Ž .
Animal Reproduction Science 59 2000 109–117
www.elsevier.comrlocateranireprosci
Natural and radiation-induced degeneration of
primordial and primary follicles in mouse ovary
Chang Joo Lee
a,b, Ho Hyun Park
b, Byung Rok Do
b,
Yong-Dal Yoon
b, Jin Kyu Kim
a,)a
Korea Atomic Energy Research Institute, Yusong P.O. Box 105, Taejon 305-600, South Korea
b
Department of Biology, College of Natural Sciences, Hanyang UniÕersity, Seoul 133-791, South Korea
Received 7 July 1999; received in revised form 30 November 1999; accepted 13 December 1999
Abstract
The present study deals with the morphological changes of the degenerating primordial and primary follicles induced byg-radiation. Prepubertal female mice of 3 weeks old ICR strain were
Ž .
g-irradiated with the dose of LD80Ž30. 8.3 Gy . The ovaries were collected at 3, 6 and 12 h after irradiation. The largest cross-sections were prepared by histological semithin sections for
micro-Ž .
scopical observations. The ratio % of normal to atretic follicles decreased with time after the irradiation in primordial follicles and in primary follicles as well. At 6 h after irradiation, the number of degenerated primordial follicles increased. Germinal vesicles disappeared and lipid droplets increased in number. Granulosa cells became round in shape and apoptotic cells started to appear. The ooplasmic membrane was not recognizable. The ratio of normal to atretic primordial follicles in the control group was 62.5. Then it became lower with time after the irradiation. It went down to 51.6, 49.0, 11.1 and 7.1 at 0, 3, 6 and 12 h, respectively. The ratio of normal to atretic primary follicles in the control mouse ovary was 81.3. It was 80.0, 75.0, 45.5 and 33.3 at 0, 3, 6 and 12 h after irradiation, respectively. It is concluded that the ionizing radiation acutely induces the degeneration of primordial and primary follicles. The pattern of degeneration is one of
Ž . Ž .
the following: 1 apoptosis of one or more granulosa cells with a relatively intact oocyte, 2 Ž .
apoptosis of an oocyte with intact follicle cells, or 3 apoptotic degenerations of both kinds of cells. These results can provide morphological clues for the identification of the degenerating
)Corresponding author. Tel.:q82-42-868-2057; fax:q82-42-868-2091.
Ž .
E-mail address: [email protected] J.K. Kim .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
primordial and primary follicles in normal and irradiated mouse ovaries.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Mouse; Ovary; Primary follicle; Primordial follicle; Radiation
1. Introduction
A primordial follicle is the smallest follicle within the ovary and contains an oocyte
Ž .
surrounded by a single layer of flattened somatic cells Meredith and Doolin, 1997 . The formation of the primordial follicles occurs around the time of birth in rodents
ŽHirshfield, 1991 . By unproved mechanisms, the primordial follicles initiate growth into.
primary, and consequently, secondary stages before acquiring an antral cavity. Except for a few primordial follicles, most of them remain as resting, quiescent, and nongrow-ing pool of follicles that will be progressively depleted throughout the reproductive life span of the female. Most of the ovarian follicles undergo a degenerative process called
Ž .
atresia during the reproductive life in mammals Byskov, 1978 . However, the precise mechanism of follicular atresia has not been elucidated yet. The atretic follicles include such morphological characteristics as gradual pyknosis of granulosa cell nuclei,
reduc-Ž
tion in granulosa cell proliferation and breakdown of the basement membrane
Hirsh-.
field and Midgley, 1978; Braw and Tsafriri, 1980 . However, most of the research works regarding the follicular atresia have been focused on the growing follicles. In the mammalian ovary, two major stages of cell degeneration can be distinguished: the degeneration of germ cells, defined as attrition, which accounts for the main loss of
Ž .
oocytes Beaumont and Mandl, 1962 and occurs prenatally; and the follicular
degenera-Ž
tion, defined as atresia, which occurs during postnatal reproductive life Kaipia and
.
Hsueh, 1997 .
Apoptosis, a regulated form of cell death, is a physiological process essential for the
Ž .
normal tissue homeostasis Kaipia and Hsueh, 1997 in the absence of immune
Ž .
surveillance Kerr et al., 1994 . Ovarian follicular degeneration or atresia is a hormon-ally controlled apoptotic process, whereby the degenerating follicles are eliminated in a
Ž .
coordinated fashion Hsueh et al., 1994 . It is now accepted that pyknosis of granulosa
Ž
cells is an apoptotic process Hughes and Gorospe, 1991; Billig et al., 1993; Hurwitz
.
and Adashi, 1993; Gougeon, 1996 . One of the atretogenic stimuli that could accelerate
Ž .
the follicular atresia was g-radiation Kim et al., 1999 . In both normal tissues and
tumors, apoptosis not only occurs spontaneously but can also be induced by irradiation
ŽHendry and West, 1997 . Radiation induced cell apoptosis Hendry and West, 1997. Ž .
Ž .
and impaired the ovarian functions Chapman, 1982 . It was reported that primordial oocytes of rats and mice were more sensitive to radiation than oocytes in the growing
Ž .
follicles Ataya et al., 1995 . However, the radiation sensitivities between the resting and nongrowing primordial and the activated primary follicles were not morphologically demonstrated. Moreover, the morphological changes of the primordial and primary follicles occurred at natural and artificial degeneration processes were also not depicted yet.
( )
C.J. Lee et al.rAnimal Reproduction Science 59 2000 109–117 111
2. Materials and methods
2.1. Experimental animals
Ž .
Prepubertal female mice ICR strain aged 3 weeks were used to avoid the interven-ing results, which could come from the reproductive cycles. Female mice were obtained from Toxicology Research Center, Korea Research Institute of Chemical Technology,
Taejon, Republic of Korea. The mice were maintained in a 238C-controlled animal care
Ž .
room with lightrdark 12r12 h . The animals had free access to tap water and
commercial chow during the experiments.
2.2. Irradiation
Five mice per group were whole-body irradiated with g-radiation from60Co isotopic
Ž
source dose rate: 6.94 cGyrmin, source strength: approximately 150 TBq, Panoramic
.
Irradiator, Atomic Energy of Canada, Limited at Korea Atomic Energy Research
Ž . Ž .
Institute KAERI as previously reported by Kim et al. 1999 . The radiation dose was
8.3 Gy, which was LD80Ž30. for the mice. The irradiation was done for 2 h. As a control,
the sham-irradiation was carried out by placing the mice in the preparation room during the irradiation period. Groups of mice were killed by cervical dislocation before irradiation, and at 0, 3, 6 and 12 h after the end of the 2-h irradiation.
2.3. Histological preparation
After irradiation, mice of each group were kept in a 238C-controlled animal care
room at KAERI. Each group of mice was killed by cervical dislocation at 0, 3, 6 and 12 h after irradiation. The ovaries were collected and fixed to observe the changes in the architecture of primordial and primary follicles.
Ž .
Postfixation using 1% of osmium tetraoxide Sigma, MO was conducted for 2 h at
Ž .
48C after prefixation with 2.5% glutaraldehyder0.1 M phosphate buffer pH 7.3 .
Embedding of specimens after an alcoholic dehydration and displacement by propylene
w Ž .
oxide was carried out in epon mixture PolyrBed 812 resin Epon 812
:dodecenylsuc-Ž . Ž
cinic anhydride:nadic methyl anhydride:2,4,6-tri dimethylaminomethyl phenol
DMP-. x Ž .
30 s19.3:12.3:9.4:0.6 ml, Polysciences . Using ultramicrotome Leica , semithin
sec-tions were prepared by 1mm in thickness and stained with 1% toluidine blue O in 1%
borax solution. The largest cross-sections were used in this study. Observation of
Ž .
morphological changes was done under a light microscope Olympus BX50 .
2.4. Identification of oÕarian status
()
C.J.
Lee
et
al.
r
Animal
Reproduction
Science
59
2000
109
–
117
Table 1
Criteria for the identification of normal and atretic primordial follicles in the present experiment
Primordial follicle Primary follicle
Normal Atretic Normal Atretic
Granulosa cells Flattened or round, Irregular or amorphous, Round, no pyknotic, Pyknosis of one or
no pyknotic apoptotic some cells mitotic more cells
Germinal vesicle Round and clear Irregular, absent Round and clear Irregular, absent
Ooplasmic membrane Clear, regular Unclear, irregular Clear, regular Unclear, irregular
Ooplasm Even, clear Uneven, unclear, dark Even, clear Uneven, unclear, dark
( )
C.J. Lee et al.rAnimal Reproduction Science 59 2000 109–117 113
pyknotic granulosa cells, andror with apoptotic oocyte or without oocyte were classified
into atretic ones based on the criteria listed in Table 1. The criteria for classification of the primary follicles were also described in Table 1. Under a microscope, the number of normal and atretic follicles was counted and their ratio was calculated. Data were
expressed as mean"SEM and the statistical differences between the experimental
groups were considered when p value was smaller than 0.05.
3. Results
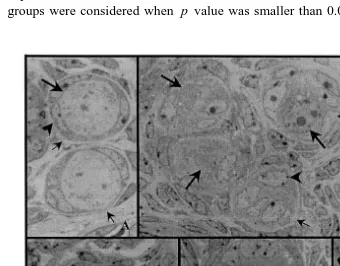
The ratio of normal to atretic follicles was significantly reduced at 6 h after irradiation. In the sham-irradiated control ovaries, the primordial follicles might be degenerated by the gradual, somewhat long-lasting, procedure. In the irradiated ones, however, it happened acutely. Since the degenerating patterns of ovarian primordial, follicles were diverse in the control mouse, various types of primordial follicles were observed. The normal primordial follicles with two or three flattened granulosa cells possessed round ooplasmic membrane, clear germinal vesicle, and one or more nucleoli as shown in Fig. 1. Mitochondria were randomly distributed throughout the ooplasm
ŽFig. 1 ..
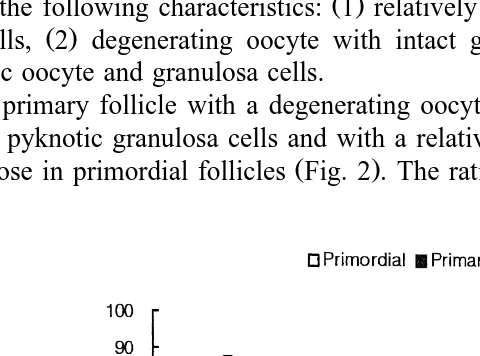
At 6 h after irradiation, the degenerated primordial follicles were considerably increased. Germinal vesicles disappeared, and lipid droplets increased. No more ooplas-mic membrane was observable. Granulosa cells became round in their shape, and apoptotic cells started to appear. Dark-stained germinal vesicles and apoptotic granulosa cells were also seen. The ratio of normal to atretic follicles in control group was 62.5. This ratio decreased with time after the irradiation. The ratio was 51.6, 49.0, 11.1 and
Ž .
7.1 at 0, 3, 6 and 12 h, respectively Fig. 2 . The degenerating primordial follicles had
Ž .
one of the following characteristics: 1 relatively normal oocyte with apoptotic
granu-Ž . Ž .
losa cells, 2 degenerating oocyte with intact granulosa cells, and 3 apoptotic or pyknotic oocyte and granulosa cells.
The primary follicle with a degenerating oocyte was observed. Follicles with apop-totic or pyknotic granulosa cells and with a relatively intact oocyte increased, similarly
Ž .
with those in primordial follicles Fig. 2 . The ratio of normal to atretic follicles in the
Fig. 2. Changes in the ratio of normal to atretic primordial and primary follicles in the control and irradiated mouse ovaries. The irradiation time in the present experiment was 2 h. Time 0 means the end of the irradiation. The number of mice in each experimental group was five. The ratio decreased steeply at 6 h after irradiation, and decreased further at 12 h after irradiation. Data were expressed as mean"SEM. a: significantly
Ž .
( )
C.J. Lee et al.rAnimal Reproduction Science 59 2000 109–117 115
control mouse ovary was 81.3. This ratio decreased down to 80.0, 75.0, 45.5 and 33.3 at 0, 3, 6 and 12 h after irradiation, respectively.
4. Discussion
Even though it has been widely accepted that radiation had a detrimental effect on the ovarian follicles in mammals, the reports concerning the changes of morphological characteristics of primordial and primary follicles induced by radiation were quite limited. In the present experiment, the changes of morphological characteristics of primordial and primary follicles caused by radiation were investigated.
Ž .
Erickson 1966 reported that primordial follicles were depleted as a consequence of either oocyte attrition or initiation of growth. The result of the present experiment
showed that g-radiation had effects of oocyte attrition, as well as the induction of
apoptosis of granulosa cells in the primordial and primary follicles. There were three kinds of degenerating patterns of primordial and primary follicles. First, follicles, which were recognized by the presence of the apoptotic oocytes had intact granulosa cells. Second, follicles had one or more apoptotic granulosa cells with relatively intact oocyte membrane. And, finally, follicles with both of the above characteristic changes were observed. Apoptotic granulosa cells and defected oocytes were easily recognized by the dark-stained nuclei. The most characteristic morphology of the degenerated primordial follicles was the lack of oocytes with two or more malformed granulosa cell-like cells. These malformed cells were placed in the basement membrane of the degenerated primordial follicles. The fact that the morphological changes were induced in a short time period after irradiation indicates that the primordial follicles have considerably greater susceptibility to ionizing radiation than primary follicles. In this regard, there is a great possibility that primary follicles, which are not degenerated, grow to secondary follicles. Also, it is thought that granulosa cells in a follicle are categorized into two groups. One type is radioresistant, and the other, radiosensitive. It can be thought that the radiation sensitivities between the resting and nongrowing primordial and the activated primary follicles were different.
Ž .
Ratts et al. 1995 reported that there were numerous primordial follicle-like struc-tures in bcl-2 deficient mouse ovaries. In the present study, the irradiated mouse ovary contained numerous oocyte-deficient primordial follicles at 12 h after irradiation. In comparison, apoptotic granulosa cells and oocytes of the primordial follicles were shown
until 6 h after irradiation. The degeneration of primordial follicles caused byg-radiation
showed an acute progression. Nearly all the primordial follicles were degenerated within 12 h after irradiation.
It was reported that there were no adequate morphological markers of degeneration in
Ž .
primordial follicles Edwards et al., 1977 . However, in the present study, the primordial
follicular degeneration was induced by g-irradiation and the morphological changes of
lies on the high radiation dose used in this study, at which 80% of the irradiated animals will die within 30 days.
The present study provides morphological clues for the microscopical identification of the degenerating primordial and primary follicles. The result indicates that the degeneration of primordial follicles goes much faster than that of primary follicles after irradiation and that the primordial follicles have higher sensitivity to the ionizing radiation than the primary follicles. In any case, the pattern of degeneration is one of the
Ž . Ž .
following: 1 apoptosis of one or more granulosa cells with relatively intact oocyte, 2
Ž .
apoptosis of oocyte with intact follicle cells, or 3 apoptotic degenerations of both cells.
5. Conclusion
The biological meaning of the present study is the identification of morphological
changes of the degenerating primordial and primary follicles in the g-irradiated mouse
ovary. The degeneration of primordial follicles is much faster than that of the primary follicles. The degenerations are mediated by apoptosis of oocytes and granulosa cells in both follicles. The present study provides morphological clues for the visual identifica-tion of the degenerating primordial and primary follicles. The result can give light on efforts to elucidate the follicular atresia mechanism.
Acknowledgements
This study has been carried out under the Nuclear R & D Program by the Ministry of
Ž .
Science and Technology MOST of Korea.
References
Ataya, K., Pydyn, E., Ramahi-Ataya, Orton, C.G., 1995. Is radiation-induced ovarian failure in rhesus monkeys preventable by luteining hormone-releasing hormone agonists? preliminary observations. J. Clin. Endocrinol. Metab. 80, 790–795.
Beaumont, H.M., Mandl, A.M., 1962. A quantitative and cytological study of oogonia and oocytes in the fetal and neonatal rat. Proc. R. Soc. London, Ser. B 155, 557–579.
Billig, H., Furuta, I., Hsueh, A.J., 1993. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 133, 2204–2212.
Braw, R.H., Tsafriri, A., 1980. Follicles explants from pentobarbitone-treated rats provide a model for atresia. J. Reprod. Fertil. 59, 259–265.
Ž .
Byskov, A.G., 1978. Follicular atresia. In: Jones, R. Ed. , The Vertebrate Ovary. Plenum, New York, pp. 533–562.
Chapman, R.M., 1982. Effect of cytotoxic therapy on sexuality and gonadal function. Semin. Oncol. 9, 84–94. Edwards, R.G., Fowler, R.E., Gore-Langton, R.E., Gosden, R.G., Jones, E.C., Readhead, C., Steptoe, P.C., 1977. Normal and abnormal follicular growth in mouse, rat, and human ovaries. J. Reprod. Fertil. 51, 237–263.
( )
C.J. Lee et al.rAnimal Reproduction Science 59 2000 109–117 117 Gougeon, A., 1996. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr.
Rev. 17, 121–155.
Hendry, J.H., West, C.M., 1997. Apoptosis and mitotic cell death: their relative contributions to normal-tissue and tumour radiation response. Int. J. Radiat. Biol. 71, 709–719.
Hirshfield, A.N., 1991. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 124, 43–101. Hirshfield, A.N., Midgley, A.R., 1978. Morphometric analysis of follicular development in the rat. Biol.
Reprod. 19, 606–611.
Hsueh, A.J., Billig, H., Tsafriri, A., 1994. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr. Rev. 15, 707–724.
Ž .
Hughes, F.M. Jr., Gorospe, W.C., 1991. Biochemical identification of apoptosis programmed cell death in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology 129, 2415–2422.
Hurwitz, A., Adashi, E.Y., 1993. Ovarian follicular atresia as an apoptotic process. In: Adashi, E.Y., Leung,
Ž .
P.C.K. Eds. , The Ovary. Raven Press, New York, pp. 473–485.
Kaipia, A., Hsueh, A.J., 1997. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 59, 349–363. Kerr, J.F., Winterford, C.M., Harmon, B.V., 1994. Apoptosis: its significance in cancer and cancer therapy.
Cancer 73, 2013–2026.
Kim, J.K., Lee, C.J., Song, K.W., Do, B.R., Yoon, Y.D., 1999.g-Radiation accelerates ovarian follicular atresia in immature mice. In Vivo 13, 21–24.
Meredith, S., Doolin, D., 1997. Timing of activation of primordial follicles in mature rats is only slightly affected by fetal stage at meiotic arrest. Biol. Reprod. 57, 63–67.