USE OF BROOK CHAR
(
SALVELINUS F ONTINALIS
)
PHY SIOLOGICAL RESPONSES TO STRESS
AS A TEACHING EX ERCISE
Boucar Diouf, Pierre Rioux, Pierre U. Blier, and Denis Rajotte
De´pa rtem ent de Biologie Chim ie et des Sciences de la Sa nte´, Universite´ du Que´bec a` Rim ousk i, Rim ousk i, Que´bec, Ca na da G5L 3A1
F
ish hematological changes during osmotic and cold stress are used to introduce the physiological reactions of the animal to an acute stress. Brook char (Sa lvelinus fontina lis) were subjected to 1 h of stress before being anesthetized and having blood taken from their caudal vein. Glucose, hemoglobin, hematocrit, and osmolarity were determined in the blood samples. Analyses showed that glucose concentration tends to increase and hematocrit tends to decrease in stressed fish. Changes in hemoglobin concentration occurred only in cold-stressed fish. A rise in blood glucose concentration is the result of cortisol secreted by the hypothalamic-pituitary-adrenal axis. The glucose produced is used as an osmolyte or energy source to resist or combat the stress. In stressed fish, changes in hematocrit could be the result of the osmoconcentra-tion of the blood plasma, as shown by the increase in osmolarity for the same group. In cold-stressed fish, a decrease in hemoglobin concentration could be the result of hemodilution by body cell water.ADV PHYSIOL EDUC 23: 18–23, 2000.
Key words:fish; plasma glucose; hemoglobin; hematocrit; osmolarity
The undergraduate biology program of the Universite´ du Que´bec a` Rimouski has two major areas of interest, marine biology and wildlife management. We there-fore adapted from research a number of experiments, dealing mainly with animal physiology and biochemis-try, to emphasize our fields of interest (5, 13–16). The use of wild species of fish, birds, and mammals has allowed us to stimulate in students an additional interest in the teaching of physiology and biochemistry.
In this paper, we present an ecophysiological experi-ment that uses basic techniques encountered in ani-mal physiology laboratories. We use the response of brook char (Sa lvelinus fontina lis) to a short-term exposure to saltwater or cold water to demonstrate
acute physiological responses to an environmental stress. Temperature and salinity changes represent stresses that fish either have to cope with (short-term reaction) or acclimate to (long-term reaction). Ther-mal stress is a prime concern in ectotherm physiology when anadromous and catadromous fish are exposed to osmotic stress during migration.
of environmental changes: pH (8, 20), handling and transportation (17), osmotic stress (19), and high stocking-density stress (24). The typical primary physi-ological stress response is increased secretion of catecholamines (epinephrine) and corticosteroids (cor-tisol) by the hypothalamic-pituitary-adrenal axis (2). This response is generally independent of the type of stressor, but the quantitative aspect of the response depends on the intensity and duration of the stressor (9). The initial endocrine responses bring several metabolic adjustments including changes in plasma osmolyte, glucose, and lactate concentrations. Corti-sol, the most important corticosteroid in teleosts, contributes to the regulation of gluconeogenesis and/or glycogenesis. The high concentration of cortisol fa-vors glucose mobilization, providing a substrate for rapid threat response (23).
Hypersecretion of catecholamines and corticosteroids also induces hematological changes (9). Decreases in hematocrit and hemoglobin have been reported in fishes subjected to an acute cold stress (3). Elevated blood epinephrine increases gill permeability to water and sodium and leads to plasma electrolyte changes in a hyperosmotic or hyposmotic environment (2, 9).
The purpose of the laboratory is to initiate students to fish handling (anesthesia and blood sampling) and hematological analysis and to introduce them to the physiological responses of an animal to a stress (1, 4). We use cold temperature and saltwater to induce changes in blood glucose, hemoglobin, hematocrit, and plasma osmolarity of brook char. This species is an anadromous salmonid; however, the specimens used in this experiment, came from a freshwater pond. The first three variables are measured easily using simple and low-cost apparatuses that are found in almost any university biology department.
MATERIAL AND METHODS
Fish and blood sampling. Brook char (Sa lvelinus fontina lis) is widespread in Canada and the northern part of the United States. This species is anadromous but can spend its life in freshwater. The specimens used in this study (males and females) were purchased from a local freshwater fishing pond during the summer and brought to the laboratory with minimal stress. All fish were 2 yr old with a total mean length of
25 cm. The pond temperature was,10–12°C. In the
laboratory, they were immediately separated into three groups in 20-liter tanks: one control and two experimental tanks were subjected to an acute stress for 1 h. Osmotic stress was achieved by transferring fish to water of 10‰ salinity (one-third strength of sea water), and cold stress was achieved by transferring fish to a 4°C tank. The controls consisted of fish maintained at,12°C. In all three groups, water was
bubbled during experimentation for oxygen supply.
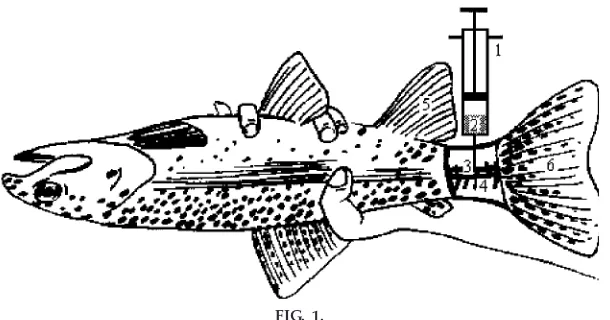
After the treatment, fish were anesthetized (5 min) in water containing 60 mg/l of 3-aminobenzoic acid ethyl ester (MS-222) and blood was sampled according to the technique described in Fig. 1. To perform blood sampling, the fish is held as shown in Fig. 1 and syringe introduced right at the back of the anal fin until the vertebral column is felt. When the syringe pierces the caudal vein lying below the vertebral column, blood will be withdrawn. Blood samples (1 ml) were placed in heparinized tubes. If such tubes are not available, regular glass tubes coated with heparin (100 units/ml) can also be used.
If one wants fish to survive after blood sampling, the anesthetizing bath temperature and pH must be ad-justed to that of pond water. For a 100- to 150-g fish, 1 ml of blood can be withdrawn without compromising survival if the specimen is returned to the pond water.
Glucose deter mination. Blood glucose was deter-mined with a blood glucose monitor, Accu-chek Easy (Boehringer Mannheim), that uses the glucose oxidase-ferrocyanide technique (11, 18). This monitor was chosen because of its low cost and high availability. However, any other glucose reflectometer may be used. The procedure for the use of this reflectometer is quite simple. A drop of blood is placed on the tagged area of the strip, which is immediately inserted into the reflectometer. Glucose concentration is dis-played in millimoles per liter.
determined using a microhematocrit reader (Interna-tional microcapillary reader, Interna(Interna-tional Equipment, Needham, MA).
The hemoglobin determination (in g/100 ml) of blood was performed using an Hb-meter (model 1010D, American Optical, Buffalo, NY). This simple and widely used apparatus compares the absorption of light by hemoglobin in a hemolyzed blood sample of known depth to that of a standardized glass plate (22). A drop of fish blood is introduced in the chamber surface and hemolyzed, and the results are then visually compared with a set of standards.
Osmolarity deter mination. We used a freezing point depression microosmometer (Advanced, model 3MO) for the measurement of the plasma osmolarity. The apparatus measures the freezing point of a 20-µl aqueous solution, which is a function of its osmolarity.
RESULTS
Changes in blood glucose, hemoglobin, hematocrit, and plasma osmolarity concentration for stressed and unstressed fish are presented in Fig. 2, A–D, respec-tively. Data shows that plasma glucose tends to increase in fish subjected to cold and osmotic stresses. Hemoglobin concentration fell only in cold-treated fish, and hematocrit tends to decreases in stressed fish. Stressed fish exhibit an increase in plasma osmo-larity. To calculate the amount of hemoglobin per red
blood cell (Hb/RBC), the total hemoglobin concentra-tion was divided by the hematocrit value. The results obtained for Hb/RBC were 0.1660.01 for the control group, 0.16 6 0.02 for the cold-stressed group, and 0.17 6 0.02 for the osmotically stressed group. No difference in Hb/RBC was observed between the control and either experimental group.
DISCUSSION
Response to cold str ess.As temperature decreases, the osmotic concentration and blood viscosity in-crease. Coping with low temperatures requires the ability to maintain homeostasis. Hematological changes are good indicators of this performance (3). The increase of blood glucose is the most studied response in this regard. It is the result of the activation of glycogenolysis that is under cortisol control (23). Hyperglycemia during cold exposure has been re-ported in many species (3, 19). In cold-treated fish, increased plasma glucose is used mainly as an osmo-lyte. Salmonid fish, including brook char, survive at low temperature by concentrating their plasma and cellular fluid electrolytes (Na1 and Cl2) and other
osmolytes like glucose (7). The plasma osmolyte concentration is negatively correlated with the freez-ing point. Decreases in hematocrit and hemoglobin could be the result of blood osmoconcentration, as shown by an increase in plasma osmolarity. This osmoconcentration leads to hemodilution by body FIG. 1.
cell water. The decrease in hemoglobin concentration in cold-treated fish could be also associated with a diminution of erythrocyte size (1). The increase in osmolyte concentration in the plasma leads to water loss from erythrocytes and thus to their shrinkage.
Hemolysis has been reported in common carp ( Cypri-nus ca rpio) during acute water temperature changes from 8°C to 4°C (3). The observed increase in plasma osmolarity following cold stress is less important than that in osmotically stressed fish (Fig. 2D).
FIG. 2.
Responses to osmotic str ess. Fish subjected to saltwater tend to increase their plasma glucose and osmolarity and lower their hematocrit (Fig. 2, A, B, andD). The transfer of salmonid species from freshwa-ter to saltwafreshwa-ter affects the wafreshwa-ter balance of the fish (10). A weight loss occurs in saltwater, whereas a weight gain occurs in freshwater. The adrenaline produced during stress increases gill water permeabil-ity in freshwater but decreases it in saltwater. These changes in gill function can be increased by 100% in a few minutes (9). Adrenaline has also been shown to affect the electrolyte balance in fish by causing a rise in gill sodium uptake in freshwater-acclimated rain-bow trout (Onchorhyncus m yk iss) (9). Combined effects of water loss, solute uptake, and an increase in plasma glucose contribute to the rise in plasma osmolarity. As a consequence of the increase in plasma osmolarity, water is withdrawn from erythro-cytes or body cells and hematocrit decreases. Similar results have been reported in other species (19). Osmoregulatory processes are of great importance for the survival of anadromous species such as Salmoni-dae or catadromous species such as eels. Moving from saltwater to freshwater and vice versa requires perfor-mative osmoregulatory processes. Decreases in hemo-globin in osmotically stressed fish are less important than those in cold-stressed fish.
Suggestions.This experiment is easy to perform and is a good tool to introduce the physiological responses an animal to acute stress in an undergraduate biology course. It allows one to observe the physiological plasticity that allows animals to cope with changing environmental factors. The hematological responses are the results of increases in plasma corticosteroids and catecholamines.
Brook char was selected for this experiment because of its large availability and its economic importance in aquaculture and sport fishing. However, big goldfish or other salmonid species can also be used.
It is possible to perform this experiment by measuring the plasma cortisol and adrenalin. One can also test the effect of intraperitoneal injections of cortisol on temporal changes of blood parameters (1).
We express appreciation to Ve´ronique Saint-Louis for assistance in producing Fig. 1, Ce´line Audet for the loan of the osmometer, and
Khaled Chatila and Richard Cloutier for helpful comments on the manuscript.
This work was supported by the De´partement de Biologie, de Chimie et de Sciences de la Sante´ of the Universite´ du Que´bec a` Rimouski as a special project.
Address for reprint requests and other correspondence: B. Diouf, De´partement de Biologie et des Sciences de la Sante´, Universite´ du Que´bec a` Rimouski, 300 Alle´e des Ursulines, Rimouski, Que´bec, Canada G5L 3A1.
Received 7 June 1999; accepted in final form 14 March 2000.
Refer ences
1. Bollar d BA, Pankhurst NW, and Wells RMG. Effects of artificially elevated plasma cortisol levels on blood parameters in the teleost fishPa grus a ura tus(Sparidae).Com p Biochem Physiol106A: 157–162, 1993.
2. Cech JJJR, Bartholow SD, Young PS, and Hopkins TE.
Striped bass exercise and handling stress in freshwater: physi-ological responses to recovery environment. Tra ns Am Fish Soc125: 308–320, 1996.
3. Chen G-R, Sun L-T, Lee Y-H, and Chang C-F.Characteristics of blood in common carp,Cyprinus ca rpio, exposed to low temperatures. J Appl Aqua c5: 21–31, 1995.
4. D’avino R, Caruso C, Car madella C, Schinna L, Rutigliano ME, Romano B, Carrator e M, Barra V, and Diprisco G.An overview of the molecular structure and functional properties of hemoglobins of the cold-adapted Antarctic teleost. In:Life Under Extrem e Conditions, edited by Diprisco G. Berlin, Germany: Springer-Verlag, 1991, p. 15–33.
5. Diouf B and Rioux P.Use of the rigor mortis process as a tool for better understanding of skeletal muscle physiology.Am Biol Tea ch, 61: 376–379, 1999.
6. Donnersber ger AB and Lesak AE.A La bora tory Textbook of Ana tom y a nd Physiology (6th ed.).Lexington, MA: Heath, 1996, p. 301–302.
7. Fletcher GL, Kao MH, and Dempson B. Lethal freezing temperatures of Arctic char and other salmonids in the pres-ence of ice.Aqua culture71: 369–378, 1988.
8. Giles MA, Majewski HS, and Hobden B.Osmoregulatory and hematological responses of rainbow trout (Sa lm o ga irdneri) to extended environmental acidification.Ca n J Fish Aqua t Sci41: 1686–1694, 1984.
9. Mazeaud MM, Mazeaud F, and Donaldson EM.Primary and secondary effects of stress in fish : some new data with a general review.Tra ns Am Fish Soc106: 201–212, 1977.
10. McCor mick SD and Naiman JR. Osmoregulation in the brook trout,Sa lvelinus fontina lis-2. Effects of size, age and photoperiod on seawater survival and ionic regulation.Com p Biochem Physiol79A: 17–28, 1984.
11. Mor J-R, and Guar naccia R. Assay of glucose using an electrochemical enzymatic sensor.Ana lyt Biochem 79: 319– 328, 1977.
13. Rioux P and Blier PU. Teaching biochemistry to wildlife management and oceanography students: kinetics of LDH isozymes in brook char.Biochem Educ23: 38–39, 1995. 14. Rioux P and Blier PU. Teaching biochemistry to wildlife
management and oceanography students: metabolism, enzy-matic activities and cold adaptation in goldfish.Biochem Educ
23: 170–172, 1995.
15. Rioux P and Blier PU.Energy metabolism and biochemical adaptation: a bird flight muscle model.Biochem Educ. In press. 16. Rioux P, Ouellet J-P, Dumont A, Blier PU, and Cr eˆte M.
Urine analyses to assess the nutritional status of deer in winter.
Biochem Educ26: 69–72, 1998.
17. Robertson L, Thomas P, and Ar nold CR.Plasma cortisol and secondary stress responses of cultured red drum (Scia enops ocella tus) to several transportation procedures.Aqua culture
68: 115–130, 1988.
18. Schla¨pfer P, Mindt W, and Racine P.Electrochemical measure-ment of glucose using various acceptors.Clin Chim Acta 57: 283–289, 1974.
19. Staur nes M, Rainuzzo JR, Sigholt T, and Jør gensen L.
Acclimation of Atlantic cod (Ga dus m orhua) to cold water:
stress response, osmoregulation, gill lipid composition and gill Na-K-ATPase activity.Com p Biochem Physiol109A: 413–421, 1994.
20. Tam WH, Birkett L, Makaran R, Payson PD, Whitney DK, and Yu CK-C.Modification of carbohydrate metabolism and liver vitellogenic function in brook trout (Sa lvelinus fontina -lis) by exposure to low pH.Ca n J Fish Aqua t Sci44: 630–635, 1987.
21. Tortora GJ and Adams HH.La bora tory Exercises in Hum a n Physiology. New York, NY: MacMillan, 1989, p. 241–248. 22. Tortora GJ and Tallitsch RB. La bo ra to ry Exercises in
Ana tom y a nd Physiology with Ca t Dissections (5th ed.). Upper Saddle River, NJ: Prentice Hall, 1996, 369–394. 23. Vijayan MM and Moon TW. Acute handling stress alters
hepatic glycogen metabolism in food-deprived rainbow trout (Oncorhynchus m yk iss).Ca n J Fish Aqua t Sci49: 2260–2266, 1992.
24. Vijayan MM, Ballantyne JS, and Leatherland JF. High stocking density alters the energy metabolism of brook char,