Brain Research 887 (2000) 187–190
www.elsevier.com / locate / bres
Short communication
Roles of urokinase type plasminogen activator in a brain stab wound
a ,
*
a a b bKazuo Kataoka
, Toshiharu Asai , Mamoru Taneda , Shigeru Ueshima , Osamu Matsuo ,
c c d
Ryotaro Kuroda , Atsufumi Kawabata , Peter Carmeliet
a
Department of Neurosurgery, Kinki University, School of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan
b
Second Department of Physiology, Kinki University, School of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan
c
Department of Pathophysiology and Therapeutics, Faculty of Pharmaceutical Sciences, Kinki University, Higashi-Osaka, Osaka 577-8502, Japan
d
Center of Trangene Technology and Gene Therapy, Vlaams Interuniversitair Instituut voor Biotechnologie, Campus Gasthuisberg, B-3000, Leuven,
Belgium
Accepted 26 September 2000
Abstract
Urokinase type plasminogen activator (uPA) may influence brain pathophysiology after injury. We studied disruption of the blood–brain barrier (BBB) and changes in the vasculature after a brain stab wound in uPA-deficient, uPA receptor-deficient, and PA inhibitor-1 (PAI-1) deficient mice. The extravasation of immunoglobulin was greater in PAI-1 deficient mice; less pronounced in uPA-deficient mice; similar to controls in uPA receptor-deficient mice. Vasculatures in the wound proliferated in PAI-1 deficient mice. Our study shows that uPA affects BBB disruption. PA enhances angiogenesis after brain injury. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Trauma
Keywords: Blood–brain barrier; Brain edema; Knock out mouse; Microglia; Plasminogen activator; Urokinase
Extracellular matrix (ECM) proteins provide structure, fibrinolysis. On the other hand, uPA binds to uPA receptors regulate the micro-environment in the brain, and maintain on the surface of various types of cells and contributes to the blood–brain barrier (BBB). For the healing process to cell-mediated fibrinolysis. However, the role of uPA in start after injury, degradation of the ECM by proteases is brain injuries remains unclear. In this study we investi-necessary [15]. Therefore, after sustaining an injury, brain gated the role of uPA, uPA receptor (uPAR), and PA tissues express increased levels of proteases to break down inhibitor-1 (PAI-1), a major inhibitor of uPA, in ex-the ECM [11]. Collagen type IV, one of ex-the ECM proteins, perimental brain stab wounds.
maintains the basement membrane of blood vessels in Mice deficient in uPA, uPAR, and PAI-1 were produced brain tissue. The breakdown of the basement membrane by by standard gene targeting in embryonic stem cells. Details proteases such as type IV collagenases may result in have been published elsewhere [3]. Ten-week-old mice of disruption of the BBB leading to brain edema, hemorrhage. both sexes were used. Age-matched male or female C57 / Plasmin is converted from plasminogen by plasminogen BL6 mice were the controls. The animals were anes-activators (PA). Because PA and plasmin contribute to the thetized with an intraperitoneal injection of 400 mg / kg conversion of inactive type IV collagenases [8], the chloral hydrate. The head of each mouse was fixed in a expression of PA may trigger a cascade of extracellular stereotaxic instrument. A small burr hole was then drilled proteolysis. There are two kinds of PA, tissue-type PA in the skull at a site on the bregma and 2.0 mm lateral to (tPA) and urokinase-type PA (uPA). Usually, tPA has a the sagittal suture on the right side. A stainless needle (0.5 high affinity for fibrin and contributes to intravascular mm diameter with a sharpened tip) was gently inserted through the hole into the cortex and the striatum to a depth of 2.5 mm from the cortical surface. A stereotaxic brain *Corresponding author. Tel.:181-723-660-221, ext. 3547; fax: 1
81-atlas of the mouse was consulted for precise positioning of 723-656-975.
E-mail address: [email protected] (K. Kataoka). the experimental brain stab wound [9]. At 3, 8 and 15 days
188 K. Kataoka et al. / Brain Research 887 (2000) 187 –190 Table 1
after placing the stab wound, the mice were deeply 2
Areas of immunoglobulin extravasation (mm ) anesthetized with sodium pentobarbital, and their brains
3 days 8 days 15 days
were fixed by transcardiac perfusion with heparinized
after lesioning physiological saline and ethanol acetic acid solution
(etha-nol:glacial acetic acid mixture 6:1, v / v). The brains were Control 3.561.4 (n56) 1.060.6 (n57) 0.460.7 (n57) PAI-1 deficient 4.260.8 (n55) 3.261.3* (n55) 0.760.8 (n56) removed and processed for paraffin embedding using
uPAR deficient 3.061.6 (n55) 0.660.9 (n55) 0.260.5 (n55) standard histological techniques. All brains were sectioned
uPA deficient 1.060.8* (n54) 1.061.0 (n55) 0.060.0 (n54) coronally at 6mm thickness. Sections including the site of
* P,0.05 (Mann–Whitney U-test) vs. controls. Values are mean6S.D. the stab wound were submitted for immunohistochemistry.
We used monoclonal rat anti-F4 / 80 as a marker of macrophage / microglia (Serotec, Oxford, UK), polyclonal
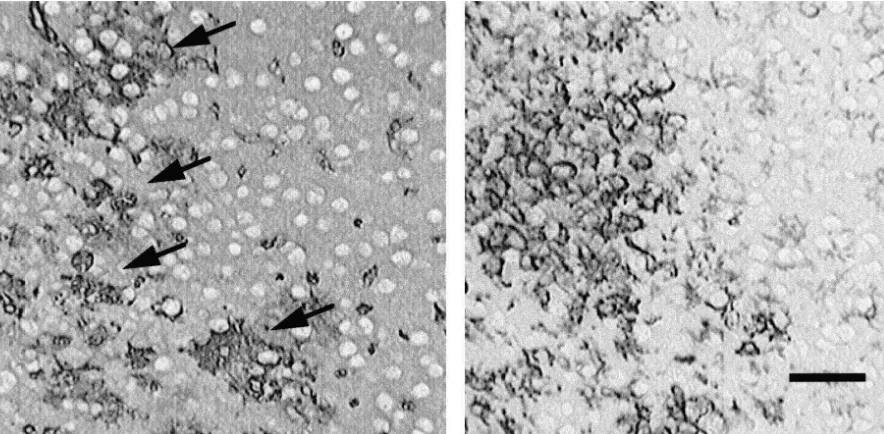
rabbit anti-mouse collagen type IV (LSL, Tokyo, Japan), 80% of PAI-1 deficient mice and in about 14% of the polyclonal rabbit anti-human von-Willebrand factor (Fac- control mice at 8 days post-injury (Fig. 1 and Table 2). tor VIII-related antigen) (DAKO, Carpinteria, CA, USA) Immunohistochemistry against von Willebrand factor and biotinylated polyclonal swine anti-mouse immuno- showed relatively large vascular structures. At 8 days, globulin (DAKO). For visualization of the primary anti- PAI-1-deficient mice had a significant increase in the
body, we used the LSAB 2 Kit (DAKO), alkaline density of von Willebrand factor-positive vascular struc-phosphatase–streptavidin complex. Then the brain sections tures surrounding the stab wound (Table 3). F4 / 80 posi-were incubated for 10 min at room temperature with tive cells were taken to be macrophages or microglia cells. non-specific binding-blocking solution (DAKO). Sections Ameboid-like strongly positive F4 / 80 cells appeared in the mounted on glass slides were incubated with these primary lesion at 3 days. These cells increased in number until 8 antibody solutions or a control solution containing non- days post-injury and decreased by day 15. The microglial specific rat or rabbit immunoglobulin for 60 min at room response and macrophage infiltration were not markedly temperature. After washing with buffer solution, all sec- different in all groups except for 80% of the PAI-1 tions except those treated with biotinylated polyclonal deficient mice and 14% of the control mice at 8 days swine anti-mouse immunoglobulin were incubated with post-injury. These animals exhibited irregular staining, biotinylated anti-rabbit or anti-rat immunoglobulin solution proliferation of collagen type IV, and the intense infiltra-for 30 min at room temperature. tion of clusters of F4 / 80-positive cells in the wound area
The specimens were then incubated with the alkaline (Fig. 1).
phosphatase–streptavidin complex solution for 10 min at Several inhibitors of PA are known such as PAI-1, room temperature. For visualization of alkaline phospha- PAI-2, and PAI-3. Among them, PAI-1 most effectively
tase, we used the New Fuchsin System (DAKO). Hema- inhibits the proteolytic activity of PA. Lipopolysaccharide toxylin was used for counterstaining. The areas stained induces an increase in the production of PAI-1 by macro-with anti-mouse immunoglobulin were quantitatively phages [6]. mRNAs encoding uPA and PAI-1 are elevated evaluated using a computer-assisted image-analyzing sys- in the mouse brain following seizure after intraperitoneal tem. The degree of von Willebrand factor immunoreactive excitotoxin injection [7]. Injury may provoke an increase tubular structures was scored; 0, nearly similar to the in the production of proteases in brain tissue [12]. We contralateral cortex (,10 immunoreactive tubular observed that the degree of immunoglobulin staining in
2
structures / 0.3 mm ); 1, moderately increased in number of uPA-deficient mice was reduced compared to control mice. von Willebrand Factor-positive tubular structures com- We also noted remarkable extravasation of immuno-pared to the contralateral cortex (10–25 immunoreactive globulin surrounding the stab wound in PAI-1 deficient
2
tubular structures / 0.3 mm ); 2, remarkably increased in mice. These findings suggest strongly that PA and tissue number of von Willebrand Factor-positive tubular struc- fibrinolysis are involved in the traumatic BBB disruption
2
tures (.25 immunoreactive tubular structures / 0.3 mm ). and that PAI-1 inhibits its spread. Similarly, excess matrix In control mice, marked extravasation of immuno- metalloproteinases lead to cerebral hemorrhage and brain globulin was observed at 3 days after lesioning; it declined edema, and their inhibitors block the development of at 8 and 15 days after lesioning. In uPA-deficient mice, the secondary brain injury [13].
K. Kataoka et al. / Brain Research 887 (2000) 187 –190 189
Fig. 1. Left, immunostaining against collagen type IV of PAI-1 deficient mouse 8 days after the stab wound. Note irregular and proliferating collagen type IV positive microvasculature (arrows). Right, immunostaining against F4 / 80 antigen of PAI-1 deficient mouse 8 days after the stab wound. Microglia cells and / or macrophages accumulated in the same area where the irregular and proliferating microvasculature is observed. Bar550mm.
Table 2 accelerated in the cell surface level via uPA receptor.
Number of mice with irregular collagen type IV (1) microvascular However, our study indicated that in uPA receptor-de-structures
ficient mice and in the controls, the same degree of BBB 3 days 8 days 15 days disruption was observed shortly after lesioning; it was
after lesioning
remarkably reduced by the 8th day post-injury. By com-Control 0 (n56) 1 (n57) 0 (n57) parison, in uPA-deficient mice, the degree of BBB disrup-PAI-1 deficient 0 (n55) 4* (n55) 2 (n56) tion was less pronounced at 3 days after the stab wound. uPAR deficient 0 (n55) 0 (n55) 0 (n55)
This shows that uPA enhances the pathophysiology of uPA deficient 0 (n54) 0 (n55) 0 (n54)
brain injury in the acute stage, and uPA works without * P,0.05 (Fisher’s exact probability test) vs. controls.
binding to its receptors in uPA receptor-deficient mice. Similarly, it is reported that uPA supplies sufficient proteolytic activities to clear fibrin deposits from the (SNR). This excess glutamate is closely related to the tissues in the absence of uPA receptor [2].
transneuronal degeneration of the SNR [14]. However, we In PAI-1-deficient mice 8 days after lesioning, the recently reported that tPA did not contribute to the number of von Willebrand factor-positive vasculatures development of transneuronal degeneration in the SNR significantly increased, and strong irregular staining following a striato-pallidal lesion [5]. Brain tissue tPA was against collagen type IV was observed. The irregular expressed only in young mice, its expression rapidly staining against collagen type IV may reflect the prolifer-declined as the animals grew older [1]. In damaged brain ation of microvasculature. If this structure reflects the tissues, the exact role of tPA remains unclear. Unlike tPA, breakdown of the microvasculature, it should be observed uPA was consistently detected in homogenized adult brain at 3 days after lesion placement. These findings suggest tissue [1]. uPA and fibrinolytic activities are regulated and that absence of PAI-1 facilitates angiogenesis. Microglia and / or macrophages accumulated in the irregular, prolifer-ating microvasculature at 8 days after brain injury. Previ-Table 3
Degree of von Willebrand Factor (1) vascular structures ous in vivo studies showed that microglia secreted uPA and expressed uPAR [10,18]. Roles of microglia on the
angio-3 days 8 days 15 days
after lesioning genesis after brain injury should be elucidated in relation to PA.
Control 1.060.0 (n56) 0.760.8 (n57) 0.360.5 (n57)
The present study clearly shows that uPA contributes to PAI-1 deficient 0.460.5 (n55) 1.860.4* (n55) 1.060.0* (n56)
uPAR deficient 0.860.4 (n55) 0.660.5 (n55) 0.260.4 (n55) the development of secondary brain damage due to the uPA deficient 0.360.6 (n54) 1.060.0 (n55) 0.560.6 (n54) breakdown of the microvascular ECM, and that uPA may
190 K. Kataoka et al. / Brain Research 887 (2000) 187 –190
[8] R. Mazzieri, L. Masiero, L. Zanetta, S. Monea, M. Onisto, S. PAI-1 inhibits traumatic brain edema. On the other hand,
Garbisa, P. Mignatti, Control of type IV collagenase activity by plasminogen activator may enhance the angiogenesis
components of the urokinase-plasmin system: a regulatory mecha-necessary for the healing process after brain injury. nism with cell-bound reactants, EMBO J. 16 (1997) 2319–2332.
[9] D.G. Montemurro, R.D. Dukelow, A Stereotaxic Atlas of the Diencephalon and Related Structures of the Mouse, Futura Publish-ing, Mount Kisco, 1972.
References [10] K. Nakajima, N. Tsuzaki, M. Shimojo, M. Hamanoue, S. Kohsaka, Microglia isolated from rat brain secrete a urokinase-type plas-minogen activator, Brain Res. 577 (1992) 285–292.
[1] M.Y. Ahn, Z.G. Zhang, L. Zhang, M. Chopp, The effect of age on
[11] G.A. Rosenberg, Matrix metalloproteinases in brain injury, J. expression of endogenous plasminogen activators after focal
cere-Neurotrauma 12 (1995) 833–842. bral ischemia in mice, Brain Res. 833 (1999) 112–116.
[12] G.A. Rosenberg, J.E. Dencoff, P.G. McGuire, L.A. Liotta, W.G. [2] T.H. Bugge, M.J. Flick, M.J. Danton, C.C. Daugherty, J. Romer, K.
Stetler-Stevenson, Injury-induced 92-kilodalton gelatinase and Dano, P. Carmeliet, D. Collen, J.L. Degen, Urokinase-type
plas-urokinase expression in rat brain, Lab. Invest. 71 (1994) 417–422. minogen activator is effective in fibrin clearance in the absence of
[13] G.A. Rosenberg, M. Navratil, Metalloproteinase inhibition blocks its receptor or tissue-type plasminogen activator, Proc. Natl. Acad.
edema in intracerebral hemorrhage in the rat, Neurology 48 (1997) Sci. USA 93 (1996) 5899–5904.
921–926. [3] P. Carmeliet, L. Schoonjans, L. Kieckens, B. Ream, J. Degen, R.
[14] M. Saji, A.D. Blau, B.T. Volpe, Prevention of transneuronal degene-Bronson, R. De Vos, J.J. van den Oord, D. Collen, R.C. Mulligan,
ration of neurons in the substantia nigra reticulata by ablation of the Physiological consequences of loss of plasminogen activator gene
subthalamic nucleus, Exp. Neurol. 141 (1996) 120–129. function in mice, Nature 368 (1994) 419–424.
[15] B.M. Schafer, K. Maier, U. Eickhoff, R.F. Todd, M.D. Kramer, [4] Z.L. Chen, S. Strickland, Neuronal death in the hippocampus is
Plasminogen activation in healing human wounds, Am. J. Pathol. promoted by plasmin-catalyzed degradation of laminin, Cell 91
144 (1994) 1269–1280. (1997) 917–925.
[16] S.E. Tsirka, A. Gualandris, D.G. Amaral, S. Strickland, Excitotoxin-[5] K. Kataoka, T. Asai, M. Taneda, S. Ueshima, O. Matsuo, R.
induced neuronal degeneration and seizure are mediated by tissue Kuroda, P. Carmeliet, D. Collen, Nigral degeneration following
plasminogen activator, Nature 377 (1995) 340–344. striato-pallidal lesion in tissue type plasminogen activator deficient
[17] Y.F. Wang, S.E. Tsirka, S. Strickland, P.E. Stieg, S.G. Soriano, S.A. mice, Neurosci. Lett. 266 (1999) 220–222.
Lipton, Tissue plasminogen activator (tPA) increases neuronal [6] S.K. Kung, H.K. Lau, Modulation of the plasminogen activation
damage after focal cerebral ischemia in wild-type and tPA-deficient system in murine macrophages, Biochim. Biophys. Acta 1176
mice, Nat. Med. 4 (1998) 228–231. (1993) 113–122.
[18] R.A. Washington, B. Becher, R. Balabanov, J. Antel, P. Dore-Duffy, [7] T. Masos, R. Miskin, mRNAs encoding urokinase-type plasminogen
Expression of the activation marker urokinase plasminogen-activator activator and plasminogen activator inhibitor-1 are elevated in the
receptor in cultured human central nervous system microglia, J. mouse brain following kainate-mediated excitation, Mol. Brain Res.