port 35, 56.
Duke, J.M., Naldrett, A.J., 1978. A numerical model of the fractionation of olivine and molten sulfide from komatiitic magma. Earth Planet. Sci. Lett. 39, 255 – 266.
Frost, K., Groves, D.I., 1989. Ocellar units at Kambalda: evidence for sediment assimilation by komatiite lavas. In: Prendergast, M.D., Jones, M.J. (Eds.), Magmatic Sul-phides: The Zimbabwe Volume. The Institution of Mining and Metallurgy, London, pp. 207 – 214.
Fyon, A., 1980. Sea water alteration of early Precambrian (Archean) volcanic rock and exploration criteria for strati-form gold deposits, Porcupine camp, Abitibi greenstone belt, northeastern Ontario. Unpublished M.Sc. Thesis, Mc-Master University, Hamilton, Ontario, pp. 238.
Ghiorso, M.S., Sack, R.O., 1995. Chemical mass transfer in magmatic processes IV: a revised and internally consistent thermodynamic model for the interpolation and extrapola-tion of liquid – solid equilibria in magmatic systems at elevated temperatures and pressures. Contrib. Miner. Petrol. 119, 197 – 212.
Gilbert, M.C., Helz, R.T., Popp, R.K., Spear, F.S., 1982. Experimental studies in amphibole stability. In: Valley, J.W., Taylor, H.P., O’Neil, J.R. (Eds.), Stable Isotopes in High Temperature Geological Processes, 9. Min. Soc. of Am. Rev. in Min, pp. 229 – 346.
Green, D.H., 1973. Experimental melting studies on a model upper mantle composition at high pressure under water-saturated and water-underwater-saturated conditions. Earth Planet. Sci. Lett. 19, 37 – 53.
Green, A.H., Naldrett, A.J., 1981. Langmuir volcanic peri-dotite-associated nickel deposits: Canadian equivalents of the Western Australian occurrences. Econ. Geol. 76, 1503 – 1523.
Hill, R.E.T., Barnes, S.J., Gole, M.J., Dowling, S.E., 1995. The volcanology of komatiites as deduced from field
rela-tionships in the Norseman – Wiluna greenstone belt, West-ern Australia. Lithos 34, 159 – 188.
Hofmann, A.W., 1988. Chemical differentiation of the earth: the relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 90, 297 – 314. Huppert, H.E., Sparks, R.S.J., Turner, J.S., Arndt, N.T., 1984.
Emplacement and cooling of komatiite lavas. Nature 309, 19 – 22.
Huppert, H.E., Sparks, S.J., 1985a. Cooling and contamina-tion of mafic and ultramafic magmas during ascent through continental crust. Earth Planet. Sci. Lett. 74, 371 – 386.
Huppert, H.E., Sparks, S.J., 1985b. Komatiites I: eruption and flow. J. Petrol. 26, 694 – 725.
Jensen, L.S., 1985. Stratigraphy and petrogenesis of Archean metavolcanic sequences, southwestern Abitibi Subprovince, Ontario. In: Ayres, L.D., Thurston, P.C., Card, K.D., Weber, W. (Eds.), Evolution of Archean Supracrustal Se-quences. Geol. Assoc. Can. Spec. Pap. 28, pp. 65 – 87. Kretz, R., 1983. Symbols for rock forming minerals. Am.
Miner. 68, 277 – 279.
Lahaye, Y., Arndt, N.T., 1996. Alteration of a komatiite flow from Alexo, Ontario. J. Petrol. 37, 1261 – 1284.
Lahaye, Y., Arndt, N.T., Byerly, G., Chauvel, C., Fourcade, C.S., Grau, G., 1995. The influence of alteration on the trace-element and Nd-isotopic compositions of komatiites. Chem. Geol. 126, 43 – 64.
Larson, M.S., 1996. Physical volcanology and petrogenesis of intrusive and extrusive komatiites in the Shaw Dome area, Abitibi greenstone belt, Ontario, Canada. Unpublished M.Sc. Thesis, University of Alabama, Tuscaloosa, Ala-bama, pp. 259.
Lesher, C.M., 1983. Localization and genesis of komatiite-hosted Fe-Ni-Cu sulfide mineralization at Kambalda, Western Australia. Unpublished Ph.D. Dissertation, Uni-versity of Western Australia, Perth, pp. 318.
Lesher, C.M., 1989. Komatiite-associated nickel sulfide de-posits. In: Whitney, J.A., Naldrett, A.J. (Eds.), Ore Depo-sition Associated With Magmas, Rev. Econ. Geol. 4. Economic Geology Publishing Company, El Paso, pp. 45 – 101.
Lesher, C.M., Arndt, N.T., 1995. REE and Nd isotope geo-chemistry, petrogenesis and volcanic evolution of contami-nated komatiites at Kambalda, Western Australia. Lithos 34, 127 – 157.
Lesher, C.M., Campbell, I.H., 1993. Geochemical and fluid dynamic modeling of compositional variations in Archean komatiite-hosted nickel sulfide ores in Western Australia. Econ. Geol. 88, 804 – 816.
Muir, T.L., 1979. Discrimination between extrusive and intru-sive Archean ultramafic rocks in the Shaw Dome area using selected major and trace elements. Can. J. Earth Sci. 16, 80 – 90.
Nisbet, E.G., 1982. The tectonic setting and petrogenesis of komatiites. In: Arndt, N.T., Nisbet, E.G. (Eds.), Komati-ites. Allen and Unwin, London, pp. 501 – 520.
M.S.Stone,W.E.Stone/Precambrian Research102 (2000) 21 – 46
46
mantle: a review of the evidence from komatiites. Lithos 30, 291 – 307.
Perring, C.S., Barnes, S.J., Hill, R.E.T., 1995. The physical volcanology of Archaean komatiite sequences from For-restania, Southern Cross Province, Western Australia. Lithos 34, 189 – 207.
Pyke, D.R., 1982. Geology of the Timmins area, District of Cochrane. Ont. Geol. Surv. Rep. 219, 141.
Pyke, D.R., Naldrett, A.J., Eckstrand, O.R., 1973. Archean ultramafic flows in Munro Township. Ont. Geol. Soc. Am. Bull. 84, 955 – 978.
Roeder, P.L., Emslie, R.F., 1970. Olivine – liquid equilibrium. Contrib. Miner. Petrol. 29, 275 – 289.
Semet, M.P., Ernst, W.G., 1981. Experimental stability rela-tions of the hornblende magnesio-hastingsite. Geol. Soc. Am. Bull. 92, 71 – 74.
Stone, W.E., Crocket, J.H., Fleet, M.E., 1995. Differentiation processes in an unusual iron-rich alumina-poor Archean ultramafic/mafic igneous body. Ont. Contrib. Miner. Petrol. 119, 287 – 300.
Stone, W.E., Deloule, Larson, M.S., Lesher, C.M., 1997. Evidence for hydrous High-MgO melts in the Precambrian. Geology 25 143 – 146.
Sun, S.S., McDonough, W.F., 1995. The composition of the Earth. Chem. Geol. 120, 223 – 253.