www.elsevier.nlrlocateraqua-online
Milt production by non-spermiating male Atlantic
ž
/
salmon Salmo salar after injection of a
commercial gonadotropin releasing hormone analog
preparation, 17
a
-hydroxyprogesterone or
17
a
,20
b
-dihydroxy-4-pregnen-3-one,
alone or in combination
Henry Robert King
a,), Graham Young
ba
Salmon Enterprises of Tasmania Pty. Ltd., PO Box 1, Wayatinah, Tasmania 7140, Australia
b
Department of Zoology, UniÕersity of Otago, PO Box 56, Dunedin, New Zealand
Received 22 November 1999; received in revised form 10 May 2000; accepted 11 July 2000
Abstract
Ž .
In three separate experiments, non-spermiating male Atlantic salmon Salmo salar were
Ž .
injected with a commercial salmon gonadotropin releasing hormone analog GnRHa preparation
Ž .
or vehicle propylene glycol . After 6 days, fish were also injected with 17a-hydroxyprogesterone
Ž17P , 17a. ,20b-dihydroxy-4-pregnen-3-one 17,20bP or vehicle. Injections were repeated on dayŽ .
7, and after a further 24 h, milt was collected by catheterisation. Blood samples were also taken and both blood and seminal plasma were retained for analysis of steroid levels. Only those fish treated with GnRHa in combination with 17P displayed a consistent increase in milt volume
Ž .
relative to sham-injected controls approximately 2–4 fold whereas treatment with GnRHa alone,
Ž .
resulted in a significant increase in milt volume relative to controls approximately two fold on only one occasion. Treatment with GnRHa in combination with 17P tended to result in lower
Ž .
blood plasma levels of 11-ketotestosterone 11KT and higher levels of 17,20bP relative to other treatments. 11KT levels in blood and seminal plasma tended to be negatively correlated with 17,20bP levels. Treatment with GnRHa in combination with 17,20bP resulted in similar blood plasma steroid profiles as combined GnRHar17P treatment. Together, these results lend further support for the role of gonadal progestogens in mediating the processes that regulate milt volume
)Corresponding author. Tel.:q61-3-6289-3280; fax:q61-3-6289-3290.
Ž .
E-mail address: harry [email protected] H.R. King .–
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
Ž .
in fish, but may also suggest additional roles for gonadotropin GtH and 17P, indicating that spermiation may not be simply 17,20bP dependent. q2001 Elsevier Science B.V. All rights reserved.
Keywords: Atlantic salmon; GnRHa; Spermiation; Plasma steroids
1. Introduction
Ž . Ž .
Gonadotropin releasing hormone analog GnRHa and gonadotropins GtHs have
Ž .
been used to stimulate milt production in a variety of teleosts see Pankhurst, 1994 . In salmonids, increased milt volume or accelerated production of milt has been observed in
Ž
response to GnRHa and GtH treatments e.g. Crim et al., 1983; Weil and Crim, 1983; .
Ueda et al., 1985 where the resulting increase in circulating levels of those compounds
Ž .
is believed to imitate endogenous changes in hormone levels Pankhurst, 1994 . In-creases in, or advancements of, milt production are unlikely to have occurred as a result of any direct effect of GtH but rather, they are elicited in response to the GtH-stimulated
Ž .
synthesis of gonadal steroids Pankhurst, 1994 . In salmonids, 17a,20b
-dihydroxy-4-Ž .
pregnen-3-one 17,20bP has been used to stimulate milt production in amago salmon,
Ž .
Oncorhynchus rhodurus Ueda et al., 1985 , and in brook trout, SalÕelinus fontinalis
ŽMarshall et al., 1989 , while GnRH and GtH-stimulated increases in 17,20. bP have been observed in other teleosts, thereby lending support to the hypothesis that milt
Ž .
production is stimulated by GtH-dependent steroid production Pankhurst, 1994 . Enhanced 17,20bP production often coincides with the decline in androgen produc-tion that occurs during the transiproduc-tion from spermatogenesis to spermiaproduc-tion in a number
Ž
of male teleosts Ueda et al., 1983, 1985; Baynes and Scott, 1985; Sakai et al., 1989; .
Miura et al., 1991; Swanson, 1991; Schulz et al., 1991, 1992 . Spermiation, the release
Ž .
of hydrated semen i.e. with lower spermatocrit than during intra-testicular storage from the genital pore, appears to be dependent on the production of 17,20bP. 17,20bP also acts to increase sperm duct pH, which in turn, increases sperm cyclic adenosine
Ž .
monophosphate cAMP, Morisawa et al., 1992, 1993; Miura et al., 1992 . cAMP is known to trigger the initiation of sperm motility in salmonids through the protein
Ž .
phosphorylation cascade Morisawa et al., 1992 .
Prior to commencement of the present study, we had attempted to control milt
Ž .
production in male Atlantic salmon Salmo salar in order to ensure that spermiation occurred in synchrony with the ovulation of naturally ripening females. These attempts generated anecdotal evidence which tended to suggest that use of a single injection of a commercial salmon GnRHa preparation resulted in a marked increase in milt production at the height of the spawning season, whereas no such increase in milt production was elicited prior to, or during, the very early stages of the recognised spawning season ŽKing, unpublished observations . The lack of effect of GnRHa injection on milt. production immediately prior to spawning could be due to several factors, including failure of GnRHa to elicit adequate GtH secretion andror lack of substrate for 17,20bP
Ž production. Kawauchi et al. have confirmed that two chemically distinct GtHs GtH-I
.
Žreviewed by Swanson, 1991 . GtH-I and GtH-II are produced by different gonadotropin.
Ž .
cell-types Nozaki et al., 1990a; Naito et al., 1993 . During vitellogenesis and spermato-genesis, large numbers of GtH-I cells were observed relative to low numbers of GtH-II cells and blood GtH-I levels increased and peaked prior to spawning while GtH-II levels remained around or below assay detection limits. In contrast, the numbers of GtH-II cells exceeded the number of GtH-I cells during spawning, a time when circulating
Ž
GtH-I declined and GtH-II displayed a rapid increase Suzuki et al., 1988; Nozaki et al., .
1990b; Swanson, 1991; Naito et al., 1993 . GtH-I and GtH-II are equipotent in
Ž .
stimulating 11-ketotestosterone 11KT and 17,20bP production during spermatogenesis
Ž .
although 11KT production exceeded 17,20bP production Planas et al., 1991 . Later, however, GtH-II was more potent than GtH-I in stimulating 17,20bP synthesis. Thus, early GnRHa treatment may have had a relatively greater impact on GtH-I cell activity and GtH-I production while the effectiveness of GnRHa injection at the height of the spawning season is consistent with increased impact on GtH-II production and 17,20bP synthesis at that stage of the reproductive cycle.
One of the primary factors limiting the early synthesis of 17,20bP may also be the Ž
lack in availability of its precursor 17a-hydroxyprogesterone 17P; Sakai et al., 1989; .
Schulz et al., 1991, 1992 due to the dominance of the androgen biosynthetic pathway. A number of authors have demonstrated increased 17,20bP production and increased
Ž
spermiation in response to exogenous 17P both in vitro and in vivo Ueda et al., 1983, .
1984, 1985; Sakai et al., 1989; Miura et al., 1991; Pankhurst, 1994 . Thus, the variation in the efficacy of GnRHa treatment as a stimulant of spermiation during different stages of reproductive development may also be related to 17P availability. However, the roles of GtH-I and GtH-II in the production of 17P, relative to androgens during
spermatogen-Ž .
esis, remain to be assessed Swanson, 1991 .
We hypothesised that providing substrate for 17,20bP synthesis would enhance the effectiveness of the GnRHa treatment. Three experiments were conducted immediately
Ž
prior to the commencement of the 1993 and 1994 spawning seasons late April in .
Tasmania in order to investigate the effect of exogenous 17,20bP, 17P and GnRHa, alone or in combination, on the spermiation response of mature non-spermiating male Atlantic salmon.
2. Materials and methods
2.1. Stock
Ž .
Experiments were conducted over two years 1993 and 1994 . Each year, maturing Ž 2qAtlantic salmon broodstock were transferred from Saltas Marine Operations Dover,
. Ž .
Tasmania to Saltas Freshwater Operations Wayatinah, Tasmania in March in order to facilitate final maturation in freshwater. Prior to transfer, fish were maintained in a single 65-m circumference polar circle-type cage at a maximum stocking density not greater than 10 kgPmy3. Fish were fed to appetite on a commercial steam-pelleted
Ž .
3 Ž .
40 m fibreglass raceways maximum 200 fish per raceway supplied with river water
Ž; y1.
15 lPs at ambient temperature. During the experiments, water temperatures ranged from 12.28C–7.38C and 14.08C–7.58C in 1993 and 1994, respectively, such that mean water temperature declined from 11.38C to 7.68C in 1993 and from 13.08C to 8.58C in 1994.
Ž
Prior to the commencement of each experiment, non-spermiating male fish mean wt.
. Ž
approx. 6.5 kg were selected from the broodstock and anaesthetised in benzocaine 25
. Ž .
mgrl . Fish were individually weighed "0.1 kg and marked by placing visible
Ž .
implant tags VI Tags, Northwest Marine Technology, Shaw Island, WA in the adipose
Ž .
eyelid according to the methods described by Bergman et al. 1992 and Kincaid and
Ž .
Calkins 1992 . Thereafter, individual fish were randomly assigned to treatment groups. As all treatment groups were housed in a single raceway, treatment groups were further
Ž .
distinguished by implantation of a colour-coded nylon dart tag Floy Tag, Seattle, WA at the base of the dorsal fin to facilitate future treatment and sampling.
2.2. Hormone solutions
Ž
A commercial salmon GnRHardomperidone preparation Ovaprim, Syndel Laborato-ries, Vancouver, BC; 20 mgD-Arg6, Pro9-Net sGnRHaq10 mg domperidone per ml
. Ž .
propylene glycol was used. Stock 10% wrv absolute ethanol 17,20bP and 17P ŽSteraloids, Wilton, NH solutions were prepared and diluted with propylene glycol to. achieve concentrations which gave a dose of 1 mgrkg body wt. at a final injection volume of 0.5 mlrkg body wt.
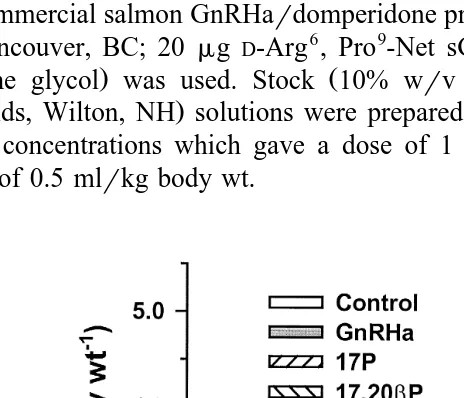
Ž y1. Ž .
Fig. 1. Milt volume mlPkg body wt. in Atlantic salmon injected with vehicle control , GnRHa, 17P,
Ž .
17,20bP or GnRHaq17HP. Values are meanqs.e. ns4 . Columns with the same superscript are not
Ž .
( ) 2.3. Experiment 1 19–27 April 1993
On day 0, 20 non-spermiating male Atlantic salmon were anaesthetised and given an
Ž . Ž .
intra-muscular i.m. injection of the commercial salmon GnRHa 10 mgrkg
prepara-Ž . Ž .
tion ns8 or vehicle alone ns12 . On both days 6 and 7, fish received a further i.m.
Ž .
injection of 17P, 17,20bP both 1 mgrkg or vehicle alone in a format resulting in the
Ž . Ž .
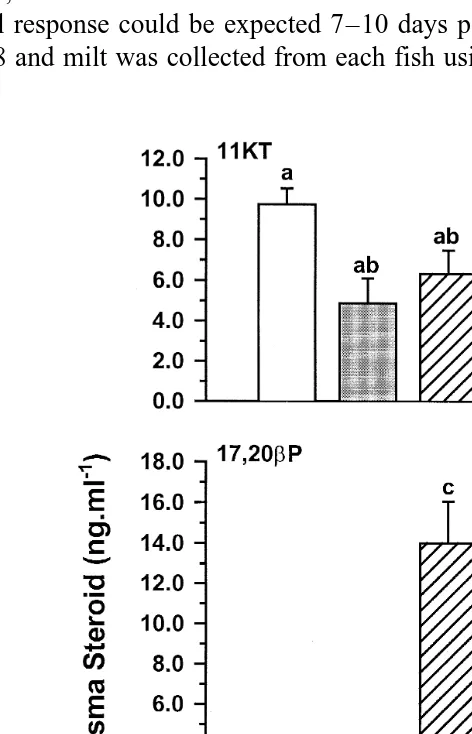
following five experimental groups ns4 : control vehicle only ; GnRHa; 17P; 17,20bP; GnRHaq17P. As the manufacturer of the GnRHa preparation indicated that a maximal response could be expected 7–10 days post treatment, fish were anaesthetised on day 8 and milt was collected from each fish using a catheterisation technique similar
Ž y1.
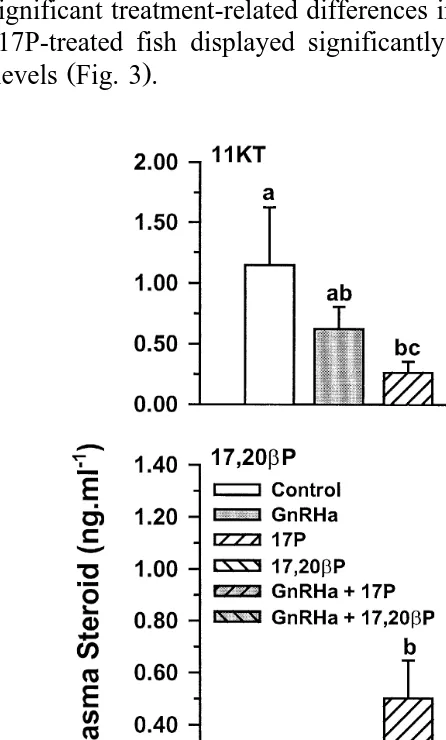
Fig. 2. Day 8 blood plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle
Žcontrol , GnRHa, 17P, 17,20. bP or GnRHaq17P. Values are meanqs.e. nŽ s4 . Columns with the same.
Ž .
Ž .
to that described by Erdahl 1982 . Milt volume was recorded and spermatocrit
Ž .
determined by the method of Miura et al. 1991 . One-millilitre blood samples from
Ž .
each fish were collected in heparinized lithium heparin syringes by puncture of the
Ž .
duct of Cuvier Lied et al., 1975 . After centrifugation, the resulting plasma was stored aty208C prior to analysis for steroid levels. Seminal plasma samples were also retained for steroid analysis.
( )
2.4. Experiment 2 5–13 April 1994
Thirty non-spermiating male Atlantic salmon were handled as before except that a blood sample was collected on day 0 before they received an i.m. injection of the
Ž y1.
Fig. 3. Day 8 seminal plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle
Žcontrol , GnRHa, 17P, 17,20. bP or GnRHaq17P. Values are meanqs.e. nŽ s4 . Columns with the same.
Ž .
Ž . Ž . Ž . commercial salmon GnRHa 10mgrkg preparation ns15 or vehicle alone ns15 .
Ž Again, on both days 6 and 7, they received an i.m. injection of 17P, 17,20bP both 1
. Ž . Ž
mgrkg or vehicle alone resulting in six experimental groups ns5 : control vehicle .
only ; GnRHa; 17P; 17,20bP; GnRHaq17P; GnRHaq17,20bP. On day 8, milt was collected from each fish and seminal and blood plasma samples were taken.
( )
2.5. Experiment 3 20–28 April 1994
Twenty non-spermiating male Atlantic salmon were subjected to the same treatment and sampling protocol as for experiment 2, except that only 17P was used.
2.6. Steroid radioimmunoassays
Levels of 11KT, 17,20bP and 17P were measured in blood and seminal plasma
Ž .
following diethyl-ether extraction using the methods of Ueda et al. 1984 and Young et
Ž .
al. 1986 .
2.7. Statistical analysis
Where appropriate, data were log-transformed in order to homogenise variances and group differences between means for milt volume and blood and seminal plasma steroid concentrations were assessed by one-way analysis of variance followed by a Tukey HSD test of pairwise multiple comparisons using the SYSTAT statistical analysis package
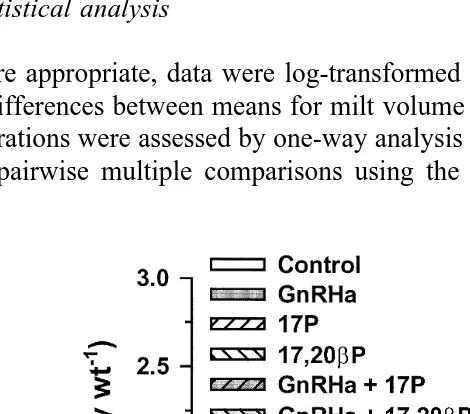
Ž y1. Ž .
Fig. 4. Milt volume mlPkg body wt. in Atlantic salmon injected with vehicle control , GnRHa, 17P,
Ž .
17,20bP, GnRHaq17P or GnRHaq17,20bP. Values are meanqs.e. ns4-5 . Columns with the same
Ž .
ŽSYSTAT, 1991 . The same package was used to derive linear correlation coefficients. between parameters.
3. Results
3.1. Experiment 1
Although all experimental treatments tended to be associated with higher mean milt
Ž .
volumes relative to controls Fig. 1 , only volumes from fish treated with GnRHa in
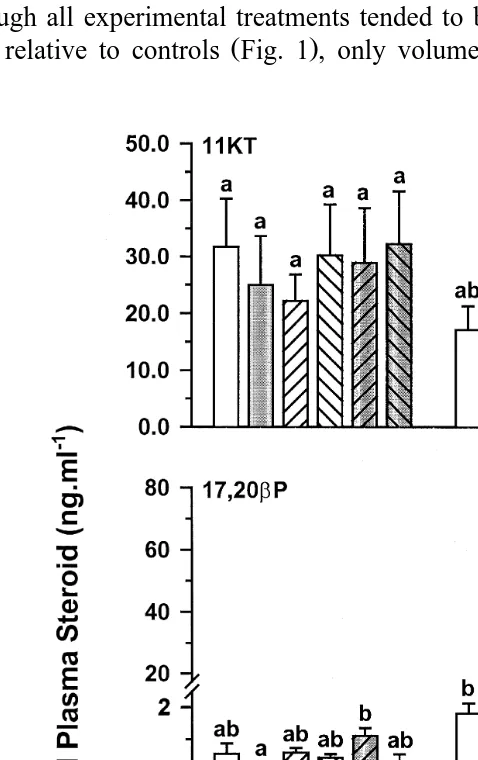
Ž y1. Ž .
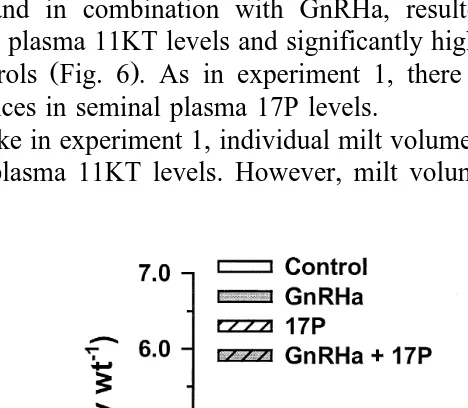
Fig. 5. Blood plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle control ,
Ž .
GnRHa, 17P, 17,20bP, GnRHaq17P or GnRHaq17,20bP. Values are meanqs.e. ns4–5 . Columns with
Ž .
Ž .
combination with 17P were significantly different from controls P-0.05 . Similarly, all experimental treatments tended to be associated with lower blood plasma 11KT
Ž .
levels relative to controls Fig. 2 although, again, the reduction was only significant ŽP-0.05 in those fish which received the combined GnRHa, 17P treatment. Treatment. with 17P or 17,20bP, alone or in combination with GnRHa, resulted in significantly
Ž . Ž .
higher P-0.05 blood plasma 17,20bP levels Fig. 2 , and 17P treatments were
Ž . Ž .
associated with significantly higher P-0.05 blood plasma 17P levels Fig. 2 . There were no significant treatment-related differences in seminal plasma 11KT or 17P, while
Ž .
only the 17P-treated fish displayed significantly P-0.05 elevated seminal plasma
Ž .
17,20bP levels Fig. 3 .
Ž y1. Ž .
Fig. 6. Seminal plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle control ,
Ž .
GnRHa, 17P, 17,20bP, GnRHaq17P or GnRHaq17,20bP. Values are meanqs.e. ns4–5 . Columns with
Ž .
Individual milt volumes were negatively correlated with blood plasma 11KT levels Žrs y0.605, P-0.01 which were in turn, negatively correlated with blood plasma.
Ž .
17,20bP levels rs y0.447, P-0.05 . Blood plasma 17,20bP and 17P levels were Ž
positively correlated with seminal plasma 17,20bP levels rs0.719 and 0.795,
respec-. Ž .
tively, P-0.01 data not shown .
3.2. Experiment 2
As in experiment 1, although all experimental treatments tended to be associated with
Ž .
higher mean milt volumes relative to controls Fig. 4 , only fish treated with GnRHa in
Ž .
combination with 17P had significantly higher P-0.05 milt volumes compared to controls. Mean blood plasma 11KT levels displayed a marked decline between days 0
Ž . Ž .
and 8 Fig. 5 . This decline was significant P-0.05 in fish receiving both 17P and 17,20bP alone and in combination with GnRHa. Blood plasma 17,20bP increased
Ž .
significantly between days 0 and 8 P-0.05 in fish receiving both 17P and 17,20bP
Ž .
either alone or in combination with GnRHa Fig. 5 . Only 17P treatment resulted in a
Ž . Ž .
significant P-0.05 increase in blood plasma 17P Fig. 5 . Progestogen treatment,
Ž .
alone and in combination with GnRHa, resulted in significantly lower P-0.05
Ž .
seminal plasma 11KT levels and significantly higher P-0.05 17,20bP levels relative
Ž .
to controls Fig. 6 . As in experiment 1, there were no significant treatment-related differences in seminal plasma 17P levels.
Unlike in experiment 1, individual milt volumes did not correlate strongly with day 8 blood plasma 11KT levels. However, milt volumes did display a negative correlation
Ž y1. Ž .
Fig. 7. Milt volume mlPkg body wt. in Atlantic salmon injected with vehicle control , GnRHa, 17P, or
Ž .
GnRHaq17P. Values are meanqs.e. ns4-5 . Columns with the same superscript are not significantly
Ž .
Ž .
with seminal plasma 11KT levels rs y0.429, P-0.05 which were in turn nega-Ž
tively correlated with day 8 blood and seminal plasma 17,20bP levels rs y0.665 and
. Ž .
y0.731, respectively, P-0.01 data not shown
3.3. Experiment 3
GnRHa treatments, alone or in combination with 17P, were associated with
signifi-Ž . Ž .
cantly higher P-0.01 mean milt volumes relative to controls Fig. 7 . However,
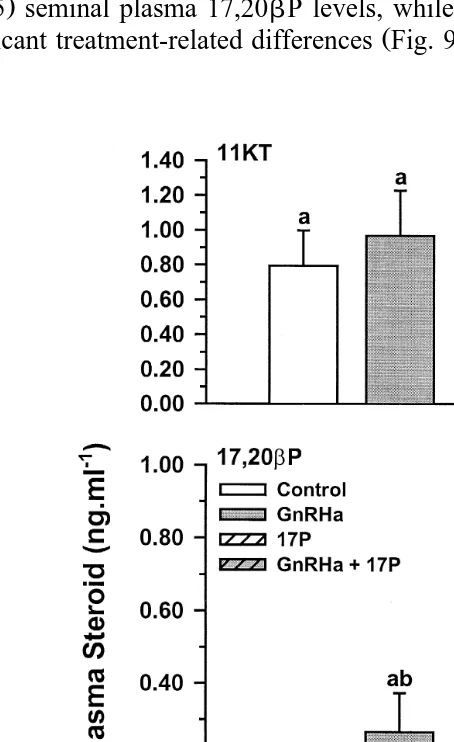
Ž y1. Ž .
Fig. 8. Blood plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle control ,
Ž .
GnRHa, 17P or GnRHaq17P. Values are meanqs.e. ns4–5 . Columns with the same superscript are not
Ž .
unlike previous experiments, treatment with 17P alone had no effect on milt volume ŽFig. 7 . As in experiment 2, mean blood plasma 11KT levels displayed a significant.
Ž . Ž .
decline P-0.05 between days 0 and 8 Fig. 8 , at which time, the levels displayed by
Ž .
both groups of 17P-treated fish were significantly lower P-0.05 than those of
Ž .
controls. These treatments also resulted in significant increases P-0.05 in 17,20bP
Ž .
and 17P between days 0 and 8 Fig. 8 . The same treatments also resulted in
Ž .
significantly lower P-0.05 seminal plasma 11KT levels and significantly higher ŽP-0.05 seminal plasma 17,20. bP levels, while seminal plasma 17P levels displayed
Ž .
no significant treatment-related differences Fig. 9 .
Ž y1. Ž .
Fig. 9. Seminal plasma 11KT, 17,20bP and 17P ngPml in Atlantic salmon injected with vehicle control ,
Ž .
GnRHa, 17P or GnRHaq17P. Values are meanqs.e. ns4–5 . Columns with the same superscript are not
Ž .
Neither the day 8 blood or the seminal plasma 11KT levels correlated with individual milt volumes. However, day 8 blood plasma 17,20bP was negatively correlated with
Ž
both blood and seminal plasma 11KT rs y0.528 andy0.618, respectively, P-0.05 .
and 0.01, respectively and was positively correlated with seminal plasma 17,20bP Žrs0.713, P-0.01 , which in turn displayed a positive correlation with milt volume. Žrs0.585, P-0.01. Ždata not shown ..
3.4. Milt quality
There were no significant treatment-related differences in spermatocrit during any of
Ž .
the experiments data not shown . Although other quantitative aspects of milt quality Že.g. sperm motility, fertilisation success were not examined, where possible, milt. collected during the experiments was used to fertilise routinely spawned ova with no discernible deleterious effects on egg performance. Furthermore, combined GnRHar17P treatment has been successfully adopted as a routine method for induced spawning of male Atlantic salmon at SALTAS Freshwater Operations, albeit with a simplified protocol involving only a single 17P injection on day 6.
4. Discussion
During the present study, treatment with GnRHa or progestational steroids alone tended to elicite only modest increases in milt production by male Atlantic salmon, whereas treatment with GnRHa in combination with 17P consistently resulted in significant increases in milt volume. Variances associated with relatively low sample numbers in each treatment group placed some constraints on statistical interpretation of the data, particularly regarding relative effectiveness of the two steroids. Nonetheless, the pattern of results was consistent in three separate experiments.
During the present study, a commercial GnRHa preparation containing the dopamine antagonist domperidone was employed. Since no GnRHa-only treatment was evaluated, it is difficult to comment upon the influence of domperidone. However, Linard et al. Ž1995. linked dopaminergic inhibition of GtH secretion in female rainbow trout ŽOncorhynchus mykiss with vitellogenesis rather than the maturational phase, and Peter.
Ž .
et al. 1993 , in a review of the literature, cited no examples of effects of dopamine antagonists on induced spawning of salmonids. The trend towards increased production of milt in previously non-spermiating Atlantic salmon treated with GnRHa alone tends to refute the anecdotal observations which prompted this study and is consistent with the results of other studies which indicate that teleost spermiation is partially GtH depen-dent. GnRHa has been observed to both advance spermiation and increase milt volume Že.g. Crim et al., 1983; Weil and Crim, 1983; Ngamvongchon et al., 1987; Chang et al.,
.
1991; Linhart et al., 1991; Pankhurst, 1994 and those studies where GtH was measured concurrently are particularly supportive of GnRHa-stimulated GtH increase as a
mediat-Ž .
ing factor in these processes e.g. Crim et al., 1983; Ngamvongchon et al., 1987 . The effects of gonadal progestogens seen in this study are indicative of their role in
Ž .
Ž . Ž . trout Marshall et al., 1989 , goldfish, Carassius auratus Ueda et al., 1985 , and
Ž .
snapper, Pagrus auratus Pankhurst, 1994 , 17P or 17,20bP treatment alone resulted in a consistent, though not statistically significant, trend towards increased milt volume.
GnRHa treatment tended not to elicit significant changes in blood or seminal plasma levels of 17,20bP. This is consistent with the observations of Ngamvongchon et al. Ž1987 and Pankhurst 1994 and, as the latter author suggests, may reflect the rapid. Ž . metabolism of that steroid in vivo rather than disproving the hypothesis that GnRHa and GtH promote spermiation by influencing gonadal steroid synthesis.
Ž .
Schulz et al. 1991, 1992 reported that, relative to 11KT, 17,20bP production in rainbow trout was highly GtH sensitive and attempted to explain this observation by suggesting that 20byHSD might have a low affinity for its substrate — 17P — and as a consequence, one action of GtH might be to stimulate production of high levels of 17P in order to facilitate increased 17,20bP synthesis. However, the levels of 11KT observed by these authors were an order of magnitude greater than those of 17,20bP. Hence, the apparent failure of GtH to stimulate further 11KT secretion relative to 17,20bP could simply be because 11KT production was already maximal. Alternatively, Sakai et al. Ž1989 suggested that poor 17,20. bP production may be due to the inability of the testes to accumulate adequate 17P. In the present study, 17P is unlikely to have been limiting in those fish which were 17P-treated and, indeed, those fish did tend to exhibit significantly elevated blood and seminal plasma 17,20bP along with increased milt volume. This observation lends some support to our initial hypothesis that the previous poor spermiation response to GnRHa might, to some extent, be related to the availability of 17P as a substrate for 20b-HSD.
The significant increase in milt production associated with the combination of GnRHa and 17P is interesting, particularly when the combination of GnRHa and 17,20bP — a treatment which resulted in largely similar blood and seminal plasma
Ž
steroid profiles — tended not to elicit a similar spermiation response although not . significant, mean milt volume was ;1.5 times higher in 17P-treated fish . While lending further support to our initial hypothesis on 17P availability, this result may also be suggestive of additional roles for GtH and 17P, tending to indicate that spermiation is not simply 17,20bP dependent. Although 20b-HSD activity is known to be controlled
Ž . Ž .
by GtH in female salmonid ovarian follicles Young et al., 1986 , Sakai et al. 1989 reported that in vitro production of 17,20bP by testicular fragments incubated with exogenous 17P was not further increased by the addition of GtH to the incubation medium, suggesting that control of 20b-HSD activity may differ between ovary and testes. It is also noteworthy that, during our first experiment, 17P treatment resulted in higher blood and seminal plasma levels of 17,20bP than direct 17,20bP treatment. During our second experiment, while the occurrence was not repeated, 17,20bP treatment did not result in very much higher levels of 17,20bP relative to 17P treatment
Ž .
as might otherwise have been expected. Pankhurst 1994 reported a similar phe-nomenon in snapper and indicated that it could simply reflect the relatively rapid clearance of 17,20bP from the plasma.
Ž
In contrast with observations in other species such as carp Ngamvongchon et al.,
. Ž .
androgens. Conversely, milt production, even in controls, was associated with reduced levels of the androgen 11KT to the extent that significant negative correlations between individual milt volumes and blood and seminal plasma 11KT levels were observed. The
Ž .
significance of these observations is difficult to comment upon. Pankhurst 1994 concluded that, in the absence of any convincing effect of exogenous androgens on milt production, the patterns reported by other authors were most likely co-incidental and insignificant. Nevertheless, our observations are consistent with the spawning-related
Ž
decline in androgens reported in amago salmon Ueda et al., 1983, 1985; Sakai et al.,
. Ž .
1989 , rainbow trout Ueda et al., 1983; Baynes and Scott, 1985 , Japanese eel ŽAnguilla japonica. ŽMiura et al., 1991 , and goldfish Ueda et al., 1983 . Furthermore,. Ž . our observations of significant negative correlations between 11KT and 17,20bP in both blood and seminal plasma are consistent with the negative in vitro effect on 11KT of
Ž .
17,20bP reported in carp by Barry et al. 1989, 1990 . More recently, Antonopoulou et
Ž .
al. 1997 have contrasted the inhibitory effect of 17,20bP on 11KT production in carp
Ž .
reported by Barry et al. 1989, 1990 with that observed in mature Atlantic salmon parr where physiological levels of 17,20bP were not associated with suppression of circulat-ing 11KT in vivo and where levels of 17,20bP exceeding 300 ngPmly1 of incubation medium were required to inhibit testicular production of 11KT in vitro. Importantly,
Ž .
while Antonopoulou et al. 1997 did not report whether they observed a decline in 11KT as maturation progressed, neither did they refute the spawning-related decline in 11KT reported elsewhere. Instead, these authors discussed the possibility that the spawning-related decline in 11KT observed in salmonids is a result of a GtH-II-induced increase in 20b-HSD activity which in turn leads to an increase in the proportion of 17P converted to 17,20bP, leaving less 17P available for conversion into androstenedione and ultimately leading to a decrease in androgen levels. However, it should be emphasised again that no evidence exists for control of testicular 20b-HSD activity by GtHs.
We were unable to measure GtH and therefore questions relating to the relative levels andror activities of GtH I and GtH II and the efficacy of GnRHa treatments could not be addressed. However, the results of this study lend further support to the argument that the processes regulating milt volume in teleost fish are mediated in part by gonadal progestogens and while there is a lack of clear evidence for the role of 17,20bP, from the perspective of commercial culture, our observations clearly demonstrate the potential of 17P as a low-cost means of augmenting GnRHa treatment protocols for the induction of spermiation in cultured Atlantic salmon.
Acknowledgements
References
Antonopoulou, E., Jakobsson, S., Mayer, I., Swanson, P., Borg, B., 1997. In vivo and in vitro effects of 17a,20b-dihydroxy-4-pregnen-3-one on testicular androgens in Atlantic salmon Salmo salar, mature parr. J. Exp. Zool. 277, 66–71.
Barry, T.P., Aida, K., Hanyu, I., 1989. Effects of 17a,20b-dihydroxy-4-pregnen-3-one on the in vitro production of 11-ketotestosterone by testicular fragments from the common carp, Cyprinus carpio. J. Exp. Zool. 251, 117–120.
Barry, T.P., Aida, K., Okumura, T., Hanyu, I., 1990. The shift from C-19 to C-21 steroid synthesis in spawning male common carp, Cyprinus carpio, is regulated by the inhibition of androgen production by progestogens produced by spermatozoa. Biol. Reprod. 43, 105–112.
Baynes, S.M., Scott, A.P., 1985. Seasonal variations in parameters of milt production and in plasma
Ž .
concentration of sex steroids of male rainbow trout Salmo gairdneri . Gen. Comp. Endocrinol. 57, 150–160.
Bergman, P.K., Haw, F., Blankenship, H.L., Buckley, R.M., 1992. Perspectives on design, use, and misuse of
Ž .
fish tags. Fisheries 17 4 , 20–25.
Chang, C.F., Yueh, W.S., Lee, M.F., 1991. Effects of LHRH-A and hCG on the steroid profiles of bisexual and mature male and female protandrous black porgy, Acanthopagrus schlegeli. Aquaculture 92, 83–92. Crim, L.W., Evans, D.M., Vickery, B.H., 1983. Manipulation of the seasonal reproductive cycle of the
Ž .
landlocked salmon Salmo salar with LHRH administration at various stages of gonadal development. Can. J. Fish. Aquat. Sci. 40, 61–67.
Erdahl, D.A., 1982. Potential application of cryobiology to aquaculture. Salmonid 6, 6–11.
Kincaid, H.L., Calkins, G.T., 1992. Retention of visible implant tags in lake trout and Atlantic salmon. Prog. Fish-Cult. 54, 163–170.
Lied, E., Gjerde, J., Braekkan, O.R., 1975. A simple and rapid technique for repeated blood sampling in
Ž .
rainbow trout Salmo gairdneri . J. Fish. Res. Bd. Can. 32, 699–701.
Linard, B., Anglade, I., Bennani, S., Salbert, G., Navas, J.M., Bailhache, T., Pakdel, F., Jego, P., Valotaire, Y.,´ Saligaut, C., Kah, O., 1995. Some insights into sex steroid feedback mechanisms in the trout. In: Goetz,
Ž .
F.W., Thomas, P. Eds. , Proceedings of the Fifth International Symposium on Reproductive Physiology of Fish, University of Texas, Austin, TX, USA. pp. 49–51.
Ž .
Linhart, O., Barth, T., Kouril, J., 1991. Stimulation of spermiation in tench Tinca tinca L. by analogues of
Ž .
GnRH and carp hypophysis. In: Scott, A.P., Sumpter, J.P., Kime, D.E., Rolfe, M.S. Eds. , Proceedings of the Fourth International Symposium on Reproductive Physiology of Fish, University of East Anglia, Norwich, UK, p. 282.
Marshall, W.S., Bryson, S.E., Idler, D.R., 1989. Gonadotropin stimulation of Kq secretion and Naq
Ž .
absorption by brook trout SalÕelinus fontinalis sperm duct epithelium. Gen. Comp. Endcrinol. 75,
118–128.
Miura, T., Yamauchi, K., Takahshi, H., Nagahama, Y., 1991. Involvement of steroid hormones in
go-Ž . Ž .
nadotropin-induced testicular maturation in male Japanese eel Anguilla japonica . Biomed. Res. 12 4 , 241–248.
Miura, T., Yamauchi, K., Takahshi, H., Nagahama, Y., 1992. The role of hormones in the acquisition of sperm motility in salmonid fish. J. Exp. Zool. 261, 359–363.
Morisawa, S., Ishida, K., Morisawa, M., 1992. The role of extracellular pH on the acquisition of sperm motility in chum salmon. Comp. Spermatol. 20 Years After, 1991 75, 503–505.
Morisawa, S., Ishida, K., Okuno, M., Morisawa, M., 1993. Role of pH and cyclic adenosine monophosphate in the acquisition of potential for sperm motility during migration from the sea to the river in chum salmon. Mol. Reprod. Dev. 34, 420–426.
Naito, N., Suzuki, K., Nozaki, M., Swanson, P., Kawauchi, H., Nakai, Y., 1993. Ultrastructural characteristics
Ž .
of two distinct gonadotropes GTH I- and GTH II-cells in the pituitary of rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 11, 241–246.
Ngamvongchon, S., Kok, L.Y., Takashima, F., 1987. Changes in endocrine profiles and spermiation response in carp after LHRH analogue injection. Bull. Jpn. Soc. Sci. Fish. 53, 229–234.
Salmonid pituitary gonadotrophs: I. Distinct cellular distribution of two gonadotropins, GTH I and GTH II. Gen. Comp. Endocrinol. 77, 348–357.
Nozaki, M., Naito, N., Swanson, P., Dickhoff, W.W., Nakai, Y., Oota, Y., Suzuki, K., Kawauchi, H., 1990b.
Ž
Salmonid pituitary gonadotrophs: II. Ontogeny of GTH I and GTH II cells in the rainbow trout Salmo
.
gairdneri irideus . Gen. Comp. Endocrinol. 77, 358–367.
Pankhurst, N.W., 1994. Effects of gonadotropin releasing hormone analogue, human chorionic gonadotropin
Ž .
and gonadal steroids on milt volume in the New Zealand snapper, Pagrus auratus Sparidae . Aquaculture 125, 185–197.
Peter, R.E., Lin, H.R., van der Kraak, G., Little, M., 1993. Releasing hormones, dopamine antagonists and
Ž .
induced spawning. In: Muir, J.F., Roberts, R.J. Eds. , Recent Adv. Aquacult., vol. IV. Blackwell Scientific Publications, Oxford, UK, pp. 25–30.
Planas, J.V., Swanson, P., Dickhoff, W.W., 1991. Regulation of testicular steroidogenesis by coho salmon
Ž .
gonadotropins, GTH I and GTH II, in vitro. In: Scott, A.P., Sumpter, J.P., Kime, D.E., Rolfe, M.S Eds. , Proceedings of the Fourth International Symposium on Reproductive Physiology of Fish, University of East Anglia, Norwich, UK, p. 103.
Ž
Sakai, N., Ueda, H., Suzuki, N., Nagahama, Y., 1989. Steroid production by Amago salmon Oncorhynchus
.
rhodurus testes at different developmental stages. Gen. Comp. Endocrinol. 75, 231–240.
Schulz, R.W., Andriske, M., Lembke, P.J., Blum, V., 1991. Sex steroids in rainbow trout: plasma levels and¨
Ž .
testicular secretion in vitro. In: Scott, A.P., Sumpter, J.P., Kime, D.E., Rolfe, M.S. Eds. , Proceedings of the Fourth International Symposium on Reproductive Physiology of Fish, University of East Anglia, Norwich, UK, pp. 83–85.
Schulz, R.W., Andriske, M., Lembke, P.J., Blum, V., 1992. Effect of salmon gonadotropic hormone on sex¨ steroids in male rainbow trout: plasma levels and testicular secretion in vitro. J. Comp. Physiol. B. 162, 224–230.
Suzuki, K., Kanamori, A., Nagahama, Y., Kawauchi, H., 1988. Development of salmon GTH I and GTH II radioimmunoassays. Gen. Comp. Endocrinol. 71, 459–467.
Swanson, P., 1991. Salmon gonadotropins: reconciling old and new ideas. In: Scott, A.P., Sumpter, J.P., Kime,
Ž .
D.E., Rolfe, M.S. Eds. , Proceedings of the Fourth International Symposium on Reproductive Physiology of Fish, University of East Anglia, Norwich, UK, pp. 2–7.
Ueda, H., Young, G., Crim, L.W., Kambegawa, A., Nagahama, Y., 1983. 17a,20b -Dihydroxy-4-pregnen-3-one: plasma levels during sexual maturation and in vitro production by the testes of Amago salmon
ŽOncorhynchus rhodurus and rainbow trout Salmo gairdneri . Gen. Comp. Endocrinol. 51, 106–112.. Ž .
Ueda, H., Kambegawa, A., Nagahama, Y., 1984. In vitro 11-ketotestosterone and 17a,20b -dihydroxy-4-preg-nen-3-one production by testicular fragments and isolated sperm of rainbow trout, Salmo gairdneri. J. Exp. Zool. 231, 435–439.
Ueda, H., Kambegawa, A., Nagahama, Y., 1985. Involvement of gonadotropin and steroid hormones in spermiation in the Amago salmon, Oncorhynchus rhodurus, and Goldfish, Carassius auratus. Gen. Comp. Endocrinol. 59, 24–30.
Weil, C., Crim, L.W., 1983. Administration of LHRH analogues in various ways: effect on the advancement of spermiation in prespawning landlocked salmon, Salmo salar. Aquaculture 35, 103–115.
Young, G., Adachi, S., Nagahama, Y., 1986. Role of ovarian thecal and granulosa layers in
gonadotropin-in-Ž .