www.elsevier.comrlocateranireprosci
Effects of ammonia during different stages of
culture on development of in vitro produced bovine
embryos
D.S. Hammon
), S. Wang, G.R. Holyoak
Department of Animal, Dairy, and Veterinary Sciences, Utah State UniÕersity, 5600 Old Main Hill, Logan, UT 84332-5600, USA
Received 25 June 1999; received in revised form 15 December 1999; accepted 22 December 1999
Abstract
The effects of various concentrations of ammonia in the media during in vitro fertilization
ŽIVF , culture IVC , and throughout maturation IVM , IVF, and IVC were evaluated using a. Ž . Ž .
randomized complete block design. Ammonia was added to the media at various concentrations
Ž . Ž .
during IVF experiment 1 , during IVC experiment 2 , and throughout IVM, IVF, and IVC
Žexperiment 3 . In the first experiment, there was a significant P. Ž -0.05 increase in embryos.
developed to blastocyst, and to expanding and hatching blastocyst, in IVF media containing moderate concentrations of ammonia compared with that in the IVF control media. In the second
Ž .
experiment, ammonia in the IVC media increased P-0.05 the proportion of degenerate ova and
Ž .
decreased P-0.05 the proportion of ova that developed to blastocysts. In experiment 3,
Ž .
cleavage rates tended Ps0.06 to be greater for control groups than for treatment groups. The
Ž .
proportion of ova developing to morula was greater P-0.05 in media containing moderate concentrations of ammonia than that in the control groups. These results indicate that the effect of ammonia on development of preimplantation bovine embryos depends on the concentration of
ammonia and the stage of development when exposure to ammonia occurs. q2000 Elsevier
Science B.V. All rights reserved.
Keywords: Cattle-reproductive technology; Oocyte maturation; In vitro fertilization; Embryo culture;
Ammo-nia
)Corresponding author. Tel.:q1-435-797-1881; fax:q1-435-797-3959.
Ž .
E-mail address: [email protected] D.S. Hammon .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
Ammonia has recently received attention as a biochemical compound that may adversely affect embryo and fetal development. Based on in vitro and in vivo embryo experiments, ammonia generated from cellular metabolism and spontaneous breakdown of amino acids in culture media, or from dietary nitrogen has been shown to be toxic to
Ž . Ž .
murine Gardner and Lane, 1993 and ovine McEvoy et al., 1997; Sinclair et al., 1998 Ž .
gametes and embryos. Lane and Gardner 1994 reported that ammonia in the embryo culture media reduced implantation following transfer, retarded fetal growth, and induced fetal exencephaly in mouse embryos in a concentration-dependent manner.
Ž . Ž .
Furthermore, studies by McEvoy et al. 1997 and Sinclair et al. 1998 indicate that ammonia may affect fetal development and fetal growth rates.
Oocyte maturation, fertilization, and early embryo development are modulated by the biochemicals in their surrounding micro environments. In addition, the biochemical composition of bovine reproductive fluids varies during the normal estrous cycle ŽSchultz et al., 1971; Wise, 1987 and is influenced by diet Jordan et al., 1983; Elrod. Ž
. Ž .
and Butler, 1993 . Hammon et al. 2000 recently reported that follicular fluid ammonia concentrations are negatively associated with follicle size and are influenced by dietary
Ž .
protein intake Hammon et al., 1997 . Although follicle size and diet influence ammonia concentration in bovine reproductive fluids, it is not clear what effect ammonia concentrations may have on bovine oocyte maturation, fertilization, and subsequent embryo development.
The objectives of the present study were to compare the effects of addition of
Ž .
ammonia into the media during in vitro fertilization IVF, experiment 1 , during culture ŽIVC, experiment 2 , and throughout in vitro maturation IVM , IVF, and IVC experi-. Ž . Ž
.
ment 3 on the development of preimplantation bovine embryos.
2. Materials and methods
Ovaries were collected from a local abattoir. Oocytes were aspirated from small and
Ž . Ž .
medium antral follicles 3–8 mm in diameter as described by Hawk and Wall 1994 . Ž .
Cumulus oocyte complexes COCs with evenly granulated ooplasm and surrounded by
Ž .
several layers at least three to 4 four layers of compact cumulus cells were selected for Ž . use according to the oocyte grading system described by Hawk and Wall 1994 .
Ž .
Oocytes were washed three times with Hepes-TALP solution Parrish et al., 1988 and once with maturation medium. The maturation medium consisted of M-199 plus 10% Žvolrvol fetal bovine serum FBS , 25 mM Hepes, 2 mM glutamine, 0.25 mM sodium. Ž .
Ž .
pyruvate, 0.5 mgrml ovine FSH F-4520, Sigma, St. Louis, MO, USA , 5.0 mgrml
Ž . Ž .
ovine LH L-5269, Sigma 1.0 mgrml 17 b-estradiol E-2258, Sigma . In vitro
Ž . Ž .
maturation IVM of oocytes followed the procedure of Sirard and Blondin 1996 .
Ž w
Polystyrene plastic four-well culture petri dishes Nunclon , Nunc, Naperville, IL, .
Cryopreserved bovine semen was used for IVF. Live sperm were separated by Percoll
Ž .
gradients 45% and 90% on the upper and lower layers, respectively and centrifuged at 500=g for 30 min. Motile spermatozoa were added to the fertilization medium
Ž . 6
Fert-TALP, Parrish et al., 1988 to provide a final concentration of 2=10 rml. Capacitation of spermatozoa occurred in Fert-TALP containing 10 mg heparinrml and 0.6% fatty acid free bovine serum albumin. IVM-matured oocytes were added to
Ž Fert-TALP containing spermatozoa and cultured in plastic four-well petri dishes
Nunc-w.
lon under paraffin oil in a humidified 5% CO atmosphere at 392 8C for 17 h. Each well contained 500ml Fert-TALP and approximately 40–65 oocytes.
Cumulus and corona cells were removed from ova by vortexing in Hepes-TALP supplemented with 0.3% bovine serum albumin for 3 min. The presumptive zygotes
Ž w.
were then cultured in plastic four-well petri dishes Nunclon under paraffin oil at Ž
398C in a humidified 5% CO2 atmosphere. A modified CR2 medium Wang et al., .
1998 comprising 108.3 mM NaCl, 2.9 mM KCl, 24.9 mM NaHCO , 2.5 mM3
Ž .
hemicalcium lactate, 0.5 mM sodium pyruvate, BME amino acids B-6766, Sigma ,
Ž .
MEM nonessential amino acids M-7145, Sigma , 0.5 mM glycine, 0.5 mM alanine, 1.0 mM glutamine, 1.0 mM glucose, and antibiotics were used to culture embryos. Each well contained 500ml CR2 medium with approximately 40–60 oocytes. During culture,
Ž .
medium was changed every other day so that it was supplemented with 5% volrvol
Ž .
fetal bovine serum on day 1 day 0sIVF , 10% on day 3, 15% on day 5, and 20% on
Ž .
day 7 of culture Zhang et al., 1992 . The cleavage rate was determined 48 h after the exposure of the in vitro matured oocytes to spermatozoa during IVF and embryos were examined for development on days 6, 8, and 10 of culture using an inverted microscope at 100=.
3. Experimental design
3.1. Experiment 1
Effects of ammonia concentration during IVF were compared in terms of oocyte cleavage, ova degeneration rate, and morula and blastocyst production, using a random-ized complete block design. A total of 1789 bovine oocytes was used. Each of the six replicates consisted of oocytes from the same collection of abattoir ovaries. Six
Ž . Ž .
treatments Table 1 consisted of ammonia A-0171, Sigma added to the fertilization
Ž . Ž . Ž . Ž . Ž .
media at concentrations of in mM : 29 A1 , 88 A2 , 132 A3 , 176 A4 , and 356 ŽA5 . Treatments were selected to simulate normal and excessive physiologic ammonia.
Ž .
concentrations. Oocytes fertilized in media with no added ammonia A0 were used as IVM controls.
3.2. Experiment 2
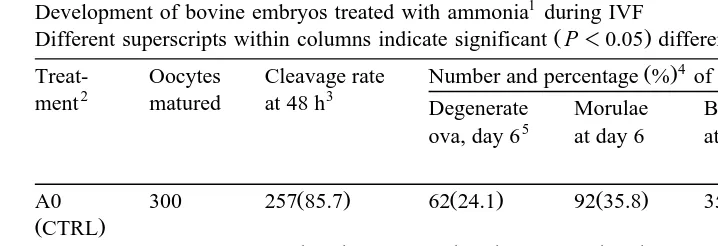
Table 1
Development of bovine embryos treated with ammonia1during IVF
Ž .
Different superscripts within columns indicate significant P-0.05 differences. 4 Ž .
Treat- Oocytes Cleavage rate Number and percentage % of cleaved ova that develop to
2 3
ment matured at 48 h Degenerate Morulae Blastocysts Expanded and 5
The IVF process was executed in medium containing various concentrations of NH CL.4 2
A0s0mM, A1s29mM, A2s88mM, A3s132mM, A4s176mM, and A5s356mM. 3
0 hsthe time when the in vitro matured oocytes were added to Fert-TALP containing spermatozoa. 4
The percentage of each treatment in this table represents seven replications. 5
Day 0sIVF.
replications consisted of oocytes from the same collection of abattoir ovaries. Treat-ments consisted of ammonia added to the culture media at the same concentrations as in experiment 1.
3.3. Experiment 3
Effects of ammonia concentration during IVM, IVF, and IVC were compared in terms of oocyte cleavage, ova degeneration rate, and morula and blastocyst production, using a randomized complete block design. A total of 2109 bovine oocytes was used. Each of seven replications consisted of oocytes from the same collection of abattoir ovaries. Treatments consisted of ammonia added to the maturation, fertilization, and culture media at the same concentrations as in experiment 1.
3.4. Statistical analysis
Data from experiments 1, 2, and 3 were angularly transformed and analyzed by the Ž .
use of a general linear model GLM ANOVA. Fisher’s least significant difference ŽLSD at 5% significant level. ŽP-0.05 was used to test the differences between.
Ž .
treatments. The NCSS Number Cruncher Statistical System Version 6.0 computer
Ž .
software package Hintze, 1997 was used for all statistical analyses.
4. Results
Exposure of ova to moderate concentrations of ammonia during IVF increased
Ž .
Table 2
Development of bovine embryos treated with ammonia1during IVC
Ž .
Different superscripts within columns indicate significant P-0.05 differences. 4 Ž .
Treat- Oocytes Cleavage rate Number and percentage % of cleaved ova that develop to
2 3
ment matured at 48 h Degenerate Morulae Blastocysts Expanded and 5
The IVC process was executed in medium containing various concentrations of NH CL.4 2
A0s0mM, A1s29mM, A2s88mM, A3s132mM, A4s176mM, and A5s356mM. 3
0 hsthe time when the in vitro matured oocytes were added to Fert-TALP containing spermatozoa. 4
The percentage of each treatment in this table represents seven replications. 5
Day 0sIVF.
moderate to high concentrations of ammonia during IVC increased the percentage of
Ž .
degenerate ova and decreased blastocyst development rate Table 2 . Exposure of ova to added ammonia throughout IVM, IVF, and IVC tended to decrease cleavage rate at 48 h
Ž .
and increased development rate to morulae at moderate concentrations Table 3 .
Table 3
Development of bovine embryos treated with ammonia1during IVM, IVF, and IVC
Ž .
Different superscripts within columns indicate significant P-0.05 differences. 4 Ž .
Treat- Oocytes Cleavage rate Number and percentage % of cleaved ova that develop to
2 3
ment matured at 48 h Degenerate Morulae Blastocysts Expanded and 5
The IVM, IVF, and IVC processes were executed in media containing various concentrations of NH CL.4 2
A0s0mM, A1s29mM, A2s88mM, A3s132mM, A4s176mM, and A5s356mM. 3
0 hsthe time when the in vitro matured oocytes were added to Fert-TALP containing spermatozoa. 4
The percentage of each treatment in this table represents seven replications. 5
Ž .
In experiment 1, there were no differences P)0.05 between treatments in cleav-ages rates, proportion of degenerate ova, or proportion of ova that developed to morula when oocytes were exposed to various concentrations of ammonia during IVF only. However, the addition of moderate concentrations of ammonia during IVF increased ŽP-0.05. embryo development to blastocyst and development to expanding and hatching blastocyst at days 8 and 10, respectively, compared with the control group ŽTable 1 ..
Ž .
In experiment 2, there were no differences P)0.05 between treatments in cleav-ages rates or proportion of ova that developed to morula when ova were exposed to various concentrations of ammonia during IVC only. However, analysis of embryo development at day 6 revealed that the addition of high concentration of ammonia in the
Ž . Ž .
culture medium A3,A5 increased P-0.05 the proportion of degenerate ova. Analy-sis of embryo development at day 8 revealed that the proportion of ova that developed to
Ž .
blastocysts was decreased P-0.05 with the addition of moderate to high
concentra-Ž .
tions of ammonia to the media A2–A5 compared with that in media containing low
Ž . Ž .
ammonia concentrations A1 and the control group A0 . Analysis of embryo develop-ment at day 10 revealed that the proportion of ova that developed to expanded and
Ž .
hatching blastocysts was decreased P-0.05 with the addition of ammonia to the
Ž . Ž .
medium A1–A5 compared with that in media containing no added ammonia Table 2 . In experiment 3, where ova were exposed to media containing ammonia throughout IVM, IVF, and IVC, analysis of embryo development at 48 h showed that cleavage rates
Ž .
tended Ps0.06 to be greater for control groups than for groups cultured in media
Ž .
containing ammonia A2–A5 . Analysis of embryo development at day 6 showed that
Ž .
the proportion of ova developing to morula was greater P-0.05 in media containing
Ž .
moderate concentrations of ammonia A2, A3 than that in the control groups. There
Ž .
were no significant P)0.05 differences between treatments in proportion of degener-ate ova or proportion of ova that developed to blastocysts and expanded and hatching
Ž .
blastocysts Table 3 .
5. Discussion
In recent years, research has suggested that ammonia is detrimental to murine ŽGardner and Lane, 1993; Lane and Gardner, 1994, 1995 and ovine embryo develop-.
Ž .
ment McEvoy et al., 1997 . Taken together, the results of the present experiments indicate that the effect of ammonia on development of preimplantation bovine embryos depends on the concentration of ammonia, the duration of exposure to ammonia, and the stage of development when exposure to ammonia occurs.
Ž .
findings of McEvoy et al. 1997 who found that ovine embryos exposed to elevated utero-oviductal ammonia concentrations up to day 3 postinsemination were more advanced in development and had increased metabolic activity when compared with embryos exposed to lower utero-oviductal ammonia concentrations. Furthermore, these
Ž .
results support the hypothesis of McEvoy et al. 1997 that a microenvironment containing elevated concentrations of ammonia may contribute to reprogramming of the embryo during the earliest stages of embryo development.
In contrast to the effects of ammonia during IVF on embryo development, continuous exposure of preimplantation bovine embryos to ammonia during IVC increases the portion of degenerate ova and has a detrimental effect on blastocyst and expanded and hatching blastocyst development in a concentration dependent manner. These results
Ž .
support those of Gardner and Lane 1993 who showed that moderate ammonia
Ž .
concentrations 75mM significantly reduced blastocyst cell numbers. Gardner and Lane Ž1993 suggested that ammonia may adversely affect the developing embryo by decreas-. ing the concentration of a-ketoglutarate by converting it to glutamate, thereby reducing the flux through the TCA cycle and depleting ATP in embryonic cells. Therefore, high ammonia in the culture media at the time of compaction and blastulation may reduce the availability of ATP for embryonic cells during a stage of development when energy demands by the embryo are high, resulting in increased degenerate ova and decreased blastocyst and expanded and hatching blastocyst development. Furthermore, the accumu-lation of hypertonic fluid within the blastocoel is in part due the action of the NaqrKq
Ž . Ž .
ATPase pump Bazer et al., 1993 . Martinelle and Haggstrom 1993 reported that active
¨
¨
intracellular transport of ammonium ions occurs via transport proteins like the NaqrKq-ATPase and the NaqKq2Cl–-cotransporter. Taken together, this suggests thatammonia may be concentrated in the fluid of the blastocoel during blastulation, which may contribute to decreased blastocyst development.
The results of the third experiment indicate that continuous exposure of bovine ova to moderate concentrations of ammonia throughout IVM, IVF, and IVC has a positive effect on morula development rate at day 6. However, continuous exposure of bovine ova to ammonia during the early stages of development may have a detrimental effect on cleavage rates at 48 h. The positive effect of moderate concentrations of ammonia on development to morula and the lack of increased development to blastocyst stage in media containing the same concentrations of ammonia may indicate that exposure of bovine embryos to ammonia beyond day 6 is detrimental to embryo development.
Ž .
Indeed, the results of McEvoy et al. 1997 indicated that day 3 in vivo produced embryos exposed to elevated utero-oviductal ammonia concentrations had increased metabolism and development, but lower pregnancy rates following transfer of embryos compared with embryos exposed to lower utero-oviductal ammonia concentrations. Collectively, these data suggest that embryos exposed to ammonia during early develop-ment may not be viable, despite having increased developdevelop-ment.
Acknowledgements
This research was supported by the Utah Agricultural Experiment Station, Utah State University, Logan, UT and approved as journal paper no. 7180. The authors thank E.A. Miller, Hyrum, UT, for the donation of ovaries.
References
Bazer, F.W., Geisert, R.D., Zavy, M.T., 1993. Fertilization, cleavage, and implantation. In: Hafez, E.S.E.
ŽEd. , Reproduction in Farm Animals. 6th edn. Lea and Febiger, Philadelphia, pp. 188–212..
Elrod, C.C., Butler, W.R., 1993. Reduction in fertility and alteration of uterine pH in heifers fed excess ruminally degradable protein. J. Anim. Sci. 71, 694–701.
Gardner, D.K., Lane, M., 1993. Amino acids and ammonia regulate mouse embryo development in culture. Biol. Reprod. 48, 377–385.
Hammon, D.S., Holyoak, G.R., Wang, S., 1997. Influence of rumen degraded protein on ammonia
concentra-Ž .
tions in the follicular fluids of early lactation dairy cows. Soc. Theriogenology Newsl. 20 6 , 5. Hammon, D.S., Wang, S., Holyoak, G.R., 2000. Ammonia concentration in bovine follicular fluid and its
effect during in vitro maturation on subsequent embryo development. Anim. Reprod. Sci. 58, 1–8. Hawk, H.W., Wall, R.J., 1994. Improved yields of bovine blastocysts from in vitro-produced oocytes.
Ž .
Theriogenology 41 8 , 1571–1583.
Ž .
Hintze, J.L., 1997. NCSS Number Cruncher Statistical System 6.0. NCSS, Kaysville, UT.
Jordan, E.R., Chapman, T.E., Holtan, D.W., Swanson, L.V., 1983. Relationship of dietary crude protein to composition of uterine secretions and blood in high-producing postpartum dairy cows. J. Dairy Sci. 66, 1854–1862.
Lane, M., Gardner, D.K., 1994. Increase in postimplantation development of cultured mouse embryos and induction of fetal retardation and exencephaly by ammonium ions. J. Reprod. Fertil. 102, 305–312. Lane, M., Gardner, D.K., 1995. Removal of embryo-toxic ammonium from the culture medium by in situ
enzymatic conversion to glutamate. J. Exp. Zool. 271, 356–363.
Martinelle, K., Haggstrom, L., 1993. Mechanisms of ammonia and ammonium ion toxicity in animal cells:¨ ¨
transport across cell membranes. J. Biotech. 30, 339–350.
McEvoy, T.G., Robinson, J.J., Aitken, R.P., Findley, P.A., Robertson, I.S., 1997. Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim. Reprod. Sci. 47, 71–90.
Parrish, J.J., Susko-Parrish, J.L., Winer, M.A., First, N.L., 1988. Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180.
Schultz, R.H., Fahning, M.L., Graham, E.F., 1971. A chemical study of the uterine fluid and blood serum of normal cows during the oestrous cycle. J. Reprod. Fert. 27, 355–367.
Sinclair, K.D., McEvoy, T.G., Carolan, C., Maxfield, E.K., Maltin, C.A., Young, L.E., Wilmut, I., Robinson, J.J., Broadbent, P.J., 1998. Conceptus growth and development following the culture of ovine embryos in
Ž .
media supplemented with sera. Theriogenology 49 1 , 218.
Sirard, M.A., Blondin, P., 1996. Oocyte maturation and IVF in cattle. Anim. Reprod. Sci. 42, 417–426. Wang, S., Holyoak, R.G., Liu, G., Bunch, J.J., Evans, R.C., Bunch, T.D., 1998. Effects of resazurin on bovine
fertilization and embryo development assessed by in vitro embryo production techniques. Anim. Reprod. Sci. 51, 205–213.
Wise, T., 1987. Biochemical analysis of bovine follicular fluid: albumin, total protein, lysosomal enzymes, ions, steroids and ascorbic acid content in relation to follicular size, rank, atresia classification and day of estrous cycle. J. Anim. Sci. 64, 1153–1169.