Three proteins from the chloroplastic outer envelope membrane and four proteins from the inner envelope membrane have been identified as components of the chloroplastic protein import apparatus. Multiple molecular chaperones and a stromal processing peptidase are also important components of the import machinery. The interactions of these proteins with each other and with the precursors destined for transport into chloroplasts are gradually being described using both biochemical and genetic strategies. Homologs of some transport components have been identified in cyanobacteria suggesting that at least some of import machinery was inherited from the cyanobacterial ancestors that gave rise to chloroplasts.
Addresses
MSU-Department of Energy Plant Research Laboratory, Michigan State University, East Lansing, MI 48824-1312, USA
*e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:471–476
1369-5266/99/$ — see front matter © 1999 Elsevier Science Ltd. All rights reserved.
Abbreviations
Tic translocon at the inner membrane of chloroplasts
Toc translocon at the outer membrane of chloroplasts
Introduction
Because chloroplasts and other types of plastids contain a genome with a limited coding capacity, the large majority of proteins contained within these organelles must be imported from the cytoplasm. The chloroplastic protein import system has received considerable attention since the first reports of its existence some 20 years ago [1,2]. This brief review focuses on recent progress in the identi-fication and characterization of components of the envelope-based transport apparatus. We briefly consider interactions between these components and precursor pro-teins that occur during protein import, and we address the evolutionary origins of the import apparatus.
Figure 1 presents a schematic representation of the protein import apparatus and a working model of the interactions that occur during the transport of precursor proteins into chloroplasts. This schematic model is based on work from several research groups over the past few years; other reviews should be consulted for details and for a considera-tion of earlier work [3–6]. Components in the outer envelope membrane are known as Toc (translocon at the outer membrane of chloroplasts) proteins, whereas compo-nents in the inner membrane are called Tic (translocon at the inner membrane of chloroplasts) proteins; the number refers to their molecular mass [7]. Molecular chaperones are also involved during protein import and putative partici-pants have been identified in several locations including
the cytoplasm, the intermembrane space between the two envelope membranes, and the stromal space.
Energy, provided by the hydrolysis of nucleoside triphos-phates, is needed for at least three, and possibly more, steps during the import process. Hydrolysis of GTP is a unique requirement for chloroplastic protein import [8••,9•]; GTP is not needed for mitochondrial protein
import [10]. The chloroplastic import apparatus contains two components that have GTP-binding sites, Toc159 and Toc34, and it is assumed that they account for the GTP requirement. ATP is needed in at least two locations: the early stages of import require ATP hydrolysis in the inter-membrane space (Figure 1c) [11], and ATP hydrolysis is required in the stroma to accomplish protein translocation (Figure 1d) [12]. In each case, it is postulated, but not proven, that this ATP is used by molecular chaperones.
Outer membrane components and the early
stages of protein import
The Toc complex fulfills three essential functions during protein import: first, it specifically recognizes the transit peptide of precursor proteins; second, after precursor pro-tein binding, the complex initiates membrane translocation; and third, it participates in the formation of contact sites between inner and outer chloroplastic mem-branes. Evidence from several laboratories indicates that the Toc complex contains three membrane proteins: Toc159, Toc34, and Toc75 [13–15]. In addition to the Toc proteins, lipids have also been thought to play a crucial role in the early stages of precursor binding to chloroplasts. For instance, transit peptides have been shown to interact with the chloroplastic-specific lipids, monogalactosyldiacylglyc-erol and digalactosyldiacylglycmonogalactosyldiacylglyc-erol [16,17]. From these studies it was proposed that the early stages of precursor targeting to the chloroplasts involved a specific interaction of the transit peptide with the lipids of the outer envelope membrane (Figure 1a). Recently, this hypothesis gained further support when Chen and Li [18•] used a genetic
approach to dissect the early stages of precursor binding to chloroplasts. They showed that chloroplasts isolated from an Arabidopsis mutant deficient in digalactosyldiacylglyc-erol are defective in their ability to import precursors destined for the interior of chloroplasts. The defect in these chloroplasts appears to occur at the energy-indepen-dent binding stage of import.
After an initial interaction of precursor with the lipids of the outer envelope membrane [16,17], the precursor inter-acts with both Toc159 and Toc34 during the energy-independent stage of binding [19,20] (Figure 1b). Both of these proteins contain GTP-binding domains [21–23]. Toc159 was identified originally as an 86 kDa pro-tein [21,22], designated Toc86; however, recent studies
Protein import into chloroplasts
have concluded that Toc86 is a proteolytic fragment of the native 159 kDa protein [8••,24]. It has been proposed that
Toc159 contains a transit peptide binding site for precursor proteins. There are two pieces of evidence for this role of Toc159: antibodies against Toc159 block precursor binding to isolated chloroplasts [21], and two different precursor proteins can be cross-linked effectively to Toc159 [14,19].
The role of Toc34 during the energy-independent stage of precursor protein binding to the Toc complex has been dif-ficult to determine. Recent experiments show that Toc34 can be cross-linked to precursor protein during the early stages of binding [19]. But the presence of either GTP or ATP prevents the formation of cross-linking to Toc34. The authors conclude that Toc34 is not a part of the transit pep-tide receptor but rather plays a regulatory role in precursor binding [19].
The third component of the Toc complex that interacts with precursor protein during the early stages of binding is Toc75 [25]. The transit peptide of precursor proteins can be cross-linked to Toc75 [14,20], thus confirming a close interaction. Taken together, the cross-linking data support the hypothe-sis that Toc75 and Toc159 form a binding site that specifically binds transit peptides. Considerable evidence indicates that Toc75 also constitutes the major component of the protein-conducting channel in the outer envelope mem-brane. The most compelling evidence comes from Hinnah et al. [26], who showed that recombinant Toc75 has voltage-sensitive ion conductance properties when reconstituted into
liposomes. The reconstituted Toc75 ion channel was calcu-lated to have a small pore with a diameter of 8–9 Å. Such a small opening would require that the precursor be complete-ly unfolded during translocation across the outer membrane. They also demonstrated that this conductance was selective-ly regulated by the transit peptide of precursor protein, supporting the role of Toc75 as a transit-peptide-regulated channel [26]. However, Toc75 may not be the only compo-nent comprising the protein-conducting channel of the outer membrane. Ma et al. [14] have shown that during transloca-tion, precursor proteins can be cross-linked to regions of Toc159 other than the cytoplasmic domain. Hence, the membrane domain of Toc159 may participate in the translo-cation process, possibly as part of the translotranslo-cation channel.
Although only three integral membrane proteins have been identified as components of the Toc complex, some of them are encoded by multiple genes that show differential expression during plastid development. For example, Arabidopsiscontains two Toc34-like genes that are differen-tially expressed [27••]. The protein products of these
genes, AtToc33 and AtToc34, have 61% amino acid sequence identity to each other, and 59% and 64% amino acid sequence identity with pea Toc34, respectively. AtToc33is preferentially expressed during the early stages of seedling development, whereas AtToc34 is expressed at constitutively low levels during all stages of leaf develop-ment. Mutant plants deficient in AtToc33 are delayed in plastid development and show a persistent chlorophyll deficiency; however, they are only partially defective in Figure 1
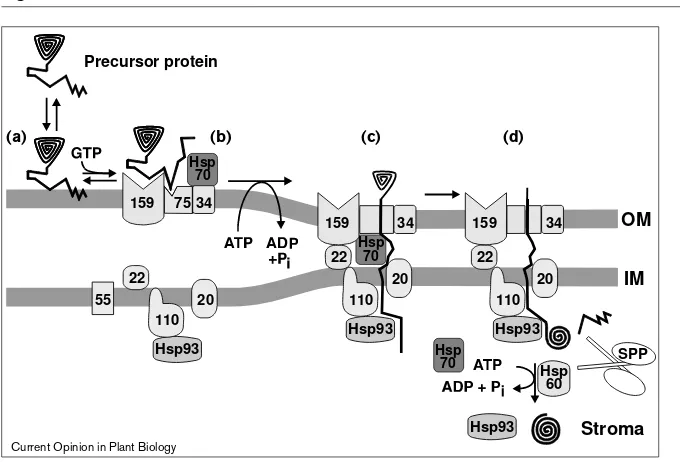
A schematic working model for protein import into chloroplasts. The numbers for the membrane components and the molecular chaperones (Hsps) represent their molecular mass. Further details about these components are given in the text. The location for the utilization of GTP is highly speculative, but the locations for the hydrolysis of ATP are based upon experimental evidence that is further described in the text. Stage (a)represents a hypothetical step where the transit peptide interacts only with the lipids of the envelope membrane. Stage (b) depicts a reversible step where the transit peptide also interacts with proteins of the transport apparatus, but prior to the hydrolysis of nucleoside triphosphates. Stage (c) depicts an early translocation intermediate, where precursor is irreversibly associated the transport apparatus, but still sensitive to exogenously added protease. Stage (d) depicts the final steps of protein transport, processing and assembly. The transit peptide located at the amino-terminus of the precursor protein is removed by the stromal processing peptidase (SPP). The differences in components present in the complexes shown in stages (b), (c), and (d) is a deliberate attempt to reflect the dynamic nature of the
composition of the translocation apparatus.
20 22
55
Hsp93
Hsp93 Hsp93 159
159 159
20 22
20 22 75 34
34 34
SPP
OM
IM
Hsp93 Hsp
60
Stroma
Hsp 70
Hsp 70
Hsp 70 110
110 110
Precursor protein
(a) (b) (c) (d)
protein import. Either AtToc34 or AtToc33 can comple-ment the mutant, demonstrating the functional redundancy of the two proteins. This work is important on several accounts. It is the first application of reverse genetics to the study of protein import into chloroplasts; furthermore, it demonstrates that some components of the transport appa-ratus are encoded by multiple genes and that these genes are differentially expressed. More work is needed to under-stand the functional significance of these multiple genes.
Although a role for GTP in protein import had been report-ed [11], the discovery that both Toc159 and Toc34 contain GTP-binding domains [21–23] required a re-evaluation of the role for GTP during import [8••,9•]. In the first stage,
the formation of early translocation intermediates is mediat-ed by Toc components that bind and hydrolyze nucleoside triphosphates. Initial interaction of the transit peptide occurs at Toc159, with the possibility of some involvement of Toc75. Further insertion of precursor protein into the import apparatus requires GTP hydrolysis. GTP hydrolysis at Toc159 and/or Toc34 may induce a conformational change in the overall Toc complex that induces the precursor to insert itself across the outer membrane through the protein-conducting channel. Therefore, two roles have been proposed for the function of GTP during import. In the first, GTP acts as a regulator for early translocation intermediate formation. In this role, Toc159 and/or Toc34 may regulate gating of the protein-conducting channel, thus ensuring the specificity of precursor binding. Through a cycle of GTP binding and hydrolysis, Toc159 and/or Toc34 may act as a switch to ensure that precursors are committed to further translocation. In the second role, GTP binding may initiate the formation of contact sites, thus forming functional translocation complexes. As the import apparatus is recon-stituted, using purified components, future investigation will begin to focus on the separate roles of Toc159 and Toc34 during the early stages of binding.
GTP hydrolysis is required for early import intermediate formation, but it is not sufficient for complete translocation across the outer membrane. The stability of early translo-cation intermediates is enhanced by ATP at low levels [11]. The presence of Hsp70 at the inner surface of the outer membrane may explain the need for ATP during early translocation intermediate formation. Young et al. [9•]
have further shown that GTP has no effect on the translo-cation steps that occur after the early import intermediate has been formed (Figure 1c,d). From this study, they con-clude that once the early import intermediates are formed, ATP hydrolysis, not GTP hydrolysis, mediates transloca-tion. This ATP is most probably utilized by stromal chaperones thought to be involved in transport across the chloroplastic envelope membranes [13,15,28].
Inner membrane components and the
formation of contact sites
Although Tic110, the first inner membrane component to be described, was identified independently by two groups
using different strategies [29,30], the two disagreed on its topology within the membrane. Jackson et al. [31] have reinvestigated this question and concluded that the large hydrophilic domain of Tic110 faces the stromal side of the inner membrane. On the basis of this topology, Tic110 is postulated to interact with stromal molecular chaperones during protein import, possibly in a manner similar to the way in which Tim44 interacts with Hsp70 in the mito-chondrial matrix [10].
Tic55, an iron-sulfur-containing protein of the inner enve-lope membrane, was identified as a translocation component based primarily on its comigration with other translocation components during blue-native polyacry-lamide gel electrophoresis [32]. However, others have not found this protein in transport complexes isolated by dif-ferent techniques [19,33••], so more work is needed to
confirm that this protein is part of the import apparatus. If Tic55 is confirmed to be a translocation component, it will be interesting to determine what role, if any, the redox center has during import.
Kouranov and Schnell [19] identified Tic22 and Tic20, two inner envelope membrane proteins, as translocation com-ponents using a chemical cross-linking strategy. More recently, Kouranov et al. [33••] isolated cDNA clones
encoding Tic22 and Tic20, provided preliminary charac-terization of these proteins, and offered hypotheses regarding their role in protein import. Tic22 is a peripher-al membrane protein associated with the outer surface of the inner envelope membrane. It does not contain readily recognizable sequence motifs, such as nucleotide binding domains, and does not possess sequence similarity to com-ponents of other transport systems; it does, however, possess 19% sequence identity over 176 amino acid residues with an open reading frame in the cyanobacteri-um Synechocystis6803 genome [34••]. Tic20 is an integral
membrane protein that is predicted to span the inner enve-lope membrane via three hydrophobic helical domains. Proteolytic digestion studies provide evidence that the amino-terminal domain, which is very basic, extends into the stroma, whereas the carboxy-terminal hydrophilic domain extends into the intermembrane space [33••].
As noted earlier, Tic22 and Tic20 were first identified as translocation components by their ability to be cross-linked to precursors during import. Kouranov et al. [33••] also used
immunoprecipitation of detergent-solubilized envelope membranes to demonstrate that a small proportion of Tic22 and Tic20 were present in complexes that also contained Tic110, Toc159, Toc75 and Toc34. Interestingly, they could not find complexes of Tic22 and Tic20 with Tic110, sug-gesting that the inner membrane components do not form complexes in the absence of association with the Toc com-plex to form putative contact sites. Kouranov et al. [33••]
enter the intermembrane space and direct them to the inner membrane import machinery. In this role, Tic22 may be the factor that connects the two envelope membranes, causing the formation of contact sites. If this is the case, one important unsolved question is what factors regulate the ability of Tic22 to mediate the apparently dynamic interac-tion between the components from the inner and outer envelope membranes.
Molecular chaperones and the translocation
process
By analogy with mitochondrial protein import where a matrix Hsp70 supports transport across the inner mem-brane [10], a stromal Hsp70 [35] was an obvious candidate for this role in chloroplasts; however, the stromal Hsp70 could not be found in translocation complexes [13,15]. Rather, translocation complexes contain Hsp93, a stromal protein in the Hsp100 family of molecular chaperones [36]. This observation has been confirmed by the recent studies of Kouranov et al. [33••]. Moreover, both groups find
evi-dence of an association between Hsp93 and Tic110, suggesting that these two proteins may have roles similar to that suggested for Hsp70 and Tim44 in mitochondria: providing the driving force for pulling proteins into the matrix [10]. Kessler and Blobel [29] also found Hsp60 asso-ciated with translocation complexes via an association with Tic110, a result that was confirmed by Kouranov et al. [33••]. Defining the role of each of the chaperones in
pro-tein translocation will require additional studies. However, it is tempting to speculate that Hsp93, and possibly Hsp70, provide the driving force for translocation across the inner envelope membrane, whereas Hsp60 is involved in folding the protein following its translocation in an unfolded state.
An Hsp70 protein present in the intermembrane space has also been identified as a component of translocation com-plexes (Figure 1c) [28]. Although this is an ideal candidate for providing the driving force for transport across the outer envelope membrane, a cDNA encoding this protein has not yet been identified. Thus, more work is needed to determine the role, if any, of this protein in the import process. Another Hsp70 associated with the surface of chloroplasts (Figure 1b) has been identified [37,38], although its role in protein import is also unclear.
Either during import or immediately afterwards, the tran-sit peptide is removed by a stromal processing protease (Figure 1d) [39]. The same metalloprotease removes the transit peptide from many different precursors, including acting on its own precursor in trans[39]. The importance of this enzyme for the import process, and consequently for the overall operation of the plant, is demonstrated by the recent generation of plants containing an antisense version of the protease gene and having reduced levels of the protein. These plants have a severe phenotype, including chlorotic leaves, reduced growth, a reduced number of plastids per cell and lower amounts of internal membranes [40•].
Evolutionary origins of the protein
import apparatus
It is widely accepted that chloroplasts evolved from cyanobacteria following an endosymbiotic event. As bacte-rial genes were transferred into the nucleus, a system was needed for delivering the proteins back into the evolving organelle. The origins of this protein transport system poses interesting questions regarding the early evolution of chloroplasts. Bölter et al. [41••] and Reumann et al. [42••]
provided evidence that at least one modern translocation component, that is Toc75, is related to a cyanobacterial outer membrane protein. Reumann et al. [42••] argued that
this cyanobacterial protein is related to a family of outer membrane proteins of unknown function whose members are widespread in the Gram-negative bacteria. This family, in turn, is related to another group of proteins involved in secretion from Gram-negative bacteria. Moreover, Bölter et al. [41••] demonstrated that the cyanobacterial protein is
capable of channel activity. Thus, it seems likely that Toc75, the putative outer membrane channel of the import system, evolved from an outer membrane channel involved in secretion from the free-living cyanobacterium. More recently, Reumann and Keegstra [34••] presented evidence
that both Tic22 and Tic20 have cyanobacterial homologs, whereas Toc159, Toc34/33 and Tic110 do not. They con-clude that the protein import apparatus has a dual origin, with some components deriving from cyanobacteria, possi-bly from an ancient protein secretion mechanism, and other components having been added to the system from other sources during the course of evolution.
Conclusions and prospects
Considerable progress has been made in recent years in identifying the components of the chloroplastic protein import system. Although it is difficult to know how many components are required for the import apparatus, it is rea-sonable to argue that the majority of the transport proteins have been identified and that relatively few additional components will be found. However, at least in Arabidopsis, several components are encoded by a small family of related genes ([27••]; D Jackson, K Keegstra,
unpublished observations). Thus, it will be important to determine the functional and developmental significance of these various family members.
define various partial reactions and reconstitute them using purified components.
A second important strategy will be the use of genetics or reverse genetics to suppress or eliminate a particular com-ponent, following on the important work of Jarvis et al. [27••], who identified Arabidopsis plants lacking Toc33. With
the new availability of several strategies for isolating plants with disruptions in a particular gene of interest, approaches similar to those employed by Jarvis et al. [27••] for Toc33 will
certainly be applied to other transport components. An alter-native is to use the antisense method, as illustrated by the work of Wan et al. [40•] with plants containing reduced
lev-els of the stromal processing protease. The availability of reverse genetic approaches and the power of in vitro recon-stitution assays should lead to models for the mechanism of protein import showing much more detail than the rather crude scheme shown in Figure 1.
Acknowledgements
The authors thank Danny Schnell for making available unpublished information from his laboratory. Work from the authors’ laboratory was supported by grants from the Cell Biology Program at the National Science Foundation and from the Energy Biosciences Program at the Department of Energy.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Chua N-H, Schmidt GW: Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase.Proc Natl Acad Sci USA1978, 75:6110-6114.
2. Highfield PE, Ellis RJ: Synthesis and transport of the small subunit of chloroplast ribulose bisphosphate carboxylase.Nature1978, 271:420-424.
3. Chen X, Schnell DJ: Protein import into chloroplasts. Trends Cell Biol1999, 9:222-227.
4. Keegstra K, Cline K: Protein import and routing systems of chloroplasts.Plant Cell1999, 11:557-570.
5. Schnell DJ: Protein targeting to the thylakoid membrane. Annu Rev Plant Physiol Plant Mol Biol1998, 49:97-126.
6. Soll J, Tien R: Protein translocation into and across the chloroplastic envelope membranes.Plant Mol Biol1998, 38:191-207.
7. Schnell DJ, Blobel G, Keegstra K, Kessler F, Ko K, Soll J:
A consensus nomenclature for the protein-import components of the chloroplast envelope.Trends Cell Biol1997, 7:303-304.
8. Chen K, Chen X, Schnell DJ: The roles of the Toc GTPases in
•• protein import into chloroplasts.Plant Phys 2000, in press. The authors demonstrate that Toc86 results from the proteolysis of a native 159 kDa protein, designated Toc159. In addition, when the GTPase domain of Toc159 was selectively removed by controlled proteolysis, binding of pre-cursor protein was reduced significantly while translocation through the pro-tein-conducting channel was reduced but not abolished. Nevertheless, translocation still remains sensitive to GTP analogs even in the absence of the Toc159 GTP-binding domain, suggesting that Toc34 may play a critical role in regulating translocation by GTP.
9. Young ME, Keegstra K, Froehlich JE: GTP promotes formation of
• early import intermediates but is not required during the translocation step of protein import into chloroplasts. Plant Physiol1999, 121:237-244.
Chromatographically purified GTP analogs were used to examine the need for GTP hydrolysis during early and late stages of protein import. The authors demonstrate that GTP hydrolysis is important during the events leading to
the formation of early-import intermediates, but GTP does not play a role dur-ing the translocation of precursors from the intermediate state.
10. Pfanner N, Craig EA, Hönlinger A: Mitochondrial preprotein translocase.Annu Rev Cell Dev Biol1997, 13:25-51.
11. Olsen LJ, Keegstra K: The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space.J Biol Chem1992, 267:433-439.
12. Theg SM, Bauerle C, Olsen LJ, Selman BR, Keegstra K: Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes.J Biol Chem1989, 264:6730-6736.
13. Akita M, Nielsen E, Keegstra K: Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking.J Cell Biol1997, 136:983-994.
14. Ma YK, Kouranov A, LaSala SE, Schnell DJ: Two components of the chloroplast protein import apparatus, IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope.J Cell Biol1996, 134:315-327.
15. Nielsen E, Akita M, Davila-Aponte J, Keegstra K: Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone.EMBO J1997, 16:935-946.
16. Pinnaduwage P, Bruce BD: In vitrointeraction between a chloroplast transit peptide and chloroplast outer envelope lipids is sequence-specific and lipid class-dependent.J Biol Chem1996, 271:32907-32915.
17. Van’t Hof R, Van Klompenburg W, Pilon M, Kozubek A,
De Korte-Kool G, Demel RA, Weisbeek PJ, de Kruijff B: The transit sequence mediates the specific interaction of the precursor of ferredoxin with chloroplast envelope membrane lipids.J Biol Chem1993, 268:4037-4042.
18. Chen LJ, Li HM: A mutant deficient in the plastid lipid DGD is
• defective in protein import into chloroplasts.Plant J1998, 16:33-39.
Chloroplasts isolated from an Arabidopsis mutant deficient in digalactosyl-diacylglycerol (DGD), were shown to be defective in importing precursor proteins into chloroplasts. The defect in these chloroplasts appears to occur at the energy-independent binding stage of import. This genetic approach provides supporting evidence for the importance of chloroplastic lipids in the precursor import.
19. Kouranov A, Schnell DJ: Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts.J Cell Biol1997, 139:1677-1685.
20. Perry SE, Keegstra K: Envelope membrane proteins that interact with chloroplastic precursor proteins.Plant Cell1994, 6:93-105.
21. Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J: A receptor component of the chloroplast protein translocation machinery. Science1994, 266:1989-1992.
22. Kessler F, Blobel G, Patel HA, Schnell DJ: Identification of two GTP -binding proteins in the chloroplast protein import machinery. Science1994, 266:1035-1039.
23. Seedorf M, Waegemann K, Soll J: A constituent of the chloroplast import complex represents a new type of GTP-binding protein. Plant J1995, 7:401-411.
24. Bölter B, May T, Soll J: A protein import receptor in pea chloroplasts, Toc86, is only a proteolytic fragment of a larger polypeptide.FEBS Lett1998, 441:59-62.
25. Tranel PJ, Froehlich J, Goyal A, Keegstra K: A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway.EMBO J1995, 14:2436-2446.
26. Hinnah SC, Hill K, Wagner R, Schlicher T, Soll J: Reconstitution of a chloroplast protein import channel.EMBO J 1997, 16:7351-7360.
27. Jarvis P, Chen LJ, Li HM, Pete CA, Fankhauser C, Chory J: An
•• Arabidopsismutant defective in the plastid general protein import apparatus.Science1998, 282:100-103.
capac-ity to import precursors and at least one precursor, NADPH:protochloro-phyllide oxidoreductase (POR), accumulates in vivo in mutant plants. 28. Schnell DJ, Kessler F, Blobel G: Isolation of components of the
chloroplast protein import machinery.Science1994, 266:1007-1012.
29. Kessler F, Blobel G: Interaction of the protein import and folding machineries in the chloroplast.Proc Natl Acad Sci USA1996, 93:7684-7689.
30. Lübeck J, Soll J, Akita M, Nielsen E, Keegstra K: Topology of IEP110, a component of the chloroplastic protein import machinery present in the inner envelope membrane.EMBO J 1996, 15:4230-4238.
31. Jackson DT, Froehlich JE, Keegstra K: The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment.J Biol Chem1998, 273:16583-16588.
32. Caliebe A, Grimm R, Kaiser G, Lübeck J, Soll J, Heins L: The chloroplastic protein import machinery contains a Rieske-type iron-sulfur cluster and a mononuclear iron-binding protein. EMBO J1997, 16:7342-7350.
33. Kouranov A, Chen XJ, Fuks B, Schnell DJ: Tic20 and Tic22 are new
•• components of the protein import apparatus at the chloroplast inner envelope membrane.J Cell Biol1998, 143:991-1002. Expanding upon the label-transfer cross-linking approach of Kouranov and Schnell [19] and Perry and Keegstra [20], the authors investigated the inter-action of a precursor protein with the chloroplastic protein import apparatus. The authors identified two new components of the import apparatus, Tic22 and Tic20, that are involved in protein translocation across the inner mem-brane. cDNA clones were isolated for two new import components from the inner envelope membrane. Tic20 is an integral membrane protein and may form part of the protein translocating channel in the inner envelope mem-brane. Tic22 is a peripheral membrane protein associated with the outer sur-face of the inner envelope membrane and may be involved in the formation of contact sites.
34. Reumann S, Keegstra K: The endosymbiotic origin of the protein
•• import machinery from chloroplastic envelope membranes. Trends Plant Sci1999, 8:302-307.
Sequence analysis programs were used to identify putative homologs of genes encoding protein import components in a cyanobacterial genome. Because not all components have homologs in cyanobacteria, the authors argue that the import apparatus has a dual origin with some components being inherited from cyanobacteria and other components arising from other origins, probably dur-ing the evolutionary pathway leaddur-ing to modern chloroplasts.
35. Marshall JS, DeRocher AE, Keegstra K, Vierling E: Identification of heat shock protein Hsp70 homologues in chloroplasts.Proc Natl Acad Sci USA1990, 87:374-378.
36. Schirmer EC, Glover JR, Singer MA, Lindquist S: Hsp100/Cpl proteins: A common mechanism explains diverse functions. Trends Biochem Sci1997, 21:289-296.
37. Ko K, Bornemisza O, Kourtz L, Ko ZW, Plaxton WC, Cashmore AR: Isolation and characterization of a cDNA clone encoding a cognate 70-kDa heat shock protein of the chloroplast envelope. J Biol Chem1992, 267:2986-2993.
38. Kourtz L, Ko K: The early stage of chloroplast protein import involves Com70.J Biol Chem1997, 272:2808-2813.
39. Richter S, Lamppa GK: A chloroplast processing enzyme functions as the general stromal processing peptidase.Proc Natl Acad Sci USA1998, 95:7463-7468.
40. Wan JX, Bringloe D, Lamppa GK: Disruption of chloroplast
• biogenesis and plant development upon down-regulation of a chloroplast processing enzyme involved in the import pathway. Plant J1998, 15:459-468.
Plants containing the gene for the chloroplast processing enzyme in an anti-sense orientation had reduced levels of this protein and reduced levels of the processing enzyme activity. These plants had severe phenotypes includ-ing chlorotic leaves, stunted growth, reduced numbers of chloroplasts and aberrant chloroplast morphology. Chloroplasts isolated from these plants had severely reduced ability to import precursors, thereby implicating the processing enzyme directly in the import process.
41. Bölter B, Soll J, Schulz A, Hinnah S, Wagner R: Origin of a
•• chloroplast protein importer.Proc Natl Acad Sci USA1998, 95:15831-15836.
A homolog of Toc75was identified in a cyanobacterial genome. Experimental evidence demonstrated the cyanobacterial protein, SynToc75, was present in the outer envelope membrane. Reconstitution of SynToc75 into liposomes produced a voltage-gated channel. These data plus the data from [42••] pro-vide the first epro-vidence that one of the chloroplastic protein import compo-nents was derived from the cyanobacterial ancestor of chloroplasts.
42. Reumann S, Davila-Aponte J, Keegstra K: The evolutionary origin of
•• the protein-translocating channel of chloroplastic envelope membranes: identification of a cyanobacterial homolog. Proc Natl Acad Sci USA1999, 96:784-789.