www.elsevier.com / locate / bres
Research report
Changes of GABA receptor binding and subunit mRNA level in rat
Abrain by infusion of subtoxic dose of MK-801
a a b c ,
*
Hack Seang Kim , Hong Serck Choi , Soo Young Lee , Seikwan Oh
a
College of Pharmacy, Chungbuk National University, Cheongju, Chungbuk 361-763, South Korea
b
Center for Cell Signaling Research, Division of Molecular Life Science, Ewha Womans University, Seoul 120-750, South Korea
c
Department of Neuroscience, Medical Research Center, College of Medicine, Ewha Womans University, Mokdong, Yangchon-ku, Seoul 158-710,
South Korea
Accepted 5 July 2000
Abstract
In the present study, we have investigated the effects of prolonged inhibition of NMDA receptor by infusion of subtoxic dose of MK-801 to examine the modulation of GABAA receptor binding and GABAA receptor subunit mRNA level in rat brain. It has been reported that NMDA-selective glutamate receptor stimulation alters GABA receptor pharmacology in cerebellar granule neurons in vitroA
by altering the levels of selective subunit. However, we have investigated the effect of NMDA antagonist, MK-801, on GABA receptorA binding characteristics in discrete brain regions by using autoradiographic and in situ hybridization techniques. The GABAA receptor
3 3 35
bindings were analyzed by quantitative autoradiography using [ H]muscimol, [ H]flunitrazepam, and [ S]TBPS in rat brain slices. Rats were infused with MK-801 (1 pmol / 10ml per h, i.c.v.) for 7 days, through pre-implanted cannula by osmotic minipumps (Alzet, model
3
2ML). The levels of [ H]muscimol binding were highly elevated in almost all of brain regions including cortex, caudate putamen,
3 35
thalamus, hippocampus, and cerebellum. However, the [ H]flunitrazepam binding and [ S]TBPS binding were increased only in specific regions; the former level was increased in parts of the cortex, thalamus, and hippocampus, while the latter binding sites were only slightly elevated in parts of thalamus. The levels of b2-subunit were elevated in the frontal cortex, thalamus, hippocampus, brainstem, and cerebellar granule layers while the levels ofb3-subunit were significantly decreased in the cortex, hippocampus, and cerebellar granule layers in MK-801-infused rats. The levels ofa6- and d-subunits, which are highly localized in the cerebellum, were increased in the cerebellar granule layer after MK-801 treatment. These results show that the prolonged suppression of NMDA receptor function by
3 3 35
MK-801-infusion strongly elevates [ H]muscimol binding throughout the brain, increases regional [ H]flunitrazepam and [ S]TBPS binding, and alters GABA receptor subunit mRNA levels in different directions. The chronic MK-801 treatment has differential effect onA various GABA receptor subunits, which suggests involvement of differential regulatory mechanisms in interaction of NMDA receptorA
with the GABA receptors. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: GABA receptors
Keywords: Autoradiography; In situ hybridization; Osmotic pump; Muscimol; Flunitrazepam
1. Introduction recognition site, but also at allosteric sites that bind benzodiazepine, barbiturates, and steroids [26,36,44]. Bio-GABA is a major inhibitory transmitter in the central chemical studies have provided evidence that there are at
2
nervous system. The GABAA receptor gates a Cl -selec- least two different conformations of the GABA receptor.A tive channel in response to the binding of transmitter and The high affinity GABAA sites can be labeled with
3
contains multiple sites for pharmacologically distinct [ H]muscimol and are associated with GABA recognition classes of allosteric modulators. GABAA receptor-medi- sites [28]. Molecular cloning has revealed a multiplicity of
2
ated Cl conductance is positively modulated at a GABA GABA receptor subunits which, based on homology, canA be divided into subunit classes with multiple members
*Corresponding author. Tel.:182-2-650-5749; fax:182-2-653-8891. [26]: a1–6, b1–4, g1–4, and d. Of the 15 GABAA
receptor subunits known to be present in the adult rodent tional (Natick, MA, USA) and all other reagents were brain, thea1, a6, b2, b3,g2, andd subunit mRNAs are purchased from Sigma (St. Louis, MO, USA).
expressed at high levels in GABA-responsive cells in the
murine cerebellum. When expressed in cell lines, different 2.2. Animals and treatment subunit combinations produce GABAA receptors result in
3
different pharmacological responses [42]. [ H]Muscimol Male Sprague–Dawley rats (Daehan Laboratory Animal, labeling revealed that the GABA recognition site is located Eumsung, South Korea) weighing 220–240 g were ac-on theb-subunit [4] or on both thea- andb-subunit [19], climatized for 1 week with free access to rat chow and tap
3
while [ H]flunitrazepam labeling indicated that the benzo- water. The temperature (24638C) and light (12-h dark) of diazepine recognition site is located mainly on the a- the housing environment were maintained constantly. All subunit [11] or on both the a- and g-subunit [16]. procedures involving rats were performed using protocols Glutamate receptors are involved in generating epi- approved by the Animal Care and Use Committee of our leptiform seizure [5]. The NMDA receptor antagonist, institution. Rats were implanted with guide cannulae for MK-801, decreases the occurrence and severity of ethanol the drug infusion. Rats were anesthetized with a self and pentobarbital withdrawal seizures [15,33], and inhibits prepared anesthetic, Equithensin (40 mM sodium pen-tolerance to ethanol [50]. There is also strong evidence in tobarbital, 250 mM chloral hydrate, 90 mM MgSO ?7H O
4 2
primary cultures of rat cerebellar granule cells that the dissolved in propylene glycol, ethanol, and distilled water), regulation of mRNA expression of various GABAA re- 3 ml / kg i.p., before standard stereotaxic surgery was ceptor subunits is controlled by activation of the NMDA performed on a Kopf stereotaxic frame. A 21-gauge receptor complex [17,29], and that GABAA receptor stainless steel cannula was implanted in the right lateral maturation is reversibly inhibited by MK-801 via modula- ventricle (L, 1.3 mm; A–P,20.5 mm; and D–V,24.5 mm) tion of receptor subunit expression [48]. Thus, gluta- of the rat brain with the bregma chosen as the stereotaxic matergic activity may be an important regulator of reference point [35]. The cannula was held in place with
GABAergic activity. rapid-setting dental acrylic (Lang Dental, Wheeling, IL,
MK-801 is a potent and selective noncompetitive an- USA) anchored to the skull by an aluminum protective cap tagonist of NMDA receptors, and MK-801 exerts various and steel screws. Rats were allowed 1 week for recovery behavioral effects such as anxiolytic, anticonvulsant, and before implantation of osmotic minipumps. The minipump neuroprotective effects [34,40]. MK-801 treatment results was implanted s.c. as described [20] with minor modi-in an modi-increase modi-in the density of radiolabeled antagonist- fication. Briefly, under ether anesthesia, a small cut was preferring conformation of the central benzodiazepine- made behind the ears of the rat and the subcutaneous space binding site [7]. However, MK-801 produces a variety of was expanded with a hemostatic forceps. Saline vehicle or behavioral effects in rodents ranging from hyperactivity to MK-801 (100 nM in saline) was filtered through a 0.2-mm stereotypic motor syndromes such as lateral head weaving, sterile syringe filter (Sterile Acrodisc) and was then used to circling, body rolls, and ataxia [21,22,24,46]. We have fill an osmotic minipump (Alzet 2ML 1, Alza, Palo Alto, therefore selected a low dose of MK-801 (1 pmol / 10 ml CA, USA). The minipump was implanted and connected per h) which does not produce these behavioral effects of directly to the cannula via 6-cm long PE-60 polyethylene the following studies. The present experiments were tubing. The infusion rate was 1 pmol MK-801 / 10ml per h designed to determine whether prolonged infusion of MK- for 7 days. The incision on the back was closed with 801 alters GABA receptor binding and whether MK-801A cyanoacrylate glue, and dental acrylic was layered on top administration alters the expression of GABAA receptor of the polyethylene tube.
subunit mRNA that is linked to the alteration of GABAA receptor in adult rats.
2.3. Tissue preparation
Rats infused with MK-801 were decapitated 2 h after the disconnection of osmotic minipumps. After the
decapita-2. Materials and methods
tion, rat brains were removed immediately and were frozen in liquid nitrogen for 20 s. Horizontal sections, 14-mm 2.1. Chemicals
thickness, were cut on an cryostat microtome at 2188C,
3 3 thaw-mounted on gelatin-coated microscope slides and
[ H]Muscimol (10.1 Ci / mmol), [ H]flunitrazepam (85.8
35 35 stored at 2808C until used.
Ci / mmol), [ S]TBPS (148.7 Ci / mmol), and [ S]dATP (1000 Ci / mmol) were purchased from New England
Nuclear (Boston, MA, USA). Nensorb 20 nuclei purifica- 2.4. Autoradiographic procedures tion cartridges and terminal transferase were purchased
3
35
modifications. In brief, tissue sections were thawed and perature air before the [ S]TBPS binding. Tissue sections dried at room temperature, pre-washed in 50 mM Tris– were incubated in 50 mM Tris–citrate buffer containing 3
35
citrate buffer (pH 7.1) which contains 150 mM NaCl for nM [ S]TBPS and 200 mM KCl for 3 h at room 30 min at 48C and blown-dry under a stream of cool air temperature, rinsed with cold 50 mM Tris–citrate buffer
3
before the [ H]muscimol binding. Tissue sections were two times for 5 min each, dipped once in distilled water at incubated in 50 mM Tris–citrate buffer containing 10 nM room temperature, and immediately dried in a stream of
3
[ H]muscimol for 40 min at 48C, rinsed with cold 50 mM cool air. Non-specific binding was determined in the Tris–citrate buffer three times for 5 min each, dipped once presence of non-radioactive 100mM picrotoxin.
in ice-cold distilled water, and immediately dried in a Dried tissue sections were placed in X-ray cassettes with 3
stream of cool air. Non-specific binding was determined in a set of tritium standards ([ H]Micro-scale RPA 510,
3 3
the presence of non-radioactive 100mM muscimol. Amersham) for [ H]muscimol and [ H]flunitrazepam bind-3
Receptor autoradiography of [ H]flunitrazepam was ing, and carbon standards (Carbon-14 standards, ARC-35
performed according to the method of Carlson et al. [3] 146C, American Radiolabeled Chemicals) for [ S]TBPS with modifications. In brief, tissue sections were thawed binding, and juxtaposed to Hyperfilm or b-max film and dried at room temperature. Then, tissue sections were (Amersham). Following a certain exposure period (6 weeks
3 3
incubated in 50 mM Tri–citrate buffer containing 150 mM for [ H]muscimol and 2 weeks for [ H]flunitrazepam
3 35
NaCl and 1 nM [ H]flunitrazepam for 90 min at 48C, binding at 48C; 2 days for [ S]TBPS binding at room rinsed with cold 50 mM Tris–citrate buffer two times for 5 temperature), the film was developed in Kodak D19 at min each, dipped once in ice-cold distilled water, and room temperature for 3 min and fixed for 5 min. Auto-immediately dried in a stream of cool air. Non-specific radiograms were analyzed by a digital scanning densitome-binding was determined in the presence of non-radioactive ter (Personal Densitometer, Molecular Dynamics,
Sunny-10mM flunitrazepam. vale, CA, USA), operating on the image acquisition and
35
Receptor autoradiography of [ S]TBPS was performed analysis program ImageQuant 3.3 (Molecular Dynamics). according to the method of Edgar and Schwartz [8] with Plastic standards exposed simultaneously with the brain modifications. In brief, tissue sections were thawed and sections were used as reference with the resulting binding dried at room temperature, pre-washed in 50 mM Tris– values given as radioactivity levels estimated for gray citrate buffer (pH 7.4) containing 200 mM KCl and 1 mM matter areas (nCi / mg tissue). Non-specific binding was EDTA to remove endogenous GABA for 10 min at room less than 5% of the total binding and was negligible for temperature and blown-dry under a stream of room-tem- analyzing the autoradiograms.
3
Fig. 1. Representative autoradiograms of [ H]muscimol binding in horizontal rat brain sections. Tissue sections were incubated in 50 mM Tris–citrate
3
Table 1 2.5. In situ hybridization
3
Changes in [ H]muscimol binding to brain regions in rats chronically infused with MK-801
In situ hybridization was carried out by our previous
3
Regions [ H]Muscimol binding described method [32]. Briefly, thaw-mounted brain sec-(nCi / mg tissue)
tions were rinsed 3 times in phosphate-buffered saline
a
Saline MK-801 (PBS) for 3 min, rinsed once in 23 SSC for 3 min and
rinsed once in deionized water. The probe was designed to
Frontal cortex
Layers II, III 1.4160.16 2.4960.21** be complementary to the mRNA of GABA receptor
A
Layers IV, V, VI 0.7960.10 1.5460.11** a6-subunit (59-TGA CTT TGC TAC CGG GGG CTT
Cingulate 1.6760.15 2.7660.19**
GGC TGC AGT CTG TGC CTG CCT-39; residues 318–
Caudate-putamen 0.4460.06 0.9460.07** 331) [25], b2-subunit (59-TCG TTC CAG GGC GTT
GCG GCC AAA ACT ATG CCT AGG CAA CCC
AGC-Thalamic nuclei
Anterior ventral 1.4060.14 2.9060.21** 39; residues 1292–1336) [51], b3-sbunit (59-CAT GTA
Ventral lateral 1.2860.13 2.7760.21** CCG CCC ATG CCC TTC CTT GGG CAT-39; residues Lateral geniculate 1.3260.17 2.5960.22**
1289–1318) [51], d-subunit (59-GAG GAC AAT GGC
Medial geniculate 1.0560.12 1.9660.11**
GTT CCT CAC GTC CAT CTG TGC CCT TGG-39;
Hippocampal formation residues 1078–1116) [41]. Probe was labeled on its 39-end
CA1 0.5960.07 1.1460.04** 35
using terminal transferase and [a- S]dATP. The labeled
Dentate gyrus 1.0160.10 1.9260.16**
probe was purified by Nensorb-20 column, and the probe
Cerebellum 5
solution (5310 dpm /ml) was diluted by 50 times with the
Molecular layer 1.7760.12 2.5160.20**
hybridization buffer. Each brain section was hybridized
Granule layer 5.0760.32 8.2860.39**
with 30 ml of hybridization buffer under a coverslip and
a
MK-801 was continuously infused (flow rate, 1 pmol / 10ml per h) into
was incubated at 388C in a high-humidity environment
rat brain (i.c.v.) by miniosmotic pumps for 7 days. The tissue sections
3 overnight. After hybridization, coverslips were removed in
were incubated with 10 nM [ H]muscimol in the presence of 150 mM
NaCl. Values are expressed as mean6S.E. from five to six rats. 13SSC, and slides were rinsed to remove excess
hybridi-**P,0.01 for difference from respective control groups. zation buffer. Finally, slides were briefly dipped in
deion-3
Fig. 2. Representative autoradiograms of [ H]flunitrazepam binding in horizontal rat brain sections. Tissue sections were incubated in 50 mM Tris–citrate
3
Table 2
ized water and dried with air. Dried tissue sections were 3
14 Changes in [ H]flunitrazepam binding to brain regions in rats chronically placed in X-ray cassettes with a set of C-standard
(ARC-infused with MK-801
146, St. Louis, MO) and juxtaposed to Hyperfilm-bmax
3
Regions [ H]flunitrazepam binding
(Amersham). Following a 2-week exposure period at room
(nCi / mg tissue)
temperature, the film was developed and analyzed as
a
Saline MK-801
described in Section 2.4.
Cortex
Layers II, III 16.0060.45 17.3360.41*
2.6. Quantification and statistics
Frontal layer IV 17.8260.47 18.7960.37 Frontal layers V, VI 13.2360.47 13.4960.35
The density in each region was recorded by marking Cingulate 19.4360.45 19.9560.33 four to 10 areas over bilateral sides of the brain according
Caudate-putamen 5.7260.26 6.1060.23
to the size and shape of the region. The mean values were
Septum 7.2660.30 8.0160.46
determined from five rats and expressed as the mean6S.E.,
in nCi / mg tissue for autoradiography and nCi / g tissue for Thalamic nuclei
in situ hybridization, respectively. Data were analyzed Ventral lateral 4.0560.27 4.7060.22 Lateral geniculate 4.8860.15 5.6260.18**
using Student’s t-test and analysis of variance followed by
Habenula 5.0660.29 5.4960.27
Newman–Keul’s test.
Hippocampal formation
CA1 13.5160.32 14.1860.24
CA3 10.8760.25 11.6760.14*
3. Results Dentate gyrus 17.9460.41 18.8760.42
Brainstem
The distribution of GABAA receptor binding (e.g., Inferior colliculus 4.9860.34 4.1560.19
3 3 35
[ H]muscimol, [ H]flunitrazepam, and [ S]TBPS binding) Central gray 13.6460.52 11.9860.24* shows regional discrepancies in the rat brain.
Autoradiog-3 Cerebellum
raphs of [ H]muscimol binding are shown in Fig. 1. When Molecular layer 13.7860.41 13.5060.33 the bindings were quantitated in 10–12 slices, the level Granule layer 5.2360.36 5.8360.42 was found to be highest in the granule layer of cerebellum, a
MK-801 was continuously infused (flow rate, 1 pmol / 10ml per h) into
moderately high in the cortex and thalamus, and low in the rat brains (i.c.v.) by miniosmotic pumps for 7 days. The tissue sections
3
hippocampus and caudate putamen (Fig. 1 and Table 1). were incubated with 1 nM [ H]flunitrazepam in the presence of 150 mM
3
Binding of [ H]muscimol was highly increased (70– NaCl. Values are expressed as mean6S.E. from five to six rats. *P,0.05, **P,0.01 for difference from respective control groups.
110%) in the cortex, caudate putamen, thalamus and hippocampus by the treatment of MK-801, and also
significantly increased (40–60%) in the cerebellum (Table mRNA in the rat brain is shown in Fig. 4. When the
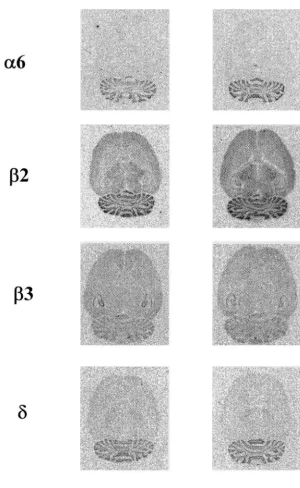
1). images were quantitated, levels of a-6 subunit were
3
When the autoradiographs of [ H]flunitrazepam were exclusively localized in cerebellar granule layers, and were quantitated, binding was found to be highest in the cortex strongly increased (87%) by the infusion of MK-801. The and hippocampus, moderately high in the caudate putamen, levels ofb2-subunit were found to be highest in cerebellar brainstem and molecular layer of the cerebellum, and low granule layers, and significant levels were observed in in the thalamus and granule layer of the cerebellum (Fig. 2 cerebral cortical areas, thalamus, hippocampus, and
in-3
and Table 2). Binding of [ H]flunitrazepam was signifi- ferior colliculus (Fig. 4 and Table 4). The levels of cantly increased (7–15%) in part of the frontal cortex b2-subunit were significantly elevated in almost all areas (layers II and III), thalamus (lateral geniculate) and of brain by the infusion of MK-801; frontal cortex (15%), hippocampus (CA3) by the treatment of MK-801, but was thalamus (14%), hippocampal area (8–17%), inferior not significantly affected in the cerebellum. However, the colliculus (16%), and cerebellar granule layers (19%). The
3
level of [ H]flunitrazepam binding was decreased (14%) in levels ofb3-subunit were found to be highest in hippocam-parts of the brain stem (central gray area) (Table 2). pal area, and significant levels were observed in frontal
35
When the autoradiographs of [ S]TBPS were quanti- cortex and cerebellar granule layers. In contrast to the tated, binding was found to be high in the frontal cortex, b2-subunit, the levels of b3-subunit were significantly thalamus, brainstem and granule layer of the cerebellum, decreased by the infusion of MK-801; frontal cortex and low in the septum and molecular layer of the cere- (34%), hippocampal area (22–39%), and cerebellar
35
bellum (Fig. 3 and Table 3). Binding of [ S]TBPS in granule layers (21%) (Fig. 4 and Table 4). The levels of almost all area of the brain was not modulated by the d-subunit were found in frontal cortex, thalamus, and treatment of MK-801, although the level was increased cerebellar granule layers. The levels of d-subunit were (7–8%) in the part of thalamus (ventral lateral and lateral significantly decreased (44%) in frontal cortex but the
geniculate) (Table 3). levels were increased (15%) in the cerebellar granule
35
Fig. 3. Representative autoradiograms of [ S]TBPS binding in horizontal rat brain sections. Tissue sections were incubated in 50 mM Tris–citrate buffer
35
containing 200 mM and 3 nM [ S]TBPS for 3 h at room temperature. Table 3
35 4. Discussion
Changes in [ S]TBPS binding to brain regions in rats chronically infused with MK-801
35 In the present study, the effect of the prolonged
ven-Regions [ S]TBPS binding (nCi / g tissue)
tricular infusion of the NMDA receptor antagonist,
MK-a
Saline MK-801
801, on GABAA receptor binding and GABAA receptor
Cortex subunit mRNA levels were determined in rat brain slices.
Frontal layers II, III 195.3069.21 199.1465.45 There is evidence that the formation of individual GABA Frontal layer IV 212.5369.53 212.9966.38
receptor assemblies may be the consequence of the
availa-Frontal layer V,VI 212.1369.63 222.3966.44 3
bility of individual subunits. The [ H]muscimol and
Entorhinal cortex 231.2165.49 232.7666.11
3
Cingulate 203.4469.29 206.3867.85 [ H]flunitrazepam photolabeling studies have revealed that the GABA and benzodiazepine recognition sites of the
Caudate-putamen 180.7664.51 185.0564.19
GABAA receptors are located on the a- and b-subunits,
Thalamic nuclei
anda- and g-subunits, respectively [16,19]. The different
Ventral lateral 194.9464.60 208.1064.64*
pharmacological and physiological profiles of GABA
Lateral geniculate 202.0262.03 219.0565.63* A
Medial geniculate 224.7366.43 232.8865.98 receptors are the consequence of the different receptor
Habenula 176.9965.24 184.5666.08 subunits that make the receptor complexes. In the current
3
studies, the binding of [ H]muscimol was increased in the
Hippocampal formation
CA1 167.0262.33 170.4664.57 frontal cortex, striatum, thalamus, hippocampus, and cere-CA3 187.9764.65 191.9866.03 bellar granule layers. Also, the levels of the b2-subunit Dentate gyrus 175.3963.05 175.2964.43
were elevated in the frontal cortex, thalamus,
hippocam-Brainstem pus, brainstem, and cerebellar granule layers, while the
Inferior colliculus 212.0064.14 207.1564.05 level of the b3-subunit was significantly decreased in the Central gray 92.5565.40 185.6265.49
cortex, hippocampus, and cerebellar granule layers. These
Cerebellum results suggest that rearrangement of GABA receptor
A
Molecular layer 182.7165.44 181.5164.16
b2-subunit composition may affect its function during
Granule layer 194.2566.54 194.8964.02
chronic MK-801 treatment which explains the elevation of
a
3
MK-801 was continuously infused (flow rate, 1 pmol / 10ml per h) into [ H]muscimol binding. In our previous studies examining rat brains (i.c.v.) by miniosmotic pumps for 7 days. The tissue sections
35 the in situ hybridization of the mRNA receptorb2-subunit,
were incubated with 3 nM [ S]TBPS in the presence of 200 mM KCl. 3
a critical subunit for [ H]muscimol binding, theb2-subunit
Values are expressed as mean6S.E. from five to six rats.
col-Fig. 4. Representative autoradiograms of GABA receptor subunit (A a6,b2,b3, andd) mRNAs in rat brain sections. Saline or MK-801 (1 pmol / 10ml per h) was continuously infused into rat brain (i.c.v.) by miniosmotic pump for 7 days.
3
liculus, and cerebellar granule layers, while [ H]muscimol as thalamus and cerebellar granule layers [2,41]. However, binding was increased only in the cingulate and cerebellar the level ofd-subunit mRNA is decreased in frontal cortex, granule layers of rats after withdrawal from pentobarbital although the levels ofa6- and d-subunit was increased in [32]. It seems probable that the binding ability of cerebellar granule layers after MK-801 infusion in the
3
[ H]muscimol does not entirely rely on theb2-subunit, and present experiment. So, the functional significance of other subunits (e.g.,a6- or d-subunit) are likely involved mRNA alterations must be interpreted with caution. in muscimol binding. Interestingly, GABAA receptor d- The chronic infusion of MK-801 increased the binding
3
subunit is most abundant in brain regions that contain high of [ H]muscimol throughout the brain while selectively 3
spe-Table 4
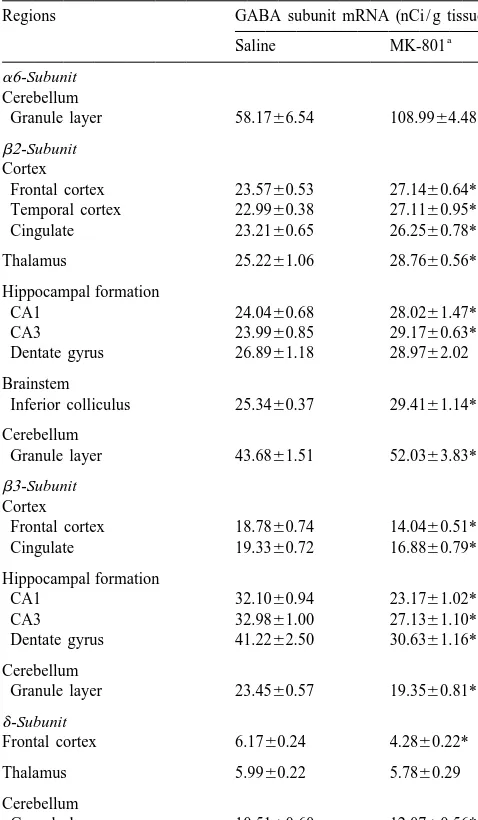
Changes of GABA receptor subunit mRNA in brain regions in rats binding and mRNA levels (b3-subunit) albeit to a different chronically infused with MK-801
GABA antagonist, suggesting that the regional type of
Regions GABA subunit mRNA (nCi / g tissue) NMDA receptor plays an important role in the GABA
a
effect. The elevation we have found within the granule cell
Saline MK-801
layer of the cerebellum might result from feedback
be-a6-Subunit
tween other cerebellar cell types that is missing in tissue
Cerebellum
Granule layer 58.1766.54 108.9964.48** culture. Recently, there was an interesting report that chronic MK-801 administration alters b2 /b3-subunit
ex-b2-Subunit
pression in a region-specific manner. This subunit was
Cortex
Frontal cortex 23.5760.53 27.1460.64** significantly decreased in hippocampus but not signifi-Temporal cortex 22.9960.38 27.1160.95** cantly changed in cerebellar cortex after repeated injection Cingulate 23.2160.65 26.2560.78*
of MK-801 (0.4 mg / kg, twice a day) for 14 days [27].
Thalamus 25.2261.06 28.7660.56* However, in the current studies, the expression of b2 and b3 was not differentially changed in the cortex and
Hippocampal formation
CA1 24.0460.68 28.0261.47* hippocampus. Although the reason for this discrepancy is CA3 23.9960.85 29.1760.63** unclear, the dose of drug or the route of administration Dentate gyrus 26.8961.18 28.9762.02
might be responsible for the differential actions of
MK-Brainstem 801. The changes of b-subunit composition are variable
Inferior colliculus 25.3460.37 29.4161.14**
after treatment of GABA agonists and antagonists. The
Cerebellum GABAA receptor antagonist, TBPS, upregulated both b
2-Granule layer 43.6861.51 52.0363.83* and b3-subunit mRNA expression in cultured cortical
b3-Subunit neurons [38], and chronic treatment with a benzodiazepine
Cortex (triazolam) resulted in a decrease of b2- and b3-subunit
Frontal cortex 18.7860.74 14.0460.51**
mRNA levels [39]. Chronic ethanol administration
pro-Cingulate 19.3360.72 16.8860.79*
duced an upregulation of b2- and b3-subunit mRNA in
Hippocampal formation cerebral cortex in rats [30]. However, different regulation
CA1 32.1060.94 23.1761.02**
of GABAA receptor subunit expression has also been
CA3 32.9861.00 27.1361.10**
observed in other experimental paradigms. Chronic ethanol
Dentate gyrus 41.2262.50 30.6361.16**
increased a6 mRNA, whereas it had no effect on the b
2-Cerebellum
and b3-subunit mRNA expression [49].
Granule layer 23.4560.57 19.3560.81**
It is unclear why there were opposing expressions ofb
2-d-Subunit
and b3-subunits. One possibility is the receptor milieu on
Frontal cortex 6.1760.24 4.2860.22*
the neurons dictates the GABA subunit. For example,
Thalamus 5.9960.22 5.7860.29
cholinergic neurons express the b3-subunit, whereas
Cerebellum GABAergic neurons express the b2-subunit [12]. In fact,
Granule layer 10.5160.60 12.0760.56* there is an interesting report that NMDA receptor blockade
a
MK-801 was continuously infused (flow rate, 1 pmol / 10ml per h) into by MK-801 reduces basal acetylcholine release and
pre-rat brains (i.c.v.) by miniosmotic pumps for 7 days. The tissue sections vents dopamine receptor-dependent stimulation of
acetyl-35
were incubated with 3 nM [ S]TBPS in the presence of 200 mM KCl.
choline release [6]. Alternatively, the specific NMDA
Values are expressed as mean6S.E. from five to six rats.
receptor subtypes expressed regionally may result in
*P,0.05, **P,0.01 for difference from respective control groups.
different responses after the prolonged treatment with MK-801.
A number of studies demonstrate the existence of 21
several [Ca ] regulation mechanisms of GABA receptor
cific brain regions. These results are consistent with the i A
21 report that 24 h after intraperitoneal injection of MK-801 function which differ in duration, source of Ca influx
21 (1.0 mg / kg), there was an increased density of the and intracellular mechanisms triggered by [Ca ]i benzodiazepine binding site along with a potentiation of [18,23,31,37]. The reduction of GABAA responses
per-21 flunitrazepam ability to block convulsions [7]. On the other sisted after cessation of NMDA-mediated Ca influx, hand, the prolonged treatment (1–2 weeks) of granule cells indicating a long-term action of NMDA on GABAA
21
21 neuronal calcium currents by prolonged changes of membrane mediates alterations in Ca signaling, protein
phosphoryl-potential, J. Neurosci. 12 (1992) 1726–1735.
ation pathways, and immediate-early gene induction
[11] K. Fuchs, H. Mohler, W. Sieghart, Various proteins from rat brain,
[1,9,10,14,47]. The addition of NMDA to cultured rat 3
specifically and irreversibly labeled by [ H]flunitrazepam, are
granule neurons affects thed-subunit transcript expression distincta-subunits of the GABA-benzodiazepine receptor complex,
21
through Ca -dependent protein kinase activation and Neurosci. Lett. 90 (1988) 314–319.
[12] B. Gao, J.P. Hornung, J.M. Fritschy, Identification of distinct
increases the d-subunit mRNA level 4-fold without
sig-GABA -receptor subtypes in cholinergic and parvalbumin-positiveA
nificantly affecting other GABA subunit levels [13].A
neurons of the rat and marmoset medial-septum, Neuroscience 65
In summary, the current results show that prolonged (1995) 101–117.
infusion of MK-801 elevates the GABAA receptor b2- [13] L.M. Gault, R.E. Siegel, NMDA receptor stimulation selectively
subunit throughout the brain as evident from increased initiates GABAA receptordsubunit mRNA expression in cultured
3 3
rat cerebellar granule neurons, J. Neurochem. 70 (1998) 1907–1915.
[ H]muscimol. The bindings of [ H]flunitrazepam and
35 [14] A. Ghosh, M.E. Greenberg, Calcium signaling in neurons: molecular
[ S]TBPS, on the other hand, are elevated only in specific
mechanisms and cellular consequences, Science 268 (1995) 239–
brain regions. The increase and decrease in b-subunit 247.
mRNA by prolonged infusion of MK-801 may underlie or [15] K.A. Grant, P. Valverius, M. Hudspith, B. Tabakoff, Ethanol at least reflect a compensation response of the GABAA withdrawal seizures and the NMDA receptor complex, Eur. J.
Pharmacol. 176 (1990) 289–296.
receptor complex to chronic occupation by NMDA
re-[16] K.L. Hadingham, P.B. Winggrove, K.A. Wafford, C. Bain, J.A.
ceptor antagonist.
Kemp, K.J. Palmer, A.W. Wilson, A.S. Wilcox, J.M. Siketa, C.I. Ragan, P.J. Whiting, Role of the b subunit in determining the pharmacology of humang-aminobutyric acid type A receptors, Mol. Pharmacol. 44 (1993) 1211–1218.
Acknowledgements [17] B.T. Harris, M.E. Charlton, E. Costa, D.R. Grayson, Quantitative
changes ina1 anda5g-aminobutyric acid type A receptor subunit
This study was supported by a grant of the Korea Health mRNAs and proteins after a single treatment of cerebellar granule neurons with N-methyl-D-aspartate, Mol. Pharmacol. 45 (1994) 21 R&D Project, Ministry of Health and Welfare, Republic
637–648.
of Korea (Grant HMP-99-N-02-0003)
[18] M. Inoue, Y. Oomura, T. Yakushiji, N. Akaike, Intracellular calcium ions decrease the affinity of the GABA receptor, Nature 324 (1986) 156–158.
[19] Z.U. Khan, A. Gutierrez, A.L. De Blas, The subunit composition of
References GABA / benzodiazepine receptor from rat cerebellum, J.
Neuro-A
chem. 63 (1994) 371–374.
[1] Y. Ben-Ari, C. Anikszetejn, P. Bregestovski, Protein kinase C [20] T. Kimura, T. Miyaoka, P.A. Saunders, M.L. Baker, A.S. Hume, I. modulation of NMDA currents: an important link for LTP induction, Yamamoto, I.K. Ho, Induction of tolerance to and physical depen-Trends Neurosci. 15 (1992) 333–339. dence on pentobarbital continuous intracerebroventricular adminis-[2] D. Benke, S. Mertens, A. Trzeciak, D. Gillessen, H. Mohler, tration, J. Pharmacol. Exp. Ther. 266 (1993) 1300–1305.
Identification and immunohistochemical mapping of GABAA re- [21] V.D. Lehman-Masten, M.A. Geyer, Spatial and temporal patterning ceptor subtypes containing the d-subunit in rat brain, FEBS Lett. distinguishes the locomotor activity effects of dizocilpine and 283 (1991) 145–149. phencyclidine in rats, Neuropharmacology 30 (1991) 629–636. [3] B.X. Carlson, A.M. Mans, R.A. Hawkins, H.A. Baghdoyan, Pen- [22] S. Liljequist, K. Ossowska, M. Grabowska-Anden, N.E. Anden,
3
tobarbital-enhanced [ H]flunitrazepam binding throughout the rat Effects of the NMDA receptor antagonist, MK-801, on locomotor brain: an autoradiographic study, J. Pharmacol. Exp. Ther. 263 activity and on the metabolism of dopamine in various brain areas of
(1992) 1401–1414. mice, Eur. J. Pharmacol. 195 (1991) 55–61.
[4] S.O. Casalotti, F.A. Stephenson, E.A. Barnard, Separate subunits for [23] I. Llano, N. Leresche, A. Marty, Calcium entry increases the agonist and benzodiazepine binding in the g-aminobutyric acidA sensitivity of cerebellar Purkinje cells to applied GABA and receptor oligomer, J. Biol. Chem. 261 (1986) 15013–15016. decreases inhibitory synaptic currents, Neuron 6 (1992) 565–574. [5] G.L. Collingridge, R.A. Lester, Excitatory amino acid receptors in [24] W. Loscher, D. Honack, The behavioral effects of MK-801 in rats:
the vertebrate central nervous system, Phramacol. Rev. 41 (1989) Involvement of dopaminergic, serotonergic, and adrenergic system,
143–210. Eur. J. Pharmacol. 215 (1992) 199–208.
[6] G. Damsma, G.S. Robertson, C.S. Tham, H.C. Fibiger, Dopa- [25] H. Luddens, B.B. Pritchett, M. Kohler, I. Killisch, K. Keinanen, H. minergic regulation of striatal acetylcholine release: importance of Monyer, R. Sprengel, P.H. Seeberg, Cerebellar GABAA receptor D1 and N-methyl-D-aspartate receptor, J. Pharmacol. Exp. Ther. 259 selective for a behavioural alcohol antagonist, Nature 346 (1990)
(1991) 1064–1072. 648–651.
[7] S.I. Deutsch, C.H. Park, L.G. Lukacs, C. Morn, L. Koetzner, J. [26] R.L. MacDonald, R.W. Olsen, GABAA receptor channels, Annu. Mastropaolo, MK-801 alters the GABAA receptor complex and Neurosci. 17 (1994) 569–602.
potentiates flunitrazepam’s antiseizure efficacy, Pharmacol. Bio- [27] D.B. Mattews, J.E. Kralic, L.L. Devaud, J.M. Fritschy, A.L. chem. Behav. 51 (1995) 909–915. Morrow, Chronic blockade of N-methyl-D-aspartate receptors alters [8] P.P. Edgar, R.D. Schwartz, Localization and characterization of g-aminobutyric acid type A receptor peptide expression and function
35
S-t-butylbicyclophosphorothionate binding in rat brain: an au- in the rat, J. Neurochem. 74 (2000) 1522–1528.
toradiographic study, J. Neurosci. 10 (1990) 603–612. [28] R.T. McCabe, J.K. Wamsley, Autoradiographic localization of
45 21
[9] A. Frandsen, J. Drejer, A. Schousboe, Glutamate-induced Ca subcomponents of the macromolecular GABA receptor complex, uptake into immature cerebral cortex neurons shows a distinct Life Sci. 39 (1986) 1937–1945.
activa-tion of N-methyl-D-aspartate-selective glutamate receptors, Mol. [42] E. Sigel, F.A. Stephenson, C. Mammalaki, E.A. Barnard, A g -Pharmacol. 39 (1991) 599–603. aminobutyric acid / benzodiazepine receptor complex of bovine [30] M. Mhatre, M.K. Ticku, Chronic ethanol treatment upregulates the cerebral cortex, J. Biol. Chem. 258 (1983) 6965–6971.
GABA receptor b subunit expression, Mol. Brain Res. 23 (1994) [43] A. Stelzer, H. Shi, Impairment of GABAA receptor function by
246–252. N-methyl-D-aspartate-mediated calcium influx in isolated CA1
[31] D. Mouginot, P. Feltz, R. Schlichter, Modulation of GABA-gated pyramidal cells, Neuroscience 62 (1994) 813–828.
21
chloride currents by intracellular Ca in cultured porcine melanot- [44] R.E. Study, J.L. Barker, Diazepam and (2)pentobarbital: fluctuation rophs, J. Physiol. 437 (1991) 109–132. analysis reveals different mechanisms for potentiation ofg
-amino-3
[32] S. Oh, I.K. Ho, Changes of [ H]muscimol binding and GABAA butyric acid responses in cultured central neurons, Proc. Natl. Acad. receptor b2-subunit mRNA level by tolerance to and withdrawal Sci. USA 78 (1981) 7180–7184.
from pentobarbital in rats, Neurochem. Res. 24 (1999) 1603–1609. [45] M.N.G. Titulaer, W. Kamphuis, C.W. Pool, J.J. van Heerikhuize, [33] S. Oh, K. Hoshi, I.K. Ho, Role of NMDA receptors in pentobarbital F.H. Lopes Da Silva, Kindling induces time-dependent and regional
3
tolerance / dependence, Neurochem. Res. 22 (1997) 767–774. specific changes in the [ H]muscimol binding in the rat hippocam-[34] G.C. Ormandy, R.S. Jope, O.C. Snead, Anticonvulsant actions of pus: a quantitative autoradiographic study, Neuroscience 59 (1994)
MK-801 on the lithium-pilocarpine model of status epilepticus, Exp. 817–826.
Neurol. 106 (1989) 172–180. [46] M.D. Tricklebank, R.J. Singh, C. Oles, C. Preston, S.D. Irversen, [35] G. Paxinos, C. Watson, in: The Rat Brain in Stereotaxic Coordinates, The behavioural effects of MK-801: A comparison with antagonists 2nd Edition, Academic Press, Orlando, FL, 1986. acting noncompetitively and competitively at the NMDA receptor, [36] J.A. Peters, E.F. Kirkness, H. Callachan, J.L. Lambert, A.J. Turner, Eur. J. Pharmacol. 167 (1989) 127–135.
Modulation of the GABA by depressant barbiturates and pregnaneA [47] F.M. Vaccarino, H. Alho, M.R. Santi, A. Guidotti, Coexistence of steroids, Br. J. Pharmacol. 94 (1988) 1257–1269. GABA receptors and GABA-modulin in primary cultures of
cerebel-21
[37] T.A. Pitler, B.E. Alger, Elevation of intracellular Ca inhibits lar granule cells, J. Neurosci. 7 (1987) 65–76.
GABAergic IPSPs in hippocampal neurons, J. Neurosci. 12 (1992) [48] X.H. Wang, W.J. Zhu, L. Corsi, S. Ikonomovic, W.R. Paljug, S.
4122–4132. Vicini, D.R. Grayson, Chronic dizocilpin (MK-801) reversibly
[38] M.O. Poulter, L. Ohannesian, Y. Larmet, P. Feltz, Evidence that delays GABA receptor maturation in cerebellar granule neurons inA
GABA receptor subunit mRNA expression during development isA vitro, J. Neurochem. 71 (1998) 693–704.
regulated by GABA receptor stimulation, J. Neurochem. 68 (1997)A [49] C.H. Wu, A. Frostholm, A.L. De Blas, A. Rotter, Differential 631–639. expression of GABA / benzodiazepine receptor subunit mRNAs andA
[39] V.A. Ramsey-Williams, D.B. Carter, Chronic trizolam and its ligand binding sites in mouse cerebellar neurons following in vivo withdrawal alters GABA receptor subunit mRNA levels: an in situA ethanol administration: an autoradiographic analysis, J. Neurochem. hybridization study, Mol. Brain Res. 43 (1996) 132–140. 65 (1995) 1229–1239.
[40] M.A. Rogawski, R.J. Porter, Antiepileptic drugs: pharmacological [50] P.H. Wu, S.J. Mihic, J.F. Liu, A.D. Le, H. Kalant, Blockade of mechanisms and clinical efficacy with consideration of promising chronic tolerance to ethanol by the NMDA antagonist, MK-801, developmental stage compounds, Pharmacol. Rev. 42 (1990) 223– Eur. J. Pharmacol. 231 (1993) 157–164.
286. [51] S. Ymer, P.R. Schofield, A. Draguhn, P. Werner, M. Kohler, P.H. [41] B.D. Shivers, I. Killisch, R. Sprengel, H. Sontheimer, M. Kohler, Seeberg, GABAA receptor b subunit heterogeneity; functional
![Fig. 1. Representative autoradiograms of [ H]muscimol binding in horizontal rat brain sections](https://thumb-ap.123doks.com/thumbv2/123dok/3140037.1382879/3.612.94.502.427.706/fig-representative-autoradiograms-muscimol-binding-horizontal-brain-sections.webp)
![Table 1Changes in [ H]muscimol binding to brain regions in rats chronically](https://thumb-ap.123doks.com/thumbv2/123dok/3140037.1382879/4.612.100.494.455.707/table-changes-muscimol-binding-brain-regions-rats-chronically.webp)
![Table 2Changes in [ H]flunitrazepam binding to brain regions in rats chronically](https://thumb-ap.123doks.com/thumbv2/123dok/3140037.1382879/5.612.307.550.91.378/table-changes-unitrazepam-binding-brain-regions-rats-chronically.webp)
![Fig. 3. Representative autoradiograms of [ S]TBPS binding in horizontal rat brain sections](https://thumb-ap.123doks.com/thumbv2/123dok/3140037.1382879/6.612.45.284.409.691/fig-representative-autoradiograms-tbps-binding-horizontal-brain-sections.webp)