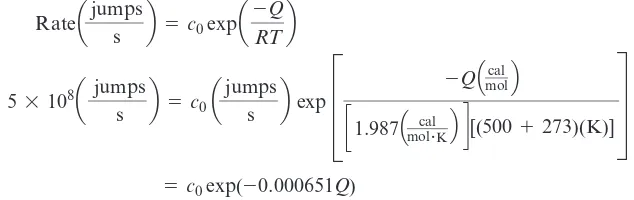Have You Ever Wondered?
• Aluminum oxidizes more easily than iron, so why do we say aluminum nor-mally does not “rust?”
• What kind of plastic is used to make carbonated beverage bottles?
• How are the surfaces of certain steels hardened?
• Why do we encase optical fibers using a polymeric coating?
• Who invented the first contact lens?
• How does a tungsten filament in a light bulb fail?
Atom and Ion Movements
in Materials
I
n Chapter 4, we learned that the atomic and ionic arrangements in materials are never perfect. We also saw that most materials are not pure elements; they are alloys or blends of different elements or compounds. Different types of atoms or ions typically “diffuse”, or move within the material, so the differences in their concen-tration are minimized. Diffusionrefers to an observable net flux of atoms or otherspecies. It depends upon the concentration gradient and temperature. Just as water flows from a mountain toward the sea to minimize its gravitational potential energy, atoms and ions have a tendency to move in a predictable fashion to eliminate concen-tration differences and produce homogeneous compositions that make the material thermodynamically more stable.
In this chapter, we will learn that temperature influences the kinetics of diffu-sion and that a concentration difference contributes to the overall net flux of diffusing species. The goal of this chapter is to examine the principles and applications of dif-fusion in materials. We’ll illustrate the concept of difdif-fusion through examples of sev-eral real-world technologies dependent on the diffusion of atoms, ions, or molecules. We will present an overview of Fick’s laws that describe the diffusion process quantitatively. We will also see how the relative openness of different crystal struc-tures and the size of atoms or ions, temperature, and concentration of diffusing species affect the rate at which diffusion occurs. We will discuss specific examples of how diffusion is used in the synthesis and processing of advanced materials as well as manufacturing of components using advanced materials.
5-1
Applications of Diffusion
Diffusion
Diffusion refers to the net flux of any species, such as ions, atoms, electrons, holes (Chapter 19), and molecules. The magnitude of this flux depends upon the concentration gradient and temperature. The process of diffusion is central to a large number of today’s important technologies. In materials processing technologies, control over the diffusion of atoms, ions, molecules, or other species is key. There are hundreds of applications and technologies that depend on either enhancing or limiting diffusion. The following are just a few examples.Carburization for Surface Hardening of Steels
Let’s say we want a surface, such as the teeth of a gear, to be hard; however, we do not want the entire gear to be hard. The carburization process can be used to increase surface hard-ness. In carburization, a source of carbon, such as a graphite powder or gaseous phase containing carbon, is diffused into steel components such as gears (Figure 5-1). In later chapters, you will learn how increased carbon concentration on the surface of the steel increases the steel’s hardness. Similar to the introduction of carbon, we can also use a process known as nitriding, in which nitrogen is introduced into the surface of a metallicmaterial. Diffusion also plays a central role in the control of the phase transformations
5 - 1 Applications of Diffusion 157
needed for the heat treatment of metals and alloys, the processing of ceramics, and the solidification and joining of materials (see Section 5-9).
Dopant Diffusion for Semiconductor Devices
The entire microelectronics industry, as we know it today, would not exist if we did not have a very good understanding of the diffusion of different atoms into silicon or other semiconductors. The creation of the p-n junction(Chapter 19) involves diffusing dopant atoms, such asphospho-rous (P), arsenic (As), antimony (Sb), boron (B), aluminum (Al), etc., into precisely defined regions of silicon wafers. Some of these regions are so small that they are best measured in nanometers. A p-njunction is a region of the semiconductor, one side of which is doped with n-type dopants (e.g., As in Si) and the other side is doped with p-type dopants (e.g., B in Si).
Conductive Ceramics
In general, polycrystalline ceramics tend to be good insulators of electricity. Diffusion of ions, electrons, or holes also plays an impor-tant role in the electrical conductivity of many conductive ceramics, such as partially orfully stabilized zirconia (ZrO2) or indium tin oxide (also commonly known as ITO). Lithium cobalt oxide (LiCoO2) is an example of an ionically conductive material that is used in lithium ion batteries. These ionically conductive materials are used for such prod-ucts as oxygen sensors in cars, touch-screen displays, fuel cells, and batteries. The ability of ions to diffuse and provide a pathway for electrical conduction plays an important role in enabling these applications.
Creation of Plastic Beverage Bottles
The occurrence of diffu-sion may not always be beneficial. In some applications, we may want to limit the occurrence of diffusion for certain species. For example, in the creation of certain plastic bottles, the dif-fusion of carbon dioxide (CO2) must be minimized. This is one of the major reasons why we use polyethylene terephthalate (PET) to make bottles which ensure that the carbonated beverages they contain will not lose their fizz for a reasonable period of time!Oxidation of Aluminum
You may have heard or know that aluminum does not “rust.” In reality, aluminum oxidizes (rusts) more easily than iron; however, the aluminum oxide (Al2O3) forms a very protective but thin coating on the aluminum’s sur-face preventing any further diffusion of oxygen and hindering further oxidation of the underlying aluminum. The oxide coating does not have a color and is thin and, hence, invisible. This is why we think aluminum does not rust.Coatings and Thin Films
Coatings and thin films are often used to limit the diffusion of water vapor, oxygen, or other chemicals.Thermal Barrier Coatings for Turbine Blades
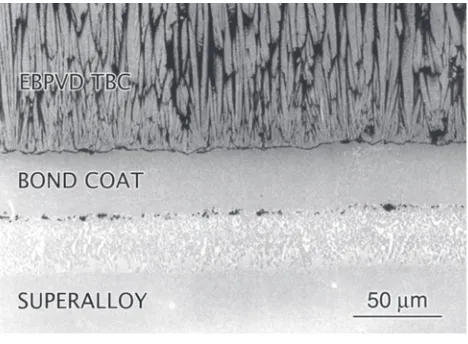
In an air-craft engine, some of the nickel superalloy-based turbine blades are coated with ceramic oxides such as yttria stabilized zirconia (YSZ). These ceramic coatings protect the under-lying alloy from high temperatures; hence, the name thermal barrier coatings (TBCs)Figure 5-2
A thermal barrier coating on a nickel-based superalloy. (Courtesy of Dr. F.S. Pettit and Dr. G.H. Meier, University of Pittsburgh.)
Figure 5-3
Illustration for diffusion of Ar He and Cu Ni (for Example 5-1).
> >
Optical Fibers and Microelectronic Components
Optical fibers made from silica (SiO2) are coated with polymeric materials to prevent diffusion of water molecules. This, in turn, improves the optical and mechanical properties of the fibers.Example 5-1
Diffusion of Ar He and Cu NiConsider a box containing an impermeable partition that divides the box into equal volumes (Figure 5-3). On one side, we have pure argon (Ar) gas; on the other side, we have pure helium (He) gas. Explain what will happen when the partition is opened? What will happen if we replace the Ar side with a Cu single crystal and the He side with a Ni single crystal?
SOLUTION
Before the partition is opened, one compartment has no argon and the other has no helium (i.e., there is a concentration gradient of Ar and He). When the partition is opened, Ar atoms will diffuse toward the He side, and vice versa. This diffusion will continue until the entire box has a uniform concentration of both gases. There may be some density gradient driven convective flows as well. If we took random samples of different regions in this box after a few hours, we would find a statisti-cally uniform concentration of Ar and He. Owing to their thermal energy, the Ar and He atoms will continue to move around in the box; however, there will be no concentration gradients.
If we open the hypothetical partition between the Ni and Cu single crys-tals at room temperature, we would find that, similar to the Ar He situation, the>
5 - 2 Stability of Atoms and Ions 159
concentration gradient exists but the temperature is too low to see any significant diffusion of Cu atoms into the Ni single crystal and vice versa. This is an example of a situation in which a concentration gradient exists; however, because of the lower temperature, the kinetics for diffusion are not favorable. Certainly, if we increase the temperature (say to 600°C) and wait for a longer period of time (e.g., !24 hours), we would see diffusion of Cu atoms into the Ni single crystal
and vice versa. After a very long time, the entire solid will have a uniform concen-tration of Ni and Cu atoms. The new solid that forms consists of Cu and Ni atoms completely dissolved in each other and the resultant material is termed a “solid solution,” a concept we will study in greater detail in Chapter 10.
This example also illustrates something many of you may know by intu-ition. The diffusion of atoms and molecules occurs faster in gases and liquids than in solids. As we will see in Chapter 9 and other chapters, diffusion has a significant effect on the evolution of microstructure during the solidification of alloys, the heat treatment of metals and alloys, and the processing of ceramic materials.
5-2
Stability of Atoms and Ions
In Chapter 4, we showed that imperfections are present and also can be deliberately intro-duced into a material; however, these imperfections and, indeed, even atoms or ions in their normal positions in the crystal structures are not stable or at rest. Instead, the atoms or ions possess thermal energy, and they will move. For instance, an atom may move from a normal crystal structure location to occupy a nearby vacancy. An atom may also move from one interstitial site to another. Atoms or ions may jump across a grain boundary, causing the grain boundary to move.
The ability of atoms and ions to diffuse increases as the temperature, or thermal energy possessed by the atoms and ions, increases. The rate of atom or ion movement is related to temperature or thermal energy by the Arrhenius equation:
(5-1)
where c0is a constant, Ris the gas constant , Tis the absolute temperature
(K), and Qis the activation energy(cal mol) required to cause Avogadro’s number of
atoms or ions to move. This equation is derived from a statistical analysis of the proba-bility that the atoms will have the extra energy Qneeded to cause movement. The rate is
related to the number of atoms that move.
We can rewrite the equation by taking natural logarithms of both sides:
(5-2)
If we plot ln(rate) of some reaction versus 1 T(Figure 5-4), the slope of the line will be
-Q Rand, consequently, Qcan be calculated. The constant c0corresponds to the
inter-cept at ln(c0) when 1>Tis zero.
> >
ln(rate) = ln(c0) - Q
RT
>
A
1.987 mol cal#K
B
Rate = c0 expa-Q
Svante August Arrhenius (1859–1927), a Swedish chemist who won the Nobel Prize in Chemistry in 1903 for his research on the electrolytic theory of dissociation applied this idea to the rates of chemical reactions in aqueous solutions. His basic idea of activation energy and rates of chemical reactions as functions of temperature has since been applied to diffusion and other rate processes.
1.0×10–3
1n(5
×
10
8) – 1n(8
×
10
10)
1n(5×108) – 1n(8
×1010)
1.2×10–3
1/T (K–1)
1.4×10–3 1.6
×10–3
6.018×10–4
20 22
In(Rate)
24 26 28
8.0×10–4
1/773 – 1/1073 –Q/R=slope
Q/R=14,032 K –Q/R=
1/773 – 1/1073
Figure 5-4
The Arrhenius plot of ln(rate) versus 1 T can be used to determine the activation energy for a reaction. The data from this figure is used in Example 5-2.
>
Example 5-2
Activation Energy for Interstitial AtomsSuppose that interstitial atoms are found to move from one site to another at the rates of 5*108jumps s at 500°C and 8*1010jumps s at 800°C. Calculate the
acti-vation energy Qfor the process.
SOLUTION
Figure 5-4 represents the data on a ln(rate) versus 1>Tplot; the slope of this line, as
> >
calculated in the figure, gives Q R!14,032 K, or Q!27,880 cal mol. Alternately, we could write two simultaneous equations:
= c0 exp(-0.000651Q)
5 * 108a jumps
s b = c0 a jumps
s b exp D
-QQmolcalR
c1.987Qmolcal#KR d[(500 + 273)(K)]
T
Ratea jumps
s b = c0 expa
-Q
RTb
5 - 3 Mechanisms for Diffusion 161
Note the temperatures were converted into K. Since
then
5-3
Mechanisms for Diffusion
As we saw in Chapter 4, defects known as vacancies exist in materials. The disorder these vacancies create (i.e., increased entropy) helps minimize the free energy and, therefore, increases the thermodynamic stability of a crystalline material. In materials containing vacancies, atoms move or “jump” from one lattice position to another. This process, known as self-diffusion, can be detected by using radioactive tracers. As an
example, suppose we were to introduce a radioactive isotope of gold (Au198) onto the
surface of standard gold (Au197). After a period of time, the radioactive atoms
would move into the standard gold. Eventually, the radioactive atoms would be uniformly distributed throughout the entire standard gold sample. Although self-diffusion occurs continually in all materials, its effect on the material’s behavior is generally not significant.
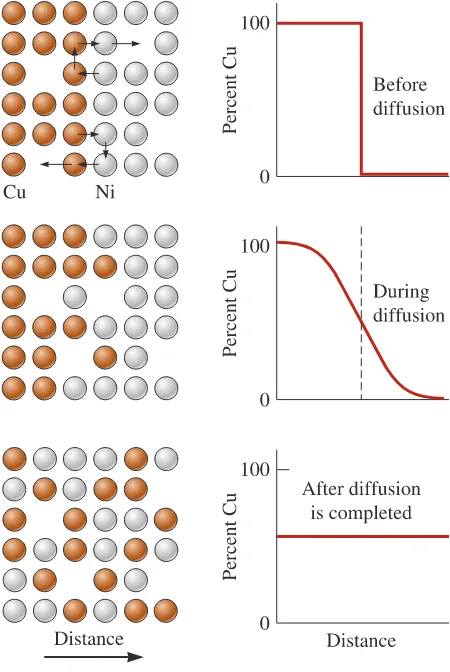
Interdiffusion
Diffusion of unlike atoms in materials also occurs (Figure 5-5). Consider a nickel sheet bonded to a copper sheet. At high temperatures, nickel atoms gradually diffuse into the copper, and copper atoms migrate into the nickel. Again, the nickel and copper atoms eventually are uniformly distributed. Diffusion of different atoms in different directions is known as interdiffusion. There are two important mechanisms bywhich atoms or ions can diffuse (Figure 5-6).
Vacancy Diffusion
In self-diffusion and diffusion involving substitutional atoms, an atom leaves its lattice site to fill a nearby vacancy (thus creating a new vacancy at the original lattice site). As diffusion continues, we have counterflows of atomsQ = 5.075
0.000182 = 27,880 cal/mol ln(160) = 5.075 = 0.000182Q
160 = exp[(0.000651 - 0.000469)Q] = exp(0.000182Q)
8 * 1010 = (5 * 10
8)exp(-0.000469Q)
exp(-0.000651Q)
c0 = 5 * 10
8
exp(-0.000651Q)a
jumps s b
= c0 exp(-0.000469Q)
8 * 1010a jumps
s b = c0 a jumps
s b exp D
-QQcal
molR
and vacancies, called vacancy diffusion. The number of vacancies, which increases as the
temperature increases, influences the extent of both self-diffusion and diffusion of sub-stitutional atoms.
Interstitial Diffusion
When a small interstitial atom or ion is present in the crystal structure, the atom or ion moves from one interstitial site to another. No vacancies are required for this mechanism. Partly because there are many more intersti-tial sites than vacancies, interstitial diffusionoccurs more easily than vacancy diffusion.Interstitial atoms that are relatively smaller can diffuse faster. In Chapter 3, we saw that Figure 5-5
Diffusion of copper atoms into nickel. Eventually, the copper atoms are randomly distributed throughout the nickel.
5 - 4 Activation Energy for Diffusion 163
the structure of many ceramics with ionic bonding can be considered as a close packing of anions with cations in the interstitial sites. In these materials, smaller cations often diffuse faster than larger anions.
5-4
Activation Energy for Diffusion
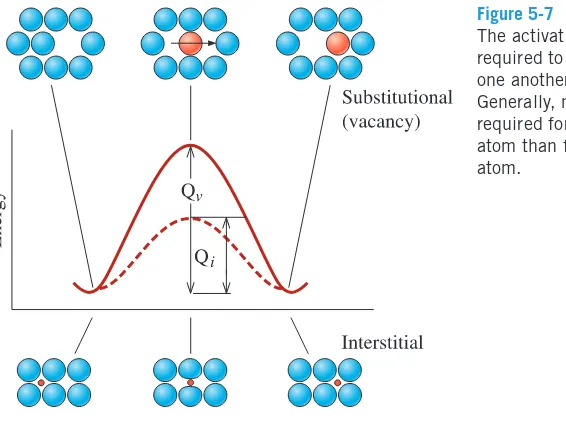
A diffusing atom must squeeze past the surrounding atoms to reach its new site. In order for this to happen, energy must be supplied to allow the atom to move to its new position, as shown schematically for vacancy and interstitial diffusion in Figure 5-7. The atom is originally in a low-energy, relatively stable location. In order to move to a new location, the atom must overcome an energy barrier. The energy barrier is the activation energy Q.
The thermal energy supplies atoms or ions with the energy needed to exceed this barrier. Note that the symbol Qis often used for activation energies for different processes (rate
at which atoms jump, a chemical reaction, energy needed to produce vacancies, etc.), and we should be careful in understanding the specific process or phenomenon to which the general term for activation energy Qis being applied, as the value of Qdepends on the
particular phenomenon.
Normally, less energy is required to squeeze an interstitial atom past the surrounding atoms; consequently, activation energies are lower for interstitial diffusion than for vacancy diffusion. Typical values for activation energies for diffusion of dif-ferent atoms in difdif-ferent host materials are shown in Table 5-1. We use the term
diffusion coupleto indicate a combination of an atom of a given element (e.g., carbon)
diffusing in a host material (e.g., BCC Fe). A low-activation energy indicates easy diffusion. In self-diffusion, the activation energy is equal to the energy needed to cre-ate a vacancy and to cause the movement of the atom. Table 5-1 also shows values of D0, which is the pre-exponential term and the constant c0from Equation 5-1,
when the rate process is diffusion. We will see later that D0is the diffusion coefficient
when 1>T!0.
Figure 5-7
5-5
Rate of Diffusion [Fick’s First Law]
Adolf Eugen Fick (1829–1901) was the first scientist to provide a quantitative descrip-tion of the diffusion process. Interestingly, Fick was also the first to experiment with contact lenses in animals and the first to implant a contact lens in human eyes in 1887–1888!
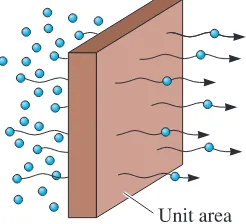
The rate at which atoms, ions, particles or other species diffuse in a material can be measured by the fluxJ. Here we are mainly concerned with diffusion of ions or atoms.
The flux Jis defined as the number of atoms passing through a plane of unit area per
unit time (Figure 5-8). Fick’s first lawexplains the net flux of atoms:
(5-3)
J = -D dc
dx
TABLE 5-1 ■ Diffusion data for selected materials
Diffusion Couple Q (cal mol) D0(cm2 s)
Interstitial diffusion:
C in FCC iron 32,900 0.23
C in BCC iron 20,900 0.011
N in FCC iron 34,600 0.0034
N in BCC iron 18,300 0.0047
H in FCC iron 10,300 0.0063
H in BCC iron 3,600 0.0012
Self-diffusion (vacancy diffusion):
Pb in FCC Pb 25,900 1.27
Al in FCC Al 32,200 0.10
Cu in FCC Cu 49,300 0.36
Fe in FCC Fe 66,700 0.65
Zn in HCP Zn 21,800 0.1
Mg in HCP Mg 32,200 1.0
Fe in BCC Fe 58,900 4.1
W in BCC W 143,300 1.88
Si in Si (covalent) 110,000 1800.0
C in C (covalent) 163,000 5.0
Heterogeneous diffusion (vacancy diffusion):
Ni in Cu 57,900 2.3
Cu in Ni 61,500 0.65
Zn in Cu 43,900 0.78
Ni in FCC iron 64,000 4.1
Au in Ag 45,500 0.26
Ag in Au 40,200 0.072
Al in Cu 39,500 0.045
Al in Al2O3 114,000 28.0
O in Al2O3 152,000 1900.0
Mg in MgO 79,000 0.249
O in MgO 82,100 0.000043
From several sources, including Adda, Y. and Philibert, J., La Diffusion dans les Solides, Vol. 2, 1966.
5 - 5 Rate of Diffusion [Fick’s First Law] 165
where Jis the flux, Dis the diffusivityor diffusion coefficient , and dc dxis the
concentration gradient . Depending upon the situation, concentration may be
expressed as atom percent (at%), weight percent (wt%), mole percent (mol%), atom fraction, or mole fraction. The units of concentration gradient and flux will change accordingly.
Several factors affect the flux of atoms during diffusion. If we are dealing with diffusion of ions, electrons, holes, etc., the units ofJ, D, and will reflect the
appropri-ate species that are being considered. The negative sign in Equation 5-3 tells us that the flux of diffusing species is from higher to lower concentrations, so that if the term is neg-ative, Jwill be positive. Thermal energy associated with atoms, ions, etc., causes the
random movement of atoms. At a microscopic scale, the thermodynamic driving force for diffusion is the concentration gradient. A net or an observable flux is created depending upon temperature and the concentration gradient.
Concentration Gradient
The concentration gradient shows how the composition of the material varies with distance: "cis the difference in concentrationover the distance "x(Figure 5-9). A concentration gradient may be created when two
materials of different composition are placed in contact, when a gas or liquid is in con-tact with a solid material, when nonequilibrium structures are produced in a material due to processing, and from a host of other sources.
dc dx dc
dx
1 atoms cm3#cm2
> 1 cm2
s 2
Figure 5-8
The flux during diffusion is defined as the number of atoms passing through a plane of unit area per unit time.
Figure 5-9
The flux at a particular temperature is constant only if the concentration gradi-ent is also constant—that is, the compositions on each side of the plane in Figure 5-8 remain unchanged. In many practical cases, however, these compositions vary as atoms are redistributed, and thus the flux also changes. Often, we find that the flux is initially high and then gradually decreases as the concentration gradient is reduced by diffusion. The examples that follow illustrate calculations of flux and concentration gradients for diffu-sion of dopants in semiconductors and ceramics, but only for the case of constant con-centration gradient. Later in this chapter, we will consider non-steady state diffusion with the aid of Fick’s second law.
Example 5-3
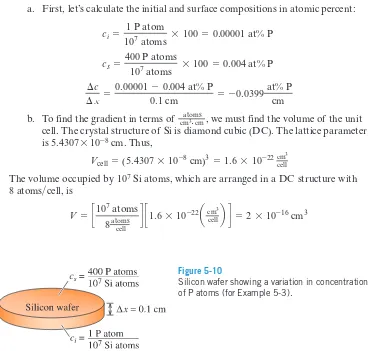
Semiconductor DopingOne step in manufacturing transistors, which function as electronic switches in inte-grated circuits, involves diffusing impurity atoms into a semiconductor material such as silicon (Si). Suppose a silicon wafer 0.1 cm thick, which originally contains one phosphorus atom for every 10 million Si atoms, is treated so that there are 400 phos-phorous (P) atoms for every 10 million Si atoms at the surface (Figure 5-10). Calculate the concentration gradient (a) in atomic percent cm and (b) in . The lattice parameter of silicon is 5.4307Å.
SOLUTION
a. First, let’s calculate the initial and surface compositions in atomic percent:
b. To find the gradient in terms of , we must find the volume of the unit cell. The crystal structure of Si is diamond cubic (DC). The lattice parameter is 5.4307*10-8cm. Thus,
The volume occupied by 107Si atoms, which are arranged in a DC structure with
8 atoms cell, is
V = c10
7 atoms
8 atoms cell d c1.6 * 10 -22
a cm3
cellb d = 2 * 10-16 cm3
>
Vcell = (5.4307 * 10-8 cm)3 = 1.6 * 10-22cm3
cell atoms
cm3#cm
¢c
¢x =
0.00001 - 0.004 at% P
0.1 cm = -0.0399 at% P
cm
cs =
400 P atoms
107 atoms * 100 = 0.004 at% P
ci =
1 P atom
107 atoms * 100 = 0.00001 at% P
atoms cm3#cm
>
Figure 5-10
5 - 5 Rate of Diffusion [Fick’s First Law] 167
The compositions in atoms cm3are
Thus, the composition gradient is
= -1.995 * 1019 PQcmatoms3#cmR ¢c
¢x =
0.005 * 1018 - 2 * 1018 P
A
atoms cm3B
0.1 cm
cs =
400 P atoms 2 * 10-16 cm3
= 2 * 1018 P
A
atoms cm3B
ci =
1 P atom 2 * 10-16 cm3
= 0.005 * 1018 P
A
atomscm3
B
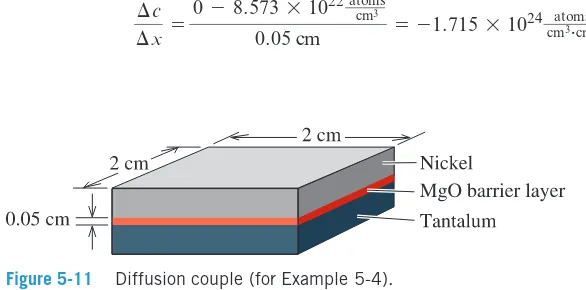
>Example 5-4
Diffusion of Nickel in Magnesium Oxide (MgO)A 0.05 cm layer of magnesium oxide (MgO) is deposited between layers of nickel (Ni) and tantalum (Ta) to provide a diffusion barrier that prevents reactions between the two metals (Figure 5-11). At 1400°C, nickel ions diffuse through the MgO ceramic to the tantalum. Determine the number of nickel ions that pass through the MgO per second. At 1400°C, the diffusion coefficient of nickel ions in MgO is 9*10-12cm2 s, and the lattice parameter of nickel at 1400°C is
3.6*10-8cm.
SOLUTION
The composition of nickel at the Ni MgO interface is 100% Ni, or
The composition of nickel at the Ta MgO interface is 0% Ni. Thus, the concentra-tion gradient is
¢c
¢x =
0 - 8.573 * 1022 atoms cm3
0.05 cm = -1.715 * 1024
atoms cm3#cm >
c Ni/MgO =
4 Ni unit cell atoms (3.6 * 10-8 cm)3
= 8.573 * 1022atoms
cm3 >
>
The flux of nickel atoms through the MgO layer is
The total number of nickel atoms crossing the 2 cm*2 cm interface per second is
Although this appears to be very rapid, in one second, the volume of nickel atoms removed from the Ni MgO interface is
The thickness by which the nickel layer is reduced each second is
For one micrometer (10-4cm) of nickel to be removed, the treatment requires
5-6
Factors Affecting Diffusion
Temperature and the Diffusion Coefficient
The kinetics of diffusion are strongly dependent on temperature. The diffusion coefficient Dis relatedto temperature by an Arrhenius-type equation,
(5-4)
where Qis the activation energy (in units of cal mol) for diffusion of the species under
consideration (e.g., Al in Si), Ris the gas constant , and Tis the absolute
temperature (K). D0is the pre-exponential term, similar to c0in Equation 5-1.
D0is a constant for a given diffusion system and is equal to the value of the
dif-fusion coefficient at 1 T!0 or T! #. Typical values for D0are given in Table 5-1, while
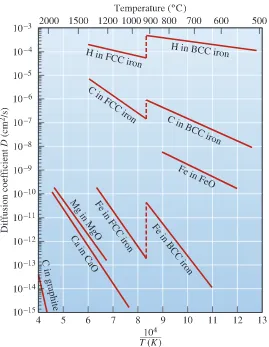
the temperature dependence ofDis shown in Figure 5-12 for some metals and ceramics.
Covalently bonded materials, such as carbon and silicon (Table 5-1), have unusually high >
A
1.987 molcal#KB
>
D = D0 expa
-Q
RTb
10-4 cm 1.8 * 10-10 cm
s
= 556,000 s = 154 h
0.72 * 10-9 cm3
s
4 cm2 = 1.8 * 10 -10 cm
s
6.17 * 1013 Ni atoms s
8.573 * 1022 Ni atoms
cm3
= 0.72 * 10-9 cm3
s
>
= 6.17 * 1013 Ni atoms/s
Total Ni atoms per second = (J)( Area) =
A
1.543 * 1013 atoms cm2#sB
(2 cm)(2 cm)J =1.543 * 1013 Ni atoms cm2#s
J = -D¢c
¢x = -(9 * 10
-12
5 - 6 Factors Affecting Diffusion 169
activation energies, consistent with the high strength of their atomic bonds. Figure 5-13 shows the diffusion coefficients for different dopants in silicon.
In ionic materials, such as some of the oxide ceramics, a diffusing ion only enters a site having the same charge. In order to reach that site, the ion must physically squeeze past adjoining ions, pass by a region of opposite charge, and move a relatively long distance (Figure 5-14 ). Consequently, the activation energies are high and the rates of diffusion are lower for ionic materials than those for metals (Figure 5-15 on page 171). We take advantage of this in many situations. For example, in the processing of silicon (Si), we create a thin layer of silica (SiO2) on top of a silicon wafer (Chapter 19). We then create a window by
remov-ing part of the silica layer. This window allows selective diffusion of dopant atoms such as phosphorus (P) and boron (B), because the silica layer is essentially impervious to the dopant atoms. Slower diffusion in most oxides and other ceramics is also an advantage in applications in which components are required to withstand high temperatures.
Dif
fusion Coef
ficient
Figure 5-13
Diffusion coefficients for different dopants in silicon. (From “Diffusion and Diffusion Induced Defects in Silicon,” by U. Gösele. In R. Bloor, M. Flemings, and S. Mahajan (Eds.), Encyclopedia of Advanced Materials, Vol. 1, 1994, p. 631, Fig. 2. Copyright © 1994 Pergamon Press. Reprinted with permission of the editor.)
Figure 5-14
Diffusion in ionic compounds. Anions can only enter other anion sites. Smaller cations tend to diffuse faster.
temperatures, the thermal energy supplied to the diffusing atoms permits the atoms to overcome the activation energy barrier and more easily move to new sites. At low temperatures—often below about 0.4 times the absolute melting temperature of the material—diffusion is very slow and may not be significant. For this reason, the heat treat-ment of metals and the processing of ceramics are done at high temperatures, where atoms move rapidly to complete reactions or to reach equilibrium conditions. Because less ther-mal energy is required to overcome the sther-maller activation energy barrier, a sther-mall activa-tion energy Qincreases the diffusion coefficient and flux. The following example illustrates
how Fick’s first law and concepts related to the temperature dependence of Dcan be
5 - 6 Factors Affecting Diffusion 171
Dif
fusion Coef
ficient
Figure 5-15 Diffusion coefficients of ions in different oxides. (Adapted from Physical Ceramics: Principles for Ceramic Science and Engineering, by Y.M. Chiang, D. Birnie, and W.D. Kingery, Fig. 3-1. Copyright © 1997 John Wiley & Sons. This material is used by permission of John Wiley & Sons, Inc.)
Example 5-5
Design of an Iron MembraneAn impermeable cylinder 3 cm in diameter and 10 cm long contains a gas that includes 0.5*1020N atoms per cm3and 0.5*1020H atoms per cm3on one side
Figure 5-16
Design of an iron membrane (for Example 5-5).
ensure a constant concentration of nitrogen and hydrogen. The gas on the other side of the membrane includes a constant 1*1018N atoms per cm3and 1*1018
H atoms per cm3. The entire system is to operate at 700°C, at which iron has the
BCC structure. Design an iron membrane that will allow no more than 1% of the nitrogen to be lost through the membrane each hour, while allowing 90% of the hydrogen to pass through the membrane per hour.
SOLUTION
The total number of nitrogen atoms in the container is
(0.5*1020N cm3)($ 4)(3 cm)2(10 cm)!35.343*1020N atoms
The maximum number of atoms to be lost is 1% of this total, or
The flux is then
Using Equation 5-4 and values from Table 5-1, the diffusion coefficient of nitrogen in BCC iron at 700°C!973 K is
= (0.0047)(7.748 * 10-5) = 3.64 * 10-7 cm s 2
D N = 0.0047 cm
2
s expC
-18,300 molcal
1.987 molcal#K (973 K)S
D = D0 expa -Q
RT b
= 0.00139 * 1018 N atoms
cm2#s
J = 0.0098
* 1018( N atoms/s)
ap
4 b(3 cm)2
= 0.0098 * 1018 N atoms/s
N atom loss per s = (35.343 * 1018 N atoms/h)/(3600 s/h)
N atom loss per h = (0.01)
A
35.34 * 1020B
= 35.343 * 1018 N atoms/h5 - 6 Factors Affecting Diffusion 173
From Equation 5-3:
"x!0.013 cm!minimum thickness of the membrane
In a similar manner, the maximum thickness of the membrane that will permit 90% of the hydrogen to pass can be calculated as
H atom loss per h!(0.90)(35.343*1020)!31.80*1020
H atom loss per s!0.0088*1020
From Equation 5-4 and Table 5-1,
Since
An iron membrane with a thickness between 0.013 and 0.073 cm will be satisfactory.
Types of Diffusion
In volume diffusion, the atoms move through the crystalfrom one regular or interstitial site to another. Because of the surrounding atoms, the acti-vation energy is large and the rate of diffusion is relatively slow.
Atoms can also diffuse along boundaries, interfaces, and surfaces in the material. Atoms diffuse easily by grain boundary diffusion, because the atom packing is
disordered and less dense in the grain boundaries. Because atoms can more easily squeeze their way through the grain boundary, the activation energy is low (Table 5-2).
Surface diffusionis easier still because there is even less constraint on the diffusing atoms
at the surface.
Time
Diffusion requires time. The units for flux are . If a large number of atoms must diffuse to produce a uniform structure, long times may be required, even at high temperatures. Times for heat treatments may be reduced by using higher temperatures or by making the diffusion distances(related to "x) as small as possible.We find that some rather remarkable structures and properties are obtained if we prevent diffusion. Steels quenched rapidly from high temperatures to prevent
atoms cm2#s = 0.073 cm = maximum thickness
¢x =
a-1.86 * 10-4cms2b a-49 * 1018H atomscm3 b
0.125 * 1018H atomscm2#s ¢x = -D¢c/J
DH = 0.0012 cm
2
s exps
-3,600 cal
mol
1.987 Kcal#mol (973 K)
t = 1.86 * 10-4 cm2/s
J = 0.125 * 1018H atoms
cm2#s
¢x = -D¢c/J =
c Q-3.64 * 10-7 cm2/ s)(1 * 1018 - 50 * 1018N atoms
cm3 R d
0.00139 * 1018N atoms
cm2#s J = -Da¢c
¢xb = 0.00139 * 10
Example 5-6
Tungsten Thorium Diffusion CoupleConsider a diffusion couple between pure tungsten and a tungsten alloy containing 1 at% thorium. After several minutes of exposure at 2000°C, a transition zone of 0.01 cm thickness is established. What is the flux of thorium atoms at this time if diffusion is due to (a) volume diffusion, (b) grain boundary diffusion, and (c) surface diffusion? (See Table 5-2.)
SOLUTION
The lattice parameter of BCC tungsten is 3.165 Å. Thus, the number of tungsten atoms cm3is
In the tungsten-1 at% thorium alloy, the number of thorium atoms is
cTh = (0.01)(6.3 * 1022) = 6.3 * 1020 Th atomscm3 W atoms
cm3 =
2 atoms/cell (3.165 * 10-8)3 cm3/ cell
= 6.3 * 1022
>
diffusion form nonequilibrium structures and provide the basis for sophisticated heat treatments. Similarly, in forming metallic glasses, we have to quench liquid metals at a very high cooling rate. This is to avoid diffusion of atoms by decreasing their thermal energy and encouraging them to assemble into nonequilibrium amorphous arrange-ments. Melts of silicate glasses, on the other hand, are viscous and diffusion of ions through these is slow. As a result, we do not have to cool these melts very rapidly to attain an amorphous structure. There is a myth that many old buildings contain win-dowpanes that are thicker at the bottom than at the top because the glass has flowed down over the years. Based on kinetics of diffusion, it can be shown that even several hundred or thousand years will not be sufficient to cause such flow of glasses at near-room temperature. In certain thin film deposition processes such as sputtering, we sometimes obtain amorphous thin films if the atoms or ions are quenched rapidly after they land on the substrate. If these films are subsequently heated (after deposition) to sufficiently high temperatures, diffusion will occur and the amorphous thin films will eventually crystallize. In the following example, we examine different mechanisms for diffusion.
TABLE 5-2 ■ The effect of the type of diffusion for thorium in tungsten and for self-diffusion in silver
Diffusion Coefficient (D)
Diffusion Type Thorium in Tungsten Silver in Silver
D0(cm2 s) Q(cal mol) D0(cm2 s) Q(cal mol)
Surface 0.47 66,400 0.068 8,900
Grain boundary 0.74 90,000 0.24 22,750
Volume 1.00 120,000 0.99 45,700
*Given by parameters for Equation 5-4.
> >
5 - 6 Factors Affecting Diffusion 175
In the pure tungsten, the number of thorium atoms is zero. Thus, the concentration gradient is
a. Volume diffusion
b. Grain boundary diffusion
c. Surface diffusion
Dependence on Bonding and Crystal Structure
Anumber of factors influence the activation energy for diffusion and, hence, the rate of diffusion. Interstitial diffusion, with a low-activation energy, usually occurs much faster than vacancy, or substitutional diffusion. Activation energies are usually lower for atoms diffusing through open crystal structures than for close-packed crys-tal structures. Because the activation energy depends on the strength of atomic bond-ing, it is higher for diffusion of atoms in materials with a high melting temperature (Figure 5-17).
We also find that, due to their smaller size, cations (with a positive charge) often have higher diffusion coefficients than those for anions (with a negative charge). In sodium chloride, for instance, the activation energy for diffusion of chloride ions (Cl-) is about twice that for diffusion of sodium ions (Na+).
J = -a1.94 * 10-7cms2 b a-6.3 * 1022cmatoms3#cmb = 12.2 * 1015Th atomscm2#s
D = 0.47 cms2 exp£
-66,400 cal
mol
Q1.987 molcal#KR(2273 K)
≥ = 1.94 * 10-7 cm2/ s
J = -a1.64 * 10-9 cm2
s b a-6.3 * 1022cmatoms3#cmb = 10.3 * 1013Th atomscm2#s
D = 0.74 cm2
s exp£
-90,000 molcal
Q1.987 molcal#KR(2273 K)
≥ = 1.64 * 10-9 cm2/ s = 18.2 * 1010Th atoms
cm2#s
J = -D¢c
¢x = -a2.89 * 10
-12cm2
s b a-6.3 * 1022cmatoms3#cmb
D = 1.0 cms2 exp £
-120,000 calmol
Q1.987 molcal#KR(2273 K)
≥ = 2.89 * 10-12 cm2/s ¢c
¢x =
0 - 6.3 * 1020 atoms
cm2
0.01 cm = -6.3 * 10
Diffusion of ions also provides a transfer of electrical charge; in fact, the electri-cal conductivity of ionielectri-cally bonded ceramic materials is related to temperature by an Arrhenius equation. As the temperature increases, the ions diffuse more rapidly, electri-cal charge is transferred more quickly, and the electrielectri-cal conductivity is increased. As men-tioned before, some ceramic materials are good conductors of electricity.
Dependence on Concentration of Diffusing Species
and Composition of Matrix
The diffusion coefficient (D) depends notonly on temperature, as given by Equation 5-4, but also on the concentration of diffusing species and composition of the matrix. The reader should consult higher-level textbooks for more information.
5-7
Permeability of Polymers
In polymers, we are most concerned with the diffusion of atoms or small molecules between the long polymer chains. As engineers, we often cite the permeability of poly-mers and other materials, instead of the diffusion coefficients. The permeability is
expressed in terms of the volume of gas or vapor that can permeate per unit area, per unit time, or per unit thickness at a specified temperature and relative humidity. Polymers that have a polar group (e.g., ethylene vinyl alcohol) have higher permeabil-ity for water vapor than for oxygen gas. Polyethylene, on the other hand, has much higher permeability for oxygen than for water vapor. In general, the more compact the structure of polymers, the lesser the permeability. For example, low-density polyethyl-ene has a higher permeability than high-density polyethylpolyethyl-ene. Polymers used for food and other applications need to have the appropriate barrier properties. For example, polymer films are typically used as packaging to store food. If air diffuses through the film, the food may spoil. Similarly, care has to be exercised in the storage of ceramic or metal powders that are sensitive to atmospheric water vapor, nitrogen, oxy-gen, or carbon dioxide. For example, zinc oxide powders used in rubbers, paints, and ceramics must be stored in polyethylene bags to avoid reactions with atmospheric water vapor.
Melting temperature
Figure 5-17
5 - 8 Composition Profile [Fick’s Second Law] 177
Diffusion of some molecules into a polymer can cause swelling problems. For example, in automotive applications, polymers used to make o-rings can absorb consid-erable amounts of oil, causing them to swell. On the other hand, diffusion is required to enable dyes to uniformly enter many of the synthetic polymer fabrics. Selective diffusion through polymer membranes is used for desalinization of water. Water molecules pass through the polymer membrane, and the ions in the salt are trapped.
In each of these examples, the diffusing atoms, ions, or molecules penetrate between the polymer chains rather than moving from one location to another within the chain structure. Diffusion will be more rapid through this structure when the diffusing species is smaller or when larger voids are present between the chains. Diffusion through crystalline polymers, for instance, is slower than that through amorphous polymers, which have no long-range order and, consequently, have a lower density.
5-8
Composition Profile [Fick’s Second Law]
Fick’s second law, which describes the dynamic, or non-steady state, diffusion of atoms,
is the differential equation
(5-5)
If we assume that the diffusion coefficient Dis not a function of location xand the
concen-tration (c) of diffusing species, we can write a simplified version of Fick’s second law as follows
(5-6)
The solution to this equation depends on the boundary conditions for a particular situa-tion. One solution is
(5-7)
where csis a constant concentration of the diffusing atoms at the surface of the material,
c0is the initial uniform concentration of the diffusing atoms in the material, and cxis the concentration of the diffusing atom at location xbelow the surface after time t. These
concentrations are illustrated in Figure 5-18. In these equations we have assumed basi-cally a one-dimensional model (i.e., we assume that atoms or other diffusing species are moving only in the direction x). The function “erf ” is the error function and can
be evaluated from Table 5-3 or Figure 5-19. Note that most standard spreadsheet
cs - cx
cs - c0
= erfa x
21Dtb
0c
0t = Da
02c
0x2b 0c
0t = 0
0xaD 0c
0xb
Example 5-7
Design of a Carburizing TreatmentThe surface of a 0.1% C steel gear is to be hardened by carburizing. In gas carbur-izing, the steel gears are placed in an atmosphere that provides 1.2% C at the surface of the steel at a high temperature (Figure 5-1). Carbon then diffuses from the sur-face into the steel. For optimum properties, the steel must contain 0.45% C at a and other software programs available on a personal computer (e.g., Excel™) also provide error function values.
The mathematical definition of the error function is as follows:
(5-8)
In Equation 5-8, yis known as the argument of the error function. We also define a
com-plementary error function as follows:
erfc(x)!1-erf(x) (5-9)
This function is used in certain solution forms of Fick’s second law.
As mentioned previously, depending upon the boundary conditions, different solutions (i.e., different equations) describe the solutions to Fick’s second law. These solu-tions to Fick’s second law permit us to calculate the concentration of one diffusing species as a function of time (t) and location (x). Equation 5-7 is a possible solutionto Fick’s law
that describes the variation in concentration of different species near the surface of the material as a function of time and distance, provided that the diffusion coefficient D
remains constant and the concentrations of the diffusing atoms at the surface (cs) and at large distance (x) within the material (c0) remain unchanged. Fick’s second law can also
assist us in designing a variety of materials processing techniques, including carburization
and dopant diffusion in semiconductors as described in the following examples. erf (x) = 2
1pL
x
0
exp (-y2)dy
TABLE 5-3 ■ Error function values for Fick’s second law
Argument of the Value of the Error Function Error Function erf
0 0
0.10 0.1125
0.20 0.2227
0.30 0.3286
0.40 0.4284
0.50 0.5205
0.60 0.6039
0.70 0.6778
0.80 0.7421
0.90 0.7969
1.00 0.8427
1.50 0.9661
2.00 0.9953
Note that error function values are available on many software packages found on personal computers.
x 21Dt x
21Dt
5 - 8 Composition Profile [Fick’s Second Law] 179
depth of 0.2 cm below the surface. Design a carburizing heat treatment that will produce these optimum properties. Assume that the temperature is high enough (at least 900°C) so that the iron has the FCC structure.
SOLUTION
Since the boundary conditions for which Equation 5-7 was derived are assumed to be valid, we can use this equation:
We know that cs!1.2% C, c0!0.1% C, cx!0.45% C, and x!0.2 cm. From Fick’s second law:
From Table 5-3, we find that
Any combination ofDand twith a product of 0.0198 cm2will work. For carbon
dif-fusing in FCC iron, the diffusion coefficient is related to temperature by Equation 5-4:
From Table 5-1:
Therefore, the temperature and time of the heat treatment are related by
Some typical combinations of temperatures and times are
IfT!900°C!1173 K, then t!116,273 s!32.3 h
IfT!1000°C!1273 K, then t!38,362 s!10.7 h
IfT!1100°C!1373 K, then t!14,876 s!4.13 h
IfT!1200°C!1473 K, then t!6,560 s!1.82 h
The exact combination of temperature and time will depend on the maximum tem-perature that the heat treating furnace can reach, the rate at which parts must be produced, and the economics of the tradeoffs between higher temperatures versus longer times. Another factor to consider is changes in microstructure that occur in the rest of the material. For example, while carbon is diffusing into the surface, the rest of the microstructure can begin to experience grain growth or other changes.
t =
0.0198 cm2
Dcms2
= 0.0198 cm
2
0.23 exp(-16,558/T) cms2
= 0.0861
exp(-16,558/T)
D = 0.23 exp£ -32,900 cal/mol
1.987 molcal#K T (K)≥
= 0.23 expa-16, 558
T b
D = D0 expa-Q
RTb
0.1 cm
1Dt = 0.71 or Dt = a
0.1 0.71b
2
= 0.0198 cm2
cs - cx
cs - c0
= 1.2% C - 0.45% C
1.2% C - 0.1% C = 0.68 = erfa
0.2 cm
21Dtb = erfa
0.1 cm
1Dt b cs - cx
cs - c0
= erfa x
Example 5-8 shows that one of the consequences of Fick’s second law is that the same concentration profile can be obtained for different processing conditions, so long as the term Dtis constant. This permits us to determine the effect of temperature on the
time required for a particular heat treatment to be accomplished.
Example 5-8
Design of a More Economical Heat TreatmentWe find that 10 h are required to successfully carburize a batch of 500 steel gears at 900°C, where the iron has the FCC structure. We find that it costs $1000 per hour to operate the carburizing furnace at 900°C and $1500 per hour to operate the fur-nace at 1000°C. Is it economical to increase the carburizing temperature to 1000°C? What other factors must be considered?
SOLUTION
We again assume that we can use the solution to Fick’s second law given by Equation 5-7:
Note that since we are dealing with only changes in heat treatment time and temper-ature, the term Dtmust be constant.
The temperatures of interest are 900°C!1173 K and 1000°C!1273 K.
To achieve the same carburizing treatment at 1000°C as at 900°C:
D1273t1273!D1173t1173
For carbon diffusing in FCC iron, the activation energy is 32,900 cal mol. Since we are dealing with the ratios of times, it does not matter whether we substitute for the time in hours or seconds. It is, however, always a good idea to use units that balance out. Therefore, we will show time in seconds. Note that temperatures must be con-verted into Kelvin.
=
D0 exp £- 32,900
cal mol
1.987 molcal#K 1173K≥
(10 hours)(3600 sec/hour)
D0 exp £- 32,900
cal mol
1.987 mol cal#K 1273K
≥
t1273 = D1173t1173
D1273
D = D0 exp(-Q/RT)
D1273t1273 = D1173t1173
>
cs - cx
cs - c0
= erfa x
5 - 8 Composition Profile [Fick’s Second Law] 181
Notice, we did not need the value of the pre-exponential term D0, since it
canceled out.
At 900°C, the cost per part is ($1000 h) (10 h) 500 parts> > !$20 part. At > t1273 = 3.299 h = 3 h and 18 min
= (10)(0.3299)(3600) s
t1273 = exp(
-14.1156)(10)(3600)
exp(-13.0068)
Example 5-9
Silicon Device FabricationDevices such as transistors are made by doping semiconductors. The diffusion coefficient of phosphorus (P) in Si is D!6.5*10-13cm2 s at a temperature of
1100°C. Assume the source provides a surface concentration of 1020atoms cm3
and the diffusion time is one hour. Assume that the silicon wafer initially con-tains no P.
Calculate the depth at which the concentration of P will be 1018atoms cm3.
State any assumptions you have made while solving this problem.
SOLUTION
We assume that we can use one of the solutions to Fick’s second law (i.e., Equation 5-7):
We will use concentrations in atoms cm3, time in seconds, and Din . Notice that
the left-hand side is dimensionless. Therefore, as long as we use concentrations in the same units for cs, cx, and c0, it does not matter what those units are.
cs - cx
cs - c0
= 10
20 atoms
cm3 - 1018 atoms cm3 1020 atoms cm3 - 0 atoms cm3
= 0.99
cm2
s
>
cs - cx
cs - c0
= erfa x
21Dtb
> > >
1000°C, the cost per part is ($1500 h) (3.299 h) 500 parts!$9.90 part.
Considering only the cost of operating the furnace, increasing the temper-ature reduces the heat-treating cost of the gears and increases the production rate. Another factor to consider is if the heat treatment at 1000°C could cause some other microstructural or other changes. For example, would increased temperature cause grains to grow significantly? If this is the case, we will be weakening the bulk of the material. How does the increased temperature affect the life of the other equipment such as the furnace itself and any accessories? How long would the cooling take? Will cooling from a higher temperature cause residual stresses? Would the product still meet all other specifications? These and other questions should be considered. The point is, as engineers, we need to ensure that the solution we propose is not only technically sound and economically sensible, it should recognize and make sense for the system as a whole. A good solution is often simple, solves problems for the sys-tem, and does not create new problems.
> >
From the error function values in Table 5-3 (or from your calculator computer), If erf(z)!0.99, z!1.82, therefore,
or
x!1.76*10-4cm
or
Note that we have expressed the final answer in micrometers since this is the length scale that is appropriate for this application. The main assumptions we made are (1) the Dvalue does not change while phosphorus (P) gets incorporated in the silicon
wafer and (2) the diffusion of P is only in one dimension (i.e., we ignore any lateral diffusion).
Limitations to Applying the Error-Function Solution
Given by Equation 5-7
Note that in the equation describing Fick’s sec-ond law (Equation 5-7):(a) It is assumed that Dis independent of the concentration of the diffusing species;
(b) the surface concentration of the diffusing species (cs) is always constant.
There are situations under which these conditions may not be met and hence the concen-tration profile evolution will not be predicted by the error-function solution shown in Equation 5-7. If the boundary conditions are different from the ones we assumed, differ-ent solutions to Fick’s second law must be used.
5-9
Diffusion and Materials Processing
We briefly discussed applications of diffusion in processing materials in Section 5-1. Many important examples related to solidification, phase transformations, heat treatments, etc., will be discussed in later chapters. In this section, we provide more information to high-light the importance of diffusion in the processing of engineered materials. Diffusional processes become very important when materials are used or processed at elevated temperatures.
x = 1.76 mm
x = (1.76 * 10-4 cm)a10
4 mm
cm b 1.82 = x
9.67 * 10-5
>
= erf a x
9.67 * 10-5b
= erf B x
22
A
6.5 * 10-13cm25 - 9 Diffusion and Materials Processing 183
Melting and Casting
One of the most widely used methods to process metals, alloys, many plastics, and glasses involves melting and casting of materials into a desired shape. Diffusion plays a particularly important role in solidification of metals and alloys. During the growth of single crystals of semiconductors, for example, we must ensure that the differences in the diffusion of dopants in both the molten and solid forms are accounted for. This also applies for the diffusion of elements during the casting of alloys. Similarly, diffusion plays a critical role in the processing of glasses. In inorganic glasses, for instance, we rely on the fact that diffusion is slow and inorganic glasses do not crystallize easily. We will examine this topic further in Chapter 9.Sintering
Although casting and melting methods are very popular for many manufactured materials, the melting points of many ceramic and some metallic materials are too high for processing by melting and casting. These relatively refractory materials are manufactured into useful shapes by a process that requires the consolidation of small par-ticles of a powder into a solid mass (Chapter 15). Sinteringis the high-temperaturetreat-ment that causes particles to join, gradually reducing the volume of pore space between them. Sintering is a frequent step in the manufacture of ceramic components (e.g., alu-mina, barium titanate, etc.) as well as in the production of metallic parts by powder metallurgy—a processing route by which metal powders are pressed and sintered into
dense, monolithic components. A variety of composite materials such as tungsten car-bide-cobalt based cutting tools, superalloys, etc., are produced using this technique. With finer particles, many atoms or ions are at the surface for which the atomic or ionic bonds are not satisfied. As a result, a collection of fine particles of a certain mass has higher energy than that for a solid cohesive material of the same mass. Therefore, the driving force for solid state sintering of powdered metals and ceramics is the reduction in the total surface areaof powder particles. When a powdered material is compacted into a shape, the
powder particles are in contact with one another at numerous sites, with a significant amount of pore space between them. In order to reduce the total energy of the material, atoms diffuse to the points of contact, bonding the particles together and eventually caus-ing the pores to shrink.
Lattice diffusion from the bulk of the particles into the neck region causes den-sification. Surface diffusion, gas or vapor phase diffusion, and lattice diffusion from curved surfaces into the neck area between particles do not lead to densification (Chapter 15). If sintering is carried out over a long period of time, the pores may be eliminated and the material becomes dense (Figure 5-20). In Figure 5-21, particles of a
powder of a ceramic material known as barium magnesium tantalate (Ba(Mg1/3Ta2/3)O3 or BMT) are shown. This ceramic material is useful in making electronic components known as dielectric resonatorsused in wireless communication systems. The
microstruc-ture of BMT ceramics is shown in Figure 5-22. These ceramics were produced by com-pacting the powders in a press and sintering the compact at a high temperature (!1500°C).
The extent and rate of sintering depends on (a) the initial density of the compacts, (b) temperature, (c) time, (d) the mechanism of sintering, (e) the average particle size, and (f) the size distribution of the powder particles. In some situations, a liquid phase forms in localized regions of the material while sintering is in process. Since diffusion of species, such as atoms and ions, is faster in liquids than in the solid state, the presence of a liquid phase can provide a convenient way for accelerating the sintering of many refractory metal and ceramic formulations. The process in which a small amount of liquid forms and assists densification is known as liquid phase sintering. For the liquid phase to be effective in
enhancing sintering, it is important to have a liquid that can “wet” the grains, similar to how water wets a glass surface. If the liquid is non-wetting, similar to how mercury does not wet glass, then the liquid phase will not be helpful for enhancing sintering. In some cases, compounds are added to materials to cause the liquid phase to form at sintering tempera-tures. In other situations, impurities can react with the material and cause formation of a liquid phase. In most applications, it is desirable if the liquid phase is transient or converted into a crystalline material during cooling. This way a glassy and brittle amorphous phase does not remain at the grain boundaries.
When exceptionally high densities are needed, pressure (either uniaxial or isostatic) is applied while the material is being sintered. These techniques are known as Figure 5-21 Particles of barium magnesium tantalate (BMT) (Ba(Mg1/3Ta2/3)O3)
5 - 9 Diffusion and Materials Processing 185
hot pressing, when the pressure is unidirectional, or hot isostatic pressing(HIP), when
the pressure is isostatic (i.e., applied in all directions). Many superalloys and ceramics such as lead lanthanum zirconium titanate (PLZT) are processed using these tech-niques. Hot isostatic pressing leads to high density materials with isotropic properties (Chapter 15).
Grain Growth
A polycrystalline material contains a large number of grain boundaries, which represent high-energy areas because of the inefficient packing of the atoms. A lower overall energy is obtained in the material if the amount of grain bound-ary area is reduced by grain growth. Grain growthinvolves the movement of grainbound-aries, permitting larger grains to grow at the expense of smaller grains (Figure 5-23). If you have watched froth, you have probably seen the principle of grain growth! Grain growth is similar to the way smaller bubbles in the froth disappear at the expense of bigger bub-bles. Another analogy is big fish getting bigger by eating small fish! For grain growth in materials, diffusion of atoms across the grain boundary is required, and, consequently, the growth of the grains is related to the activation energy needed for an atom to jump across the boundary. The increase in grain size can be seen from the sequence of micro-graphs for alumina ceramics shown in Figure 5-23. Another example for which grain growth plays a role is in the tungsten (W) filament in a lightbulb. As the tungsten filament gets hotter, the grains grow causing it to get weaker. This grain growth, vaporization of tungsten, and oxidation via reaction with remnant oxygen contribute to the failure of tungsten filaments in a lightbulb.
The driving forcefor grain growth is reduction in grain boundary area. Grain
boundaries are defects and their presence causes the free energy of the material to increase. Thus, the thermodynamic tendency of polycrystalline materials is to transform Figure 5-22 The microstructure of BMT ceramics obtained by compaction and
into materials that have a larger average grain size. High temperatures or low-activation energies increase the size of the grains. Many heat treatments of metals, which include holding the metal at high temperatures, must be carefully controlled to avoidexcessive
grain growth. This is because, as the average grain size grows, the grain-boundary area decreases, and there is consequently less resistance to motion of dislocations. As a result, the strength of a metallic material will decrease with increasing grain size. We have seen this concept before in the form of the Hall-Petch equation (Chapter 4). In normal grain growth, the average grain size increases steadily and the width of the grain size
distribu-tion is not affected severely. In abnormal grain growth, the grain size distribution tends
to become bi-modal (i.e., we get a few grains that are very large and then we have a few grains that remain relatively small). Certain electrical, magnetic, and optical properties of materials also depend upon the grain size of materials. As a result, in the processing of these materials, attention has to be paid to factors that affect diffusion rates and grain growth.
Diffusion Bonding
A method used to join materials, called diffusion bonding, occurs in three steps (Figure 5-24). The first step forces the two surfaces togetherat a high temperature and pressure, flattening the surface, fragmenting impurities, and producing a high atom-to-atom contact area. As the surfaces remain pressed together at high temperatures, atoms diffuse along grain boundaries to the remaining voids; the atoms condense and reduce the size of any voids at the interface. Because grain boundary diffu-sion is rapid, this second step may occur very quickly. Eventually, however, grain growth isolates the remaining voids from the grain boundaries. For the third step—final elimina-tion of the voids—volume diffusion, which is comparatively slow, must occur. The diffu-sion bonding process is often used for joining reactive metals such as titanium, for joining dissimilar metals and materials, and for joining ceramics.
Summary 187
Figure 5-24 The steps in diffusion bonding: (a) Initially the contact area is small; (b) application of pressure deforms the surface, increasing the bonded area; (c) grain boundary diffusion permits voids to shrink; and (d) final elimination of the voids requires volume diffusion.
Summary
• The net flux of atoms, ions, etc., resulting from diffusion depends upon the initial con-centration gradient.
• The kinetics of diffusion depend strongly on temperature. In general, diffusion is a thermally activated process and the dependence of the diffusion coefficient on temper-ature is given by the Arrhenius equation.
• The extent of diffusion depends on temperature, time, the nature and concentration of diffusing species, crystal structure, composition of the matrix, stoichiometry, and point defects.
• Encouraging or limiting the diffusion process forms the underpinning of many impor-tant technologies. Examples include the processing of semiconductors, heat treatments of metallic materials, sintering of ceramics and powdered metals, formation of amor-phous materials, solidification of molten materials during a casting process, diffusion bonding, and barrier plastics, films, and coatings.
• Fick’s laws describe the diffusion process quantitatively. Fick’s first law defines the rela-tionship between the chemical potential gradient and the flux of diffusing species. Fick’s second law describes the variation of concentration of diffusing species under non-steady state diffusion conditions.
• For a particular system, the amount of diffusion is related to the term Dt. This term
permits us to determine the effect of a change in temperature on the time required for a diffusion-controlled process.
• The two important mechanisms for atomic movement in crystalline materials are vacancy diffusion and interstitial diffusion. Substitutional atoms in the crystalline mate-rials move by the vacancy mechanism.
• The activation energy Qdescribes the ease with which atoms diffuse, with rapid
fusion occurring for a low activation energy. A low-activation energy and rapid dif-fusion rate are obtained for (1) interstitial difdif-fusion compared to vacancy difdif-fusion, (2) crystal structures with a smaller packing factor, (3) materials with a low melting temperature or weak atomic bonding, and (4) diffusion along grain boundaries or surfaces.
• The total movement of atoms, or flux, increases when the concentration gradient and temperature increase.
• Diffusion of ions in ceramics is usually slower than that of atoms in metallic materi-als. Diffusion in ceramics is also affected significantly by non-stoichiometry, dopants, and the possible presence of liquid phases during sintering.
• Atom diffusion is of paramount importance because many of the materials processing techniques, such as sintering, powder metallurgy, and diffusion bonding, require diffu-sion. Furthermore, many of the heat treatments and strengthening mechanisms used to control structures and properties in materials are diffusion-controlled processes. The stability of the structure and the properties of materials during use at high temperatures depend on diffusion.
Glossary
Abnormal grain growthA type of grain growth observed in metals and ceramics. In this mode
of grain growth, a bimodal grain size distribution usually emerges as some grains become very large at the expense of smaller grains. See “Grain growth” and “Normal grain growth.”
Activation energyThe energy required to cause a particular reaction to occur. In diffusion,
the activation energy is related to the energy required to move an atom from one lattice site to another.
CarburizationA heat treatment for steels to harden the surface using a gaseous or solid source
of carbon. The carbon diffusing into the surface makes the surface harder and more abrasion resistant.
Concentration gradientThe rate of change of composition with distance in a nonuniform
mate-rial, typically expressed as or .
Conductive ceramicsCeramic materials that are good conductors of electricity as a result of
their ionic and electronic charge carriers (electrons, holes, or ions). Examples of such materials are stabilized zirconia and indium tin oxide.
DiffusionThe net flux of atoms, ions, or other species within a material caused by temperature and
a concentration gradient.
Diffusion bondingA joining technique in which two surfaces are pressed together at high
pres-sures and temperatures. Diffusion of atoms to the interface fills in voids and produces a strong bond between the surfaces.
Diffusion coefficient(D) A temperature-dependent coefficient related to the rate at which atoms,
ions, or other species diffuse. The diffusion coefficient depends on temperature, the composition and microstructure of the host material and also the concentration of the diffusing species.
Diffusion coupleA combination of elements involved in diffusion studies (e.g., if we are
consid-ering diffusion of Al in Si, then Al-Si is a diffusion couple).
Diffusion distanceThe maximum or desired distance that atoms must diffuse; often, the
dis-tance between the locations of the maximum and minimum concentrations of the diffusing atom.
Diffusi