Germination, senescence and pathogenic attack in soybean
(
Glycine max
. L.): identification of the cytosolic aconitase
participating in the glyoxylate cycle
Joaquim Cots, Franc¸ois Widmer *
Laboratory of Plant Biology and Physiology,Biology Building,Uni6ersity of Lausanne,CH-1015Lausanne,Switzerland
Received 5 May 1999; received in revised form 30 June 1999; accepted 30 June 1999
Abstract
During our study of the glyoxylate cycle in soybean (Glycine max. L. var. Maple arrow), two mitochondrial and three cytosolic aconitase molecular species (EC 4.2.1.3) were detected, designated as M1, M2, C1, C2 and C3 isoforms, respectively, according to their intracellular locations and electrophoretic mobilities. Using the glyoxylate cycle marker enzymes isocitrate lyase (ICL, EC 4.1.3.1) and malate synthase (MS, EC 4.1.3.2), the activity of this pathway providing the essential link betweenb-oxidation and gluconeogenesis was confirmed during germination (cotyledons) and senescence (leaves). It was then established that, in both cases, the activity of the C1 aconitase isoform developed concomitantly with the transcription and translation levels of theicland
msgenes. This strongly suggests that C1 aconitase is constitutive of the glyoxylate cycle. In addition, the same isoform was found to be active during pathogenic attack as well (hypocotyls). It might be assumed that in such a case the glyoxylate cycle is reinitiated as a part of a carbon reallocation system feeding on the diseased tissue cellular components. © 1999 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Glycine max. L.; Aconitase; Germination; Glyoxylate cycle; Pathogenic attack; Senescence
www.elsevier.com/locate/plantsci
1. Introduction
The glyoxylate cycle was first described in Pseu
-domonasspp, where it was shown to allow growth of these organisms on acetate as the sole carbon source [1,2]. Numerous microorganisms were sub-sequently shown to operate this carbon-conserving pathway, including archaea [3], with the recent assumption that it may also allow growth on glycerol, lactate, propionate, oleate and n-alkanes [4,5]. In higher plants, this 5-enzyme short cut of the citric acid cycle was first detected occurring in the glyoxysomes of endosperm in castor bean seedlings during early postgerminative growth [6]. Many investigators subsequently reported finding glyoxylate cycle enzyme activities and transcripts
encoding these enzymes in the cotyledons of ger-minating oilseeds, where the cycle is involved in the mobilisation of lipid reserves (for a review, see Ref. [7]). The classical view considers that in this case the cycle achieves the net conversion of two molecules of acetyl-CoA produced by b-oxidation to one molecule of succinate. This C4 compound is converted to malate via the citric acid cycle in the mitochondria and then feeds into the gluco-neogenic pathway in the cytosol to reallocate the carbon source to the rest of the plant. Such en-zyme activities have also been detected during embryogenesis in plants [8 – 10], in pollen develop-ment [11], as well as in various animal tissues (for a review and critical comments, see Refs. [12] and [13], respectively). Reallocation of carbon through the glyoxylate cycle and gluconeogenesis similarly occurs in senescing plant tissues, derived from the catabolism of structural lipids (for a review, see Ref. [12]).
* Corresponding author. Tel.:+41-21-692-4190; fax:+ 41-21-692-4195.
E-mail address:[email protected] (F. Widmer)
The postulated exclusive glyoxysomal/ peroxiso-mal location of the glyoxylate cycle [14] has been challenged by the experimental evidence that gly-oxysomes are devoid of aconitase activity [15,16], and that a cytosolic aconitase form might partici-pate in the pathway [17]. A strict organellar com-partmentation has also been assumed to be dispensable in various unicellular green algae [18 – 20] and in Saccharomyces cere6isiae [21]. In
addi-tion, it has been postulated that the in vivo occurrence of a glyoxylate cycle stricto sensu might be questioned in plant glyoxysomes, in view of the possible role of the glyoxysomal malate dehydrogenase in the NAD+ regeneration system sustaining b-oxidation [12]. However, this has no bearing on the intrinsic involvement of the five considered enzymes in the reactional intricacies linking lipid catabolism to gluconeogenesis.
The assessment of aconitase isoforms in soybean now indicates that it possesses two mitochondrial and three cytosolic such molecular species. This distribution pattern is displayed under the physio-logical conditions of germination and senescence, where in both cases the same aconitase isoform appears to participate in the glyoxylate cycle. In addition, evidence is also presented that an identi-cal situation prevails under conditions of patho-genic attack.
2. Materials and methods
2.1. Biological material and growth conditions
Soybean seeds (Glycine max. L., cv. Maple ar-row) were obtained from Schweizer Samen AG, CH-3600 Thun, Switzerland. Seed imbibition oc-curred without prior surface sterilisation on soaked vermiculite. The details of germination and growth conditions are given in the Results and discussion section. Foliar senescence was induced by placing plants first grown for 20 days in phy-totron (16 h light/8 h darkness) in total darkness for up to 12 days.
2.2. Organelle preparation
The organelles were isolated essentially as de-scribed by Courtois-Verniquet and Douce [15], and all steps were carried out at 4°C. Cotyledons were minced and homogenised with razor blades
(Petri dish) in a medium containing 400 mM su-crose, 10 mM KCl, 2 mM DTT, 1 mM MgCl2, 1 mM citric acid, 1 mM PMSF, 5% BSA, 5% PVP 40 and 150 mM Tricine pH 7.8 (2 ml medium per g cotyledons). After filtration through 2 layers of cheesecloth, the homogenate was first cleared by centrifugation at 280 g for 5 min, and the or-ganelles were then sedimented by centrifugation at 10 800×g for 30 min. After recovery of the super-natant (cytosol), the pellet (organelles) was resus-pended in the homogenisation medium and layered onto a discontinuous sucrose gradient (33 – 60%). Fractions of 1.5 ml were collected after centrifugation for 3 h 30 at 24 000 rpm (Centrikon T-2080 centrifuge using a TST 28.38 swing-out rotor).
2.3. Crude extract preparation
Fresh plant material was frozen in liquid nitro-gen, pulverised in a mortar and homogenised at 4°C in the above described medium (2 ml per g material). After filtration through two layers of cheesecloth, the homogenates were cleared by cen-trifugation at 30 000×g for 30 min at 4°C. Pellets were discarded and the floating lipid layers were removed by filtration through cheesecloth.
2.4. Enzymatic assays
All assays were performed in triplicate at 25°C and monitored with Ultrospec III spectrophoto-meters (Pharmacia-LKB). Aconitase (EC 4.2.1.3) activity was measured by following the disappear-ance of cis-aconitate essentially as described by Fansler and Lowenstein [22]. Isocitrate lyase (ICL, EC 4.1.3.1), and malate synthase (MS, EC 4.1.3.2) were assayed according to Ruchti and Widmer [23] and Miernyk et al. [24], respectively. The methods of Gerhardt [25] and of Pontremoli [26] were used for 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) and fructose-1,6-bisphosphatase (EC 3.1.3.11), respectively.
2.5. Zymograms
Bevers-dorf [27]. The evolution of activity staining was carefully observed, and photographs were taken at the appropriate times before extensive diffusion into the gels.
2.6. Polyacrylamide gel electrophoresis and Western blotting
SDS-PAGE was performed as described by Laemmli [28], using a 4% stacking gel and a 12.5% resolving gel. Proteins were denatured prior to electrophoresis by boiling the samples for 2 min in an medium containing 1% 2-mercaptoethanol, 1% SDS, 0.125 mM bromophenol blue, 5% glycerol, 10 mM EDTA and 10 mM Tris – HCl pH 8.0. After electrophoresis, proteins were electrically transferred to Trans-Blot® nitrocellulose mem-branes (Bio-Rad mini trans-blot cell). ICL and MS detection was performed with specific anti-soybean ICL or MS antibodies.
2.7. Nucleotide probe labelling
DIG-labelled probes were synthesised by PCR from ICL and MS cDNA clones (Genbank access numbers L02329 and L01629). The reaction medium contained Taq buffer 1X (Pharmacia), 1 ng DNA, 1 mM primers, 1.5 mM MgCl2, 2 mM DIG-labelling mix (Boehringer Mannheim) and 1 U Taq polymerase (Pharmacia).
Primers 41+ (tgagaggttcaggctaacaaa) and 333− (ctcaaccttgtttgggacagt) were used for ICL probe synthesis. Primers 51+ (tatgatgtggcca-gagggagtg) and 675− (cttgggaagataaaagaaagg) were used for MS probe synthesis.
2.8. Northern blot analysis
Total RNA from mature and senescent leaves harvested at 0 to 9 days and at 12 days after initiation of dark treatment was extracted using a modification of the single-step method [29]. The isolated RNA was electrophoresed on a 1.2% agarose gel containing formaldehyde and then transferred (capillarity) to a Hybond-N+ nylon membrane (Amersham). The blots were hybridised at 50°C with each probe in a buffer containing 50% formamide, 7% SDS, 5 X SSC, 2% blocking reagent (Boehringer) and 0.1% lauroylsarcosine, washed twice in 2 X SSC/0.1% SDS, and then twice in 0.5 X SSC/0.1% SDS.
2.9. Miscellaneous
Sucrose concentration was determined by refractometry.
Polyclonal antibodies against soybean ICL and MS were obtained as described by Guex et al. [30]. The prestained SDS-PAGE standards (low range) from Bio-Rad were used for Western blot analysis.
The leaf chlorophyll content was determined as described by Borrell [31].
3. Results and discussion
3.1. Cotyledons at germination
Fig. 1. Localisation of mitochondria and glyoxysomes after sedimentation on a discontinuous sucrose gradient (5-day dark-germi-nated cotyledons). The various fractions were tested for the marker enzyme activities (cco and MS), as well as for aconitase activity. The cco and aconitase activity peaks coincide at a 38% sucrose concentration (1.17 density characterising mitochondria), whereas the main MS activity peak corresponds to a 55% sucrose concentration (1.25 density characterising glyoxysomes).
An extraglyoxysomal aconitase may thus partic-ipate in the glyoxylate cycle, and this possibility was further investigated on etiolated cotyledons using zymograms obtained from cytosolic, mito-chondrial and glyoxysomal fractions subjected to agarose electrophoresis. Five chromatic bands are observed on gels stained for aconitase activity (Fig. 2). This confirms the results of Cardy and Beversdorf [27] obtained on the same material using starch zymograms. However, these authors did not elucidate the intracellular locations of the five isoforms. The present results demonstrate that three isoforms are cytosolic (designated as C1, C2 and C3 isoforms according to their electrophoretic mobilities), whereas the two others are mitochon-drial (M1 and M2). The same zymogram also shows that glyoxysomes are indeed devoid of aconitase activity. At least one of the detected cytosolic aconitases might participate in the gly-oxylate cycle, as was proposed for etiolated pump-kin cotyledons [17,35].
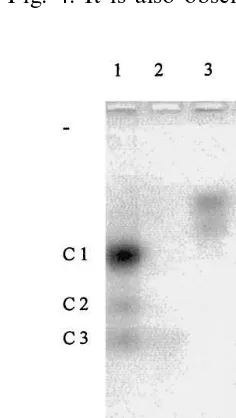
In order to verify this hypothesis, several crude extracts of etiolated cotyledons from plants grown for various periods were analyzed on agarose zy-mograms to identify which aconitase participates in the glyoxylate cycle. Glyoxysomal enzyme activ-ities increase in fatty tissues of germinating seedlings during the first days of growth, and thereafter decline as reserve lipids are consumed. Subsequent illumination initiates photosynthesis and accordingly accelerates the disappearance of glyoxysomal enzyme activities [36]. It can now be noted that the C1 isoform is not detected in the
cotyledons of freshly imbibed seeds (Fig. 3), whereas the C2 and C3 isoforms are present at low levels. The increase of C1 aconitase activity during growth in the dark then parallels that of MS and ICL activities (markers of the glyoxylate cycle) seen on Fig. 4. It is also observed that the
Fig. 3. Evolution of the aconitase isoform activities during germination (zymogram). Extracts from dark-germinated cotyledons were loaded on a 1.2% agarose gels, and migration occurred at 60 V for 5 h. Day 0 corresponds to the initiation of germination after seed imbibition.
ual cells or specific organs to the entire plant. These events can be considered as a recycling program, which is particularly active in the case of structural lipid dismantling.
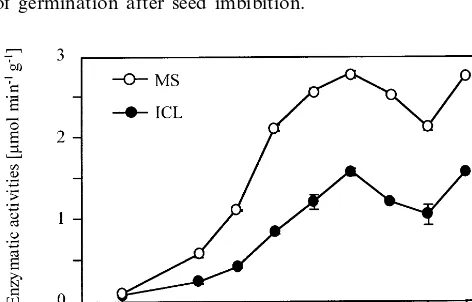
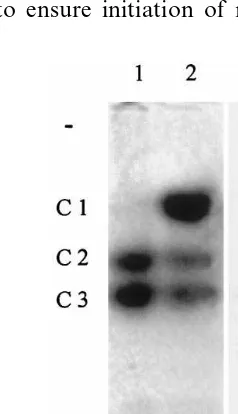
ICL and MS activities have already been de-tected in various senescing tissues of several higher plant species [37 – 40], and the evolution of aconi-tase activity in leaves subjected to dark-induced senescence has now been studied. Leaf extracts from soybean plants placed in total darkness for various periods up to 12 days were subjected to agarose electrophoresis for zymogram analysis. The M1, M2, C2 and C3 aconitase isoforms are detected in nonsenescing mature leaves (day 0 on Fig. 5). In contrast, the C1 isoform is not observed until 5 – 6 days of dark-induced senescence, and is thereafter detected at a moderate level until day 12. A parallel can be drawn between the evolution of this chromatic band and the increase of MS activity during dark-induced leaf senescence (Fig. 6), whereas surprisingly no ICL activity could be detected during this process. Such an apparent inactivity had already been observed during seed maturation for cotton [41] as well as for cucumber [42], even though the glyoxylate cycle is presumed to be active during embryogenesis. In addition, it has to be noted that the MS activity observed here during leaf senescence does not exceed 2.5 – 3% of that characterising cotyledons during germination (Fig. 4). The glyoxylate cycle nonetheless appears to be reinduced in senescing leaves, as this is supported by the results on Fig. 7, which indicate de novo synthesis for the MS protein under dark
Fig. 4. Evolution of ICL and MS activities in dark-germi-nated cotyledons. The enzymatic activities were monitored in crude extracts, and are expressed per g fresh weight.
Fig. 5. Separation and detection of the aconitase isoforms in leaves from dark-treated plants (zymogram). Plants were put in the dark after a 20-day growth in phytotron. The various extracts underwent a phase partition (Triton X-114) before application to a 1.2% agarose gel. Migration occurred at 60 V for 5 h.
tion of C1 aconitase activity in cotyledons of seedlings growing in the greenhouse is concomi-tant with the increases and declines of MS and ICL activities (data not shown). In contrast, aconitase C1 is not detected in mature leaves, roots, or hypocotyls. These results strongly suggest that a cytosolic aconitase, namely isoform C1 in soybean cotyledons, participates in the glyoxylate cycle during seedling growth.
3.2. Leaf senescence
individ-Fig. 6. MS activities detected in leaves from dark-treated plants. Plants were put in the dark after a 20-day growth in phytotron.
scription of both genes does not occur in mature leaves, but is initiated after 2 days of darkness and strongly persists until day 12 (Fig. 8). The above noted absence of ICL activity may thus be due to enzymatic inhibition, as was already observed in spinach, wheat and maize leaves [43], in banana [44], or in cauliflower cotyledons [45].
The chlorophyll content of dark-stressed leaves is still high until days 5 – 6 (data not shown), when the genes of the two glyoxylate cycle specific en-zymes are strongly expressed. This is at variance with natural senescence, where ICL and MS syn-thesis occurs late in the process, when the thy-lakoids are already disassembled and when chlorophyll is almost totally absent [46].
Fig. 9 shows the evolution of 3-hydroxyacyl-CoA dehydrogenase (marker enzyme of b -oxida-tion) and fructose-1,6-bisphosphatase activities in the material already described by Figs. 5 – 8. Hy-drolytic dephosphorylation of fructose-1,6-bispho-sphate to yield fructose-6-phosphate is a by-reaction of the Calvin-Benson cycle as well as an essential step of gluconeogenesis. Fructose-1,6-bisphosphatase activity is therefore significant in mature leaves, and subsequently falls off when senescence is initiated. This is confirmed by the results on Fig. 9, which furthermore indicate that this enzymatic activity is reinduced when all pho-tosynthetic processes are indeed nonexistent. This might be explained by an active gluconeogenetic pathway induced by the prolonged dark treatment (i.e. by the senescence process).
Mitochondrial b-oxidation is active in mature leaves, and provides short chain fatty acids for biosynthetic purposes (e.g. for chlorophyll synthe-sis), whereas its peroxisomal/glyoxysomal counter-part reaches completion and produces acetyl-CoA. The results pertaining to 3-hydroxyacyl-CoA-de-hydrogenase seen on Fig. 9 might be explained by an induction of glyoxysomal b-oxidation at the onset of senescence, as an essential downstream step in the process of structural lipid dismantling. A similar case has already been observed for senescing pumpkin petals [47].
The results (Figs. 5 – 8) pertaining to the C1 aconitase isoform as well as to the glyoxylate cycle specific enzymes ICL and MS strongly suggested the implication of the pathway in the senescence process. Bearing in mind that the glyoxylate cycle is intricately connected to b-oxidation and gluco-neogenesis, the hypothesis is now substantiated by
Fig. 7. Western blot analysis (MS protein) for leaves from dark-treated plants. Plants were put in the dark after a 20-day growth in phytotron. Similar results were obtained for the ICL protein.
Fig. 8. Analysis ofmsandicltranscripts abundance in leaves from dark-treated plants (northern blots). Plants were put in the dark after a 20-day growth in phytotron. Transfer on Hybond-N+ was carried out after electrophoresis of each sample (20 mg total RNA, denaturing conditions). DIG-la-belled probes from partial cDNA clones were used for tran-script detection.
tran-Fig. 9. 3-Hydroxyacyl-CoA dehydrogenase and fructose-1,6-bisphosphatase activities in leaves from dark-treated plants. Plants were put in the dark after a 20-day growth in phytotron.
the evolution of 3-hydroxyacyl-CoA-dehydroge-nase and fructose-1,6-bisphosphatase activities (Fig. 9).
3.3. Pathogenic attack
Gradual necroses of hypocotyls unexpectedly and infrequently occurred for various cultivation sets (germination and seedling growth in the dark up to 5 days), and were identified as due to bacterial developments (mostly Bacillus spp and
Erwinia herbicola). In such cases, root regenera-tion was usually observed above the necrotic spots and evidenced the vital necessity to maintain a functional root system through a ‘bypass’ of the diseased tissues. This survival strategy may imply a reallocation system feeding on the cellular com-ponents of the diseased necrotising tissues. In ad-dition, the dismantling of cellular elements in the affected tissues might hold up the pathogen advance.
Theiclandmsgenes are considered to belong to the class 6 of senescence enhanced genes [48,49], which is in agreement with the fact that they are instrumental to the carbon reallocation system provided by the glyoxylate cycle in senescing tis-sues (see Section 3.2). In this connection, it is worth reminding that the genes coding for the so called pathogenesis related proteins are also ex-pressed during senescence, but at late stages [49]. The activation of these genes coding for proteins involved in defense mechanisms might be induced
by pathogen attack on senescing tissues whose mechanical and chemical defenses are irretrievably weakened, and not be senescence stricto sensu.
The survival strategy now observed in the case of necrotising hypocotyls may somehow corre-spond to the opposite situation, where a pseudo-senescence could be established after a pathogenic attack to ensure initiation of reallocation
nisms. Indeed, Fig. 10 shows that such might be the case for the glyoxylate cycle, whose signature (the aconitase C1 isoform) is present in the by-passed hypocotyls. The possibility of an artifact (bacterial aconitase) was ruled out by the fact that the four aconitase forms detected in the contami-nating microorganisms were characterised by elec-trophoretic mobilities (zymograms) equivalent or higher than that of the soybean C3 aconitase.
4. Concluding remarks
The involvement of the C1 aconitase isoform in the glyoxylate cycle under conditions of germina-tion, senescence or pathogenic attack is strongly suggested by the present results. This leads to the conclusion that the same aconitase gene might be expressed under various physiological conditions. The isolation of the gene is now a prerequisite to the investigation of its regulation intricacies.
The present results show that the C2 and C3 cytosolic aconitase isoforms are essentially consti-tutive. This might evidence the necessity of the continuous conversion of cytosolic citrate, as al-ready suggested for sycamore cell suspensions [50]. The well documented occurrence of cytosolic NADP-isocitrate dehydrogenase [51,52] further substantiates this hypothesis, in agreement with the fact that citrate and isocitrate are at branch point positions in plant metabolism. The fate of the produced 2-oxoglutarate is nevertheless still unclear. Its complete oxidation in the TCA cycle is difficult to rationalise, since most of the extramito-chondrial citrate originates from the mitochon-drion [50]. Furthermore, the presumed role of 2-oxoglutarate in ammonia assimilation via the GS/GOGAT system can no longer be envisaged as a prevalent mechanism, since amino acid an-abolism occurs in plastids (with permanent recy-cling of 2-oxoglutarate), as recently reaffirmed [53]. On the other hand, it should not be over-looked that the consecutive actions of aconitase(s) and NADP-isocitrate dehydrogenase also results in the production of NADPH, which might be a significant alternative source for reductive biosyn-theses in the cytosol, thus complementing the pen-tose phosphate pathway [54].
A number of aspects of biomolecular flows via citrate and isocitrate remain unknown [54], but the fact that an additional cytosolic aconitase (C1
isoform) is specifically induced concomitantly with the (re)initiation of the glyoxylate cycle under various physiological conditions now suggests a differentiated integration of the three isoforms in the cytosolic substructure.
Acknowledgements
The authors thank Dr Olivier Cazelles (Swiss Federal Research Station for Plant Production, Changins, CH-1260 Nyon, Switzerland) for bacte-rial identification, and Armand Ducraux for his technical help.
References
[1] H.J. Saz, E.P. Hillary, The formation of glyoxylate and succinate from tricarboxylic acids by Pseudomonas aeruginosa, Biochem. J. 62 (1956) 563 – 569.
[2] H.L. Kornberg, N.B. Madsen, Synthesis of C4-dicar-boxylic acids from acetate by a glyoxylate bypass of the tricarboxylic acid cycle, Biochim. Biophys. Acta 24 (1957) 651 – 653.
[3] J.A. Serrano, M. Camacho, M.J. Bonete, Operation of glyoxylate cycle in halophilic archaea: presence of malate synthase and isocitrate lyase inHaloferax6olcanii, FEBS
Letters 434 (1998) 13 – 16.
[4] H. Atomi, K. Umemura, M. Ueda, A. Tanaka, Tran-scriptional regulation of peroxisomal glyoxylate cycle enzymes of ann-alkane assimilating yeast,Candida trop
-icalis, Ann. NY Acad. Sci. 804 (1996) 684 – 686. [5] K. Umemura, H. Atomi, M. Izuta, T. Kanai, S.
Takeshita, M. Ueda, A. Tanaka, Analysis of carbon source-regulated gene expression by the upstream region of theCandida tropicalismalate synthase gene inSaccha
-romyces cere6isiae, Biochim. Biophys. Acta 1350 (1997)
80 – 88.
[6] H.L. Kornberg, H. Beevers, The glyoxylate cycle as a stage in the conversion of fat to carbohydrate in castor beans, Biochim. Biophys. Acta 26 (1957) 531 – 537. [7] H. Kindl, Plant peroxisomes: recent studies on function
and biosynthesis, Cell Biochem. Funct. 10 (1992) 153 – 158.
[8] R.B. Turley, R.N. Trelease, Development and regulation of three glyoxysomal enzymes during cotton seed matu-ration and growth, Plant Mol. Biol. 14 (1990) 137 – 146. [9] J.Z. Zhang, M. Gomez-Pedrozo, C.S. Baden, J.J. Harada, Two classes of isocitrate lyase genes are ex-pressed during late embryogeny and postgermination in
[11] J.Z. Zhang, D.L. Laudencia-Chingcuanco, L. Comai, M. Li, J.J. Harada, Isocitrate lyase and malate synthase genes fromBrassica napusL. are active in pollen, Plant Physiol. (1994) 857 – 864.
[12] C.L. Escher, F. Widmer, Lipid mobilization and gluco-neogenesis in plants: do glyoxylate cycle enzyme activi-ties constitute a real cycle ? A hypothesis, Biol. Chem. 378 (1997) 803 – 813.
[13] R.P. Holmes, The absence of glyoxylate cycle enzymes in rodent and embryonic chick liver, Biochim. Biophys. Acta 1158 (1993) 47 – 51.
[14] R.W. Breidenbach, H. Beevers, Association of the gly-oxylate cycle enzymes in a novel subcellular particle from castor bean endosperm, Biochim. Biophys. Res. Com-mun. 27 (1967) 462 – 469.
[15] F. Courtois-Verniquet, R. Douce, Lack of aconitase in glyoxysomes and peroxisomes, Biochem. J. 294 (1993) 103 – 107.
[16] L. De Bellis, M. Hayashi, P.P. Biagi, I. Hara-Nishimura, A. Alpi, M. Nishimura, Immunological analysis of aconitase in pumpkin cotyledons: the absence of aconi-tase in glyoxysomes, Physiol. Plant. (1994) 757 – 762. [17] L. De Bellis, M. Hayashi, M. Nishimura, A. Alpi,
Sub-cellular and developmental changes in distribution of aconitase in pumpkin cotyledons, Planta 195 (1995) 464 – 468.
[18] H. Stabenau, Verteilung von Microbody-Enzymen aus
Chlamydomonas in Dichtegradienten, Planta 118 (1974) 35 – 42.
[19] H. Stabenau, Microbodies in different algae, in: W. Wiessner, D. Robinson, R. Starr (Eds.), Compartments in Algal Cells and their Interaction, Springer Verlag, Berlin, 1984, pp. 183 – 190.
[20] P. Zheng, Cloning and characterization of a Chlamy
-domonas NAD-dependent malate dehydrogenase gene and localization of its gene product by cd-tagging, Ph.D. Thesis, Department of Biological Sciences, University of Iowa, 1996, pp. 110 – 112.
[21] C. Brocard, F. Kragler, M. Binder, A. Hartig, Regula-tion of malate synthase activity, Ann. New York Acad. Sci. 804 (1996) 694 – 695.
[22] B. Fansler, J.M. Lowenstein, Aconitase from pig heart, Methods Enzymol. 13 (1969) 26 – 31.
[23] M. Ruchti, F. Widmer, Isocitrate lyase from germinating cotyledons: purification and characterization, J. Exp. Bot. 37 (1986) 1685 – 1690.
[24] J.A. Miernyk, R.N. Trelease, J.S. Choinski Jr., Malate synthase activity in cotton and other ungerminated oil-seeds: a survey, Plant Physiol. 63 (1979) 1068 – 1071. [25] B. Gerhardt, Peroxisomes and fatty acid degradation,
Methods Enzymol. 148 (1987) 516 – 525.
[26] S. Pontremoli, Fructose-1,6-diphosphatase, Methods En-zymol. 9 (1966) 625 – 631.
[27] B.J. Cardy, W.D. Beversdorf, Identification of soybean cultivars using isoenzyme electrophoresis, Seeds Sci. Technol. 12 (1984) 943 – 954.
[28] U.K. Laemmli, Cleavage of structural proteins during assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[29] C. Venugopalan, H.C. Kapoor, Single step isolation of plant RNA, Phytochemistry 46 (1997) 1303 – 1305.
[30] N. Guex, H. Henry, J. Flach, H. Richter, F. Widmer, Glyoxysomal malate dehydrogenase and malate synthase from soybean cotyledons (Glycine max. L.): enzyme asso-ciation, antibody production and cDNA cloning, Planta 197 (1995) 369 – 375.
[31] A. Borrell, L. Carbonell, R. Farra`s, P. Puig-Parellada, A.F. Tiburcio, Polyamines inhibit lipid peroxidation in senescing oat leaves, Physiol. Plant. 99 (1997) 385 – 390. [32] A.H.C. Huang, Comparative studies of glyoxysomes from various fatty seedlings, Plant Physiol. 55 (1975) 870 – 874.
[33] L. De Bellis, R. Tsugeki, A. Alpi, M. Nishimura, Purifi-cation and characterization of aconitase isoforms from etiolated pumpkin cotyledons, Physiol. Plant. 88 (1993) 485 – 492.
[34] F. Verniquet, J. Gaillard, M. Neuburger, R. Douce, Rapid inactivation of plant aconitase by hydrogen perox-ide, Biochem. J. 89 (1991) 693 – 698.
[35] M. Hayashi, L. De Bellis, A. Alpi, M. Nishimura, Cyto-solic aconitase participates in the glyoxylate cycle in etiolated pumpkin cotyledons, Plant Cell Physiol. 36 (1995) 669 – 680.
[36] A.H.C. Huang, R.N. Trelease, T.S. Moore, Plant Perox-isomes, Academic Press, New York, 1983.
[37] L. De Bellis, M. Nishimura, Development of enzymes of the glyoxylate cycle during senescence of pumpkin cotyledons, Plant Cell Physiol. 32 (1991) 555 – 561. [38] L. Pistelli, P. Perata, A. Alpi, Effect of leaf senescence on
glyoxylate cycle enzyme activities, Aust. J. Plant Physiol. 19 (1992) 723 – 729.
[39] I.A. Graham, C.J. Leaver, S.M. Smith, Induction of malate synthase gene expression in senescent and de-tached organs of cucumber, Plant Cell 4 (1992) 349 – 357.
[40] J.C. McLaughlin, S.M. Smith, Glyoxylate cycle enzyme synthesis during the irreversible phase of senescence of cucumber cotyledons, J. Plant Physiol. 146 (1995) 133 – 138.
[41] J.S. Choinski, R.N. Trelease, Control of enzyme activi-ties in cotton cotyledons during maturation and germina-tion: II. Glyoxysomal enzyme development in embryos, Plant Physiol. 62 (1978) 141 – 145.
[42] W. Ko¨ller, J. Frevert, H. Kindl, Incomplete glyoxysomes appearing at a late stage of maturation of cucumber seeds, Z. Naturforsch. 34 (1979) 1232 – 1236.
[43] H.R. Godavari, S.S. Badour, E.R. Waygood, Isocitrate lyase in green leaves, Plant Physiol. 51 (1973) 863 – 867. [44] K.K. Surendranathan, P.M. Nair, Purification and char-acterization of a natural inhibitor for isocitrate lyase, present in g-irradiated preclimateric banana, Plant Sci. Lett. 12 (1978) 169 – 175.
[45] W.F. Ettinger, J.J. Harada, Translational or posttransla-tional processes affect differentially the accumulation of isocitrate lyase and malate synthase proteins and enzyme active seedlings ofBrassica napus, Arch. Biochem. Bio-phys. 281 (1990) 139 – 143.
[46] J.C. Mc Laughlin, S.M. Smith, Metabolic regulation of glyoxylate-cycle enzyme synthesis in detached cucumber cotyledons and protoplasts, Planta 195 (1994) 22 – 28. [47] L. De Bellis, R. Tsugeki, M. Nishimura, Glyoxylate cycle
(Cucurbitasp.) during senescence, Plant Cell Physiol. 32 (1991) 1227 – 1235.
[48] C.M. Smart, Gene expression during leaf senescence, New Phytol. 126 (1994) 419 – 448.
[49] V. Buchanan-Wallaston, The molecular biology of leaf senescence, J. Exp. Bot. 48 (1997) 181 – 199.
[50] R. Brouquisse, M. Nishimura, J. Gaillard, R. Douce, Characterization of a cytosolic aconitase in higher plant cells, Plant Physiol. 84 (1987) 1402 – 1407.
[51] H.W. Heldt, Plant Biochemistry and Molecular Biology, Oxford University Press, Oxford, 1997, p. 261.
[52] R. Chen, Plant NADP-dependent isocitrate dehydroge-nases are predominantly localized in the cytosol, Planta 207 (1998) 280 – 285.
[53] K.A. Pyke, Plastid division and development, Plant Cell 11 (1999) 549 – 556.
[54] T.N. Popova, M.A.A. Pinheiro de Carvalho, Biochim. Biophys. Acta 1364 (1998) 307 – 325.