Corresponding author: [email protected]
Cytotoxic activity of simvastatin in T47D
breast cancer cell lines and its effect on
cyclin D1 expression and apoptosis
Bayu Putra1, Mae Sri Hartati Wahyuningsih2*, Eti Nurwening Sholikhah2
1Postgraduate Program in Basic Medical and Biomedical Sciences, 2Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
DOI: http://dx.doi.org/10.19106/JMedSci004901201701
ABSTRACT
Statins is HMG-CoA inhibitors which used for decreasing plasma cholesterol levels and preventing coronary artery disease. Preclinical and clinical studies showed that statin could decrease the risk of cancer. This study was performed to evaluate the cytotoxic activity of simvastatin on T47D breast cancer cell lines and its effect in cyclin D1 expression and apoptosis. This was quasi experiment using post test with non-equivalent control group design. Simvastatin cytotoxic activity was evaluated using MTT assay. Furthermore, the effect of simvastatin on cyclin D1 expression and apoptosis were evaluated using low cytometry using antibody monoclonal anti-cyclin D1 and annexin V-Pi, respectively. The results showed that simvastatin had cytotoxic activity on T47D breast cancer cell lines with an IC50 value of 25.25 ± 1.61 µg/mL. Moreover, simvastatin in concentration range from 6.31 to 50.5 µg/mL decreased the cyclin D1 expression with an EC50 value of 18.96±4.42 µg/mL and induced apoptosis with an EC50 value of 26.96 ± 6.05 µg/mL. In conclusion, simvastatin inhibits T47D breast cancer cell growth through reduction of cyclin D1 expression and induction of apoptosis.
ABSTRAK
Statin (HMG-CoA inhibitor) merupakan golongan obat yang digunakan untuk menurunkan kadar kolesterol plasma dan untuk mencegah jantung koroner. Penelitian praklinik dan klinik menunjukkan bahwa terapi statin dapat menurunkan risiko terjadinya kanker. Penelitian ini bertujuan mengkaji aktivitas sitotoksik simvastatin terhadap kultur sel kanker payudara T47D dan efeknya terhadap ekspresi cyclin D1 dan apoptosis. Jenis penelitian ini adalah eksperimenal semu dengan rancangan post test with non equivalent control group. Aktivitas sitotoksi simvastatin diuji dengan metode MTT assay. Selanjutnya efek simvastatin terhadap ekspresi cyclin D1 dan apoptosis dikaji menggunakan lowcytometry menggunakan antibody monoclonal anti-cyclin D1 dan annexin V-Pi. Hasil penelitian menunjukkan simvastatin mempunyai aktivitas sitotoksik terhadap sel kanker payudara T47D dengan nilai IC50 sebesar 25,25 ± 1,61 µg/mL. Simvastatin dengan kisaran konsentrasi 6,31 sampai 50,5 µg/mL mampu menurunkan ekspresi cyclin D1 dengan nilai EC50 sebesar 18,96 ± 4,42 µg/mL dan menginduksi apoptosis dengan nilai EC50 sebesar 26,96 ± 6,05 µg/mL. Sebagai kesimpulan, simvastatin menghambat pertumbuhan sel kanker payudara T47D dengan menurunkan ekspresi cyclin D1 dan menginduksi apoptosis.
INTRODUCTION
Cancer can be deined as the rapid growth of body cells cause disorders thus growth beyond the limits of necessity and then invade and spread to other parts of the body. Breast cancer is one of the most common cancers that cause death in women in the world. In 2013 Ministry of Health, Republic of Indonesia reported that the prevalence of breast cancer was the second highest in Indonesia, approximately 0.5% of total population, with the highest prevalence in Yogyakarta Special Region (2.4%).1
Statin (HMG-CoA Inhibitors) is drugs used for decreasing cholesterol level and prevent heart coroner disease.2 In Indonesia, 82.3% patients with hypercholesterolemia still use statin as irst choice drug with the most frequently used is simvastatin (42.8%) followed by rosuvastatin (27.9%) and atorvastatin (19.2%).3 Statin use is associated with 20% risk reduction of cancer. Statin have protective effect on cancer survivors therapy for more than 4 years.4 Cohort study found that using simvastatin in patients diagnosed with breast cancer, could reduce the death rate of breast cancer.5
Furthermore, the statins such as luvastatin, atorvastatin and simvastatin can inhibit cell proliferation associated with decreasing synthesis of DNA and cell cycle arrest in G1 and G2/M by increasing the expression of p53 and p21 proteins and induces cell death with oxidative stress.6,7 Simvastatin could also induce antiproliferative effects and increase expression of caspase 3 in order to stimulate apoptosis.8,9 This study was conducted to prove the cytotoxicity of simvastatin against T47D breast cancer cell lines and its effect on cyclin D1 expression and apoptosis.
MATERIALS AND METHODS
Materials
50% (IC50) of the cell lines was determined by probit analysis.
Expression of cyclin D1 assay
The T47D breast cancer cell lines were cultured in 6 well plates at 5x105 cells per well. Each well was added with 2000 µL of each concentration series of simvastatin 0; 6.31; 12.62; 25.25 and 50.48 µg/mL, and 400 µL of lowcytometry reagent. Cells suspension was transferred into the lowcytometry-tube through ilter (nylon/glass cloth fabrics) using 1 mL micropipette. The proile of cyclin D1 expression was measured with lowcytometer and analyzed using FASC-Calibur program. Data were displayed in percentage of cyclin D1 expression in each treatment groups. The effect on cyclin D1 expression was assessed on the concentration inhibiting 50% expression (EC50) which determined by probit analysis.
Apoptosis induction assay
The T47D breast cancer cell lines were cultured at 5x105 in 6 well plates. Each well was added with 2000 µL from each concentration series of simvastatin 0; 6.31; 12.62; 25.25 and 50.48 µg/mL and doxorubicin 0.15 µg/mL. Solutions were prepared using incubation buffer 10 mM Hepes/NaOH pH 7.4; 140 mM NaCl and 5 mM CaCl2. Labeling solution for
sample consisting of 15 to 30 μL of annexin V plus 1.5 mL of incubation buffer and 30 µL of PI. Incubation cells were placed at the dark room for 10-15 minutes at a temperature of 15-25°C. Apoptosis was analyzed using FACS-Calibur program to obtain distribution of the survived cell, apoptotic and necrotized cell on all treatment group. The apoptosis induction activity was assessed on the concentration inducing 50% apoptosis (EC50) which determined by probit analysis.
Statistical analysis
Data of IC50 an EC50 were presented as mean ± SD. Data of cyclin D1 expression and apoptosis induction were presented as percentage and compared using analysis of variance (ANOVA) followed by post hoc Bonferroni or Tamhane. A p value <0.05 was considered as statistically signiicant.
RESULTS
Cytotoxic activity assay
TABLE 1. The percentage of T47D cell lines proliferation inhibition (%) and the IC50 (mean ± SD) of simvastatin and doxorubicin
Drug Concentration (µg/mL)
Replication
Mean IC50
1 2 3
Inhibition IC50 Inhibition IC50 Inhibition IC50
Simvastatin 50 89.45
27.03
90.44
24.78
91.39
23.92 25.25±1.61
25 51.28 45.73 58.52
12.5 21.25 20.66 20.92
6.3 12.68 26.45 18.60
3.1 1.98 26.22 11.76
1.6 2.46 5.56 9.06
Doxorubicin 0.25 75.71
0.17
80.53
0.14
80.15
0.15 0.15±0.016
0.13 38.51 69.17 56.64
0.06 16.19 14.67 5.14
0.03 2.46 2.74 -6.25
0.02 -10.84 -9.49 -9.02
Expression of cyclin D1 assay
The cyclin D1 expression of T47D breast cancer cell line after incubation with simvastatin is presented in FIGURE 1. At
the left side showed population of cells that did not express cyclin D1, and the right side showed population of cells that expressed cyclin D1.
FIGURE 1. Cyclin D1 expression measured with lowcytometry in (a) control group without simvastatin, (b) simvastatin 6.31 µg/mL, (c) simvastatin 12.62 µg/mL, (d) simvastatin 25.25 µg/mL (e) simvastatin 50.48 µg/mL.
1-TABLE 2. Cyclin D1 expression of T47D cells (%) after incubation with simvastatin for 24 hours.
Treatment Concentration (µg/mL) Cyclin D1 expression
(Mean ± SD %) p EC50 (µg/mL)
Control 0 65.89±0.73
0.000*
Simvastatin 6.31 59.98±1.81b 18.96±4.42
12.62 54.97±3.42b
25.25 45.01±3.96ab
50.48 25.23±13.72a
*:ANOVA; a: p<0.05, ANOVA followed by post hoc Bonferroni, compared with control; b: p<0.05, ANOVA followed by post hoc Bonferroni, compared with simvastatin 50.48 µg/mL.
Apoptosis induction assay
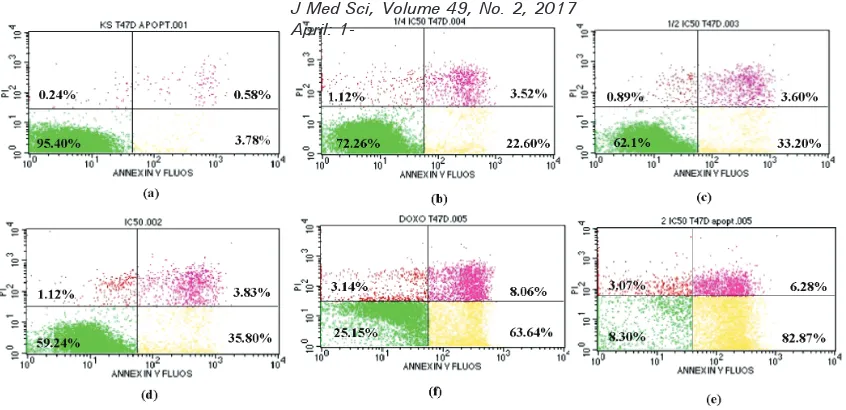
The apoptosis of T47D breast cancer cell line after incubation with simvastatin is presented in FIGURE 2. Lower left showed
live cells population, lower right showed apoptotic cells, and upper right showed necrotic cells.
FIGURE 2. Apoptosis induction measured with lowcytometry after 24 hours incubation in (a) control group without simvastatin, (b) simvastatin 6.31 µg/mL, (c) simvastatin 12.62 µg/mL, (d) simvastatin 25.25 µg/mL, (e) simvastatin 50.48 µg/mL, and (f) doxorubicin 0.15 µg/mL as positive control. Lower left: live cells pop-ulation, lower right: apoptotic cells, upper right: necrotic cells
TABLE 3 shows the T47D breast cancer cells apoptosis after incubation with simvastatin for 24 hours. The simvastatin
assay. Simvastatin increased cells apoptosis in a concentration-dependent manner with the EC50 value of 26.96 ± 6.05 µg/mL.
This inding indicated that incubation with simvastatin induces apoptosis in T47D breast cancer cells.
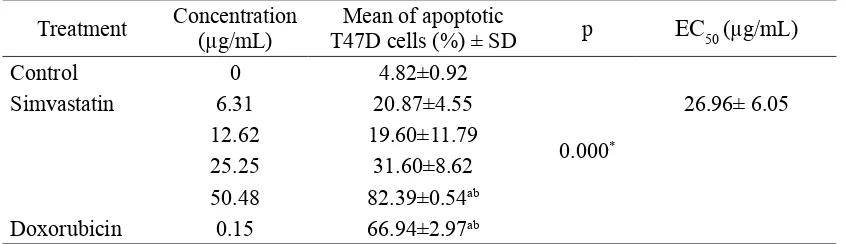
TABLE 3. Mean of apoptotic cell of T47D cells (%) after giving simvastatin or doxorubicin for 24 hours
Treatment Concentration (µg/mL) T47D cells (%) ± SDMean of apoptotic p EC50 (µg/mL)
Control 0 4.82±0.92
0.000*
Simvastatin 6.31 20.87±4.55 26.96± 6.05
12.62 19.60±11.79
25.25 31.60±8.62
50.48 82.39±0.54ab
Doxorubicin 0.15 66.94±2.97ab
*: ANOVA Test; a: p<0.05, ANOVA continued post hoc Tamhane, compared with control; b: p<0.05, ANOVA continued post hoc Tamhane, compared with simvastatin 6.31 µg/mL.
DISCUSSION
Cytotoxic activity of simvastatin
The T47D breast cancer cell lines was used in this study due to its high homogeneity and its simplicity replaceable with frozen stock contamination observed. Therefore these cells are often used for in vitro cancer studies.10 The results showed that incubation with simvastatin at concentrations from 50 to 1.5625 µg/mL (119.45-3.73 µM) for 24 hours inhibited T47D cell lines proliferation. This inding was consistent with other in vitro
studies conducted by some authors. Lee et al.11 showed that incubation with simvastatin
at concentrations from 0 to 500 µM (0-209.3 µg/mL) for 24 and 48 hours inhibited the bile duct cancer cells proliferation. The incubation with simvastatin at concentration from 15 to 120 µM (6.27 to 50.22 µg/mL) for 24 and 28 also inhibited lung cancer A549 cells proliferation.12,13 Furthermore, Huang et al. 14 reported that incubation with simvastatin at concentration from 2 to 16 µM for 24-72 hours inhibited the HepG2 and Huh7
cell line growth. The proliferation inhibition of ECC-1 and Ishikawa cell lines were also observed after incubation with simvastatin at concentration from 0.01 to 50 µM (0.0041 to 20.92 µg/mL).15
cancer cells.18 In addition, atorvastatin was active against NCI-H292 cancer cells with an IC50 value of 5.54 µg/mL20 and lovastatin was active against MDAMB468 and MDAMB231 cancer cells with IC50 values of 8 μg/mL and 5 μg/mL, respectively.21
Effect of simvastatin on cyclin D1 expression Simvastatin decreased cyclin D1 expressi-on of T47D breast cancer cell lines in a concentration-dependent manner (TABLE 2). This inding was similar with study conducted by Liang et al. 22 which showed
that simvastatin at concentration range from 12.5 to 50 µM (5.23 to 20.92 µg/mL) induced cell cycle arrest of NCI-H460 cancer cells through reduction of cyclin D1 and CDK4 expression and enhancement of P21 inhibitors CKD expression. Simvastatin also decreased cyclin D1 expression and CDK on hepatocellular cancer cell lines (Hep3B and Huh-7).23 Another study reported that simvastatin induced apoptosis in the HepG2 and Huh7 cancer cell lines and its activity was accompanied by inhibition of CDK and cyclin D1, whereas CDK inhibitors p19 and p27 were enhanced.24 Other statins such as lovastatin also could affect the cell cycle by decreased cyclin D1-CDK 4 expression and increased p21WAF1/CIP1 expression on MCF-7 cells.2
Apoptosis induction activity of simvastatin Simvastatin increased cells apoptosis in a concentration-dependent manner. This inding indicated that simvastatin induced apoptosis in T47D breast cancer cell lines. This inding similar with previous studies conducted by some authors. Gopalan et al.25
demonstrated that incubation with simvastatin at concentration range from 0.625-5.0 µM for three days induced apoptosis of MCF7 and MDA-MB-231 cancer cell line via activation
of JNK/CHOP/DR5 signaling pathway. Ghosh-Choudury et al.26 also demonstrated
that simvastatin attenuated the antiapoptotic BclXL expression and induced depression of phosphatase and tensin homologous (PTEN) expression through NFκB to inhibit breast cancer growth. In addition, Koyuturk et al.27
reported that simvastatin induced apoptosis through involvement of JNK in breast cancer cells independent of their ER or p53 expression status. Simvastatin also suppressed PI3K/Akt/mTOR pathway by enhancing PTEN expression and by further sequentially dephosphorylating downstream cascades including Akt, mTOR, p70S6K, S6RP and 4E-BP1. Furthermore, simvastatin inhibited MAPK/ERK pathway by dephosphorylating sequential cascades such as c-Raf, MEK1/2 and ERK1/2.28
CONCLUSION
Simvastatin shows cytotoxic activity on T47D breast cancer cell lines with an IC50 value of 25.25 µg/mL. Furthermore, simvastatin decrease cyclin D1 expression with an EC50 value of 18.96 µg/mL, and induce apoptosis with an EC50 value of 26.96 µg/mL.
ACKNOWLEDGEMENTS
Authors would like to thank all technicians for their valuable assistance during laboratory works.
REFERENCES
1. Kementerian Kesehatan Republik Indonesia.
Stop kanker. Jakarta: Pusat Data dan
Informasi. Kementerian Kesehatan RI, 2015. 2. Sewester CS, Dombeck C, Olin BR, Kastrup
3. Munawar M, Hartono B, Rifqi S. LDL cholesterol goal attainment in hypercholesterolemia: CEPHEUS Indonesian survey. Acta Cardiol Sin 2013; 29(1):71-81. 4. Graaf MR, Beiderbeck AB, Egberts AC,
Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol 2004; 22(12):2388-94. http://dx.doi.org/10.1200/ JCO.2004.02.027
5. Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology 2015; 26(1):68-78. http:// dx.doi.org/10.1097/EDE.0000000000000189 6. Horiguchi A, Sumitomo M, Asakuma J,
Asano T, Asano T, Hayakawa M. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, luvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin Cancer Res 2004; 10(24):8648-55. http:// dx.doi.org/10.1158/1078-0432.CCR-04-1568 7. Sanchez CA, Rodrıguez E, Varela E, Zapata
E, Paez A, Masso FA, et al. Statin-induced
inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Cancer Invest 2008; 26(7):698-707. http://dx.doi. org/10.1080/07357900701874658
8. Lee SK, Kim YC, Song SB, Kim S. Stabilization and translocation of p53 to mitochondria is linked to Bax translocation to mitochondria in simvastatin-induced apoptosis. Biochem Biophys Res Commun 2010; 391(4):1592-7. http://dx.doi.org/10.1016/j.bbrc.2009.12.077 9. Gallelli L, Falcone D, Scaramuzzino M,
Pelaia G, D’Agostino B, Mesuraca M, et
al. Effects of simvastatin on cell viability
and proinlammatory pathways in lung adenocarcinoma cells exposed to hydrogen peroxide. BMC Pharmacol Toxicol 2014; 15:67. http://dx.doi.org/10.1186/2050-6511-15-67
10. Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res 2003; 5(2):89-95. 11. Lee J, Hong EM, Jang JA, Park SW, Koh DH,
Choi MH, et al. Simvastatin induces apoptosis
and suppresses insulin-like growth factor 1 receptor in bile duct cancer cells. Gut Liver 2016; 10(2):310-7. http://dx.doi.org/10.5009/ gnl15195
12. Kim YS, Seol CH, Jung JW, Oh SJ, Hwang
KE, Kim HJ, et al. Synergistic effect of
sulindac and simvastatin on apoptosis in lung cancer A549 cells through AKT-Dependent down regulation of survivin. Cancer Res Treat 2015; 47(1):90-100. http://dx.doi. org/10.4143/crt.2013.194
13. Li Y, Fu J, Yuan X, Hu C. Simvastatin inhibits the proliferation of A549 lung cancer cells through oxidative stress and up-regulation of SOD2. Pharmazie 2014; 69(8):610-4.
14. Huang X, Ma J, Xu J, Su Q, Zhao J. Simvastatin induces growth inhibition and apoptosis in HepG2 and Huh7 hepatocellular carcinoma cells via upregulation of Notch1 expression. Mol Med Rep 2015; 11(3):2334-0. http://dx.doi.org/111(3):2334-0.3892/ mmr.2014.2976 15. Schointuch MN, Gilliam TP, Stine JE, Han
X, Zhou C, Gehrig PA, et al. Simvastatin,
an HMG-CoA reductase inhibitor, exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer. Gynecol Oncol 2014;
134(2):346-55. http://dx.doi.org10.1016/
j.ygyno.2014.05.015
16. Nurani LH, Widyarini S, Mursyidi A. Uji sitotoksik dan uji kombinasi fraksi etil asetat
ekstrak etanol akar pasak bumi (Eurycoma
longifolia Jack) dan doxorubicin pada sel
limfosit. J Trop Pharm Chem2015;
3(2):138-47.
17. Xu YJ, Yip SC, Kosela S, Fitri E, Hana M,
Goh SH, et al. Novel cytotoxic polyprenylated
18. Sławińska-Brych A, Zdzisińska B, Kandefer-Szerszeń M. Fluvastatin inhibits growth and alters the malignant phenotype of the C6 glioma cell line. Pharmacol Rep 2014;
66(1):121-9. http://dx.doi.org/10.1016/j.
pharep.2014.01.00221
19. Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res Pharm Sci 2010; 5(2):127-33.
20. Barros ALS, Aguiar JS, Araújo, LCC, Peixoto
CA, de Medeiros PL, Catanho MTJA, et al.
Synergistic anticancer effects of valproic acid, atorvastatin and pioglitazone in human malignant and murine cells. Afr J Pharm Pharmacol 2014; 8(2):31-39. http://dx.doi. org/10.5897/AJPP2013.3797
21. Klawitter J, Shokati T, Moll V, Christians U, Klawitter J. Effects of lovastatin on breast cancer cells: a proteo-metabonomic study. Breast Cancer Res 2010; 12(2):1-20. http:// dx.doi.org/10.1186/bcr2485
22. Liang YW, Chang CC, Hung CM, Chen TY, Huang TY, Hsu YC. Preclinical activity of simvastatin induces cell cycle arrest in G1 via blockade of cyclin D-Cdk4 expression in non-small cell lung cancer (NSCLC). Int J Mol Sci 2013; 14(3):5806-16. http://dx.doi. org/10.3390/ijms14035806
23. Lee SJ, Hwang JW, Yim H, Yim HJ, Woo SU, Suh SJ, et al. Synergistic effect of simvastatin
plus NS398 on inhibition of proliferation and survival in hepatocellular carcinoma cell line.
J Gastroenterol Hepatol 2014;
29(6):1299-307. http://dx.doi.org/10.1111/jgh.12503 24. Relja B, Meder F, Wilhelm K, Henrich D,
Marzi I, Lehnert M. Simvastatin inhibits cell growth and induces apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int J Mol
Med2010; 26(5):735-41.
25. Gopalan A, Yu W, Sanders BG, Kline K. Simvastatin inhibition of mevalonate pathway induces apoptosis in human breast cancer cells via activation of JNK/CHOP/ DR5 signaling pathway. Cancer Lett 2013; 329(1):9-16. http://dx.doi.org/ 10.1016/j. canlet.2012.08.031
26. Choudhury N, Mandal CC, Ghosh-Choudhury N, Ghosh-Ghosh-Choudhury G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell. Signal 2010; 22(5):749-58. http://dx.doi.org/ 10.1016/j. cellsig.2009.12.010
27. Koyuturk M, Ersoz M, Altiok N. Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent
pathway requiring signalling through JNK.
Cancer Lett 2007; 250(2):220-8. http://dx.doi. org/ 10.1016/j.canlet.2006.10.009
28. Wang T, Seah S, Loh X, Chan CW, Hartman
M, Goh BC, et al. Simvastatin-induced breast