www.elsevier.com / locate / bres
Research report
The over-expression of somatostatin in the gerbil entorhinal cortex
induced by seizure
a a b b a
Tae-Cheon Kang , Seung-Kook Park , Seon-Gil Do , Jun-Gyo Suh , Seung Mook Jo ,
b c a ,
*
Yang-Seok Oh , Young-Gil Jeong , Moo Ho Won
a
Department of Anatomy, Collage of Medicine, Hallym University, Chunchon, Kangwon-Do 200-702, South Korea b
Experimental Animal Center, College of Medicine, Hallym University, Chunchon, Kangwon-Do 200-702, South Korea c
Department of Anatomy, College of Medicine, Konyang University, Nonsan 320-711, South Korea Accepted 8 August 2000
Abstract
In present study, we investigated the immunohistochemical distribution of somatostatin (SRIF) in the hippocampal complex of the Mongolian gerbil and its association with different sequelae of spontaneous seizures, in an effort to identify the roles of SRIF in the
1
self-recovery mechanisms in these animals. In the dentate gyrus and subiculum, SRIF immunoreactive (SRIF ) cells were similar in both the seizure resistant and the pre-seizure group of seizure sensitive gerbils. Interestingly, SRIF immunoreactivity was markedly decreased until 12 h postictal. Twenty-four hours after the on-set of seizure, the distribution of SRIF immunoreactivity in these regions had slightly
1
increased. In contrast, in the entorhinal cortex the population of SRIF cells and their density were significantly elevated compared to
1
pre-seizure group 30 min postictal. Twelve hours after the on-set of seizure, however, the population of SRIF cells and their density declined, approximately 70–80% compared to the situation at 30 min postictal. These findings suggest that the enhancement of SRIF expression in gerbil entorhinal cortex may affect tissue excitability and have a role in modulating recurrent excitation following seizures.
2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Peptides: anatomy and physiology
Keywords: Somatostatin; Hippocampus; Dentate hilus; Subiculum; Mongolian gerbil; Epilepsy
1. Introduction expression of various neuropeptides in distinct forebrain areas and their release from neurons, and the density The Mongolian gerbil provides an opportunity for and / or affinity of their receptor subtypes are profoundly studying the mechanisms of epileptogenesis, because natu- changed by seizures. Many previous studies have indicate rally epileptic and non-epileptic animals can be compared that somatostatin (SRIF) containing neurons in the hip-directly to detect differences in brain anatomy and elec- pocampus play an important role in hippocampal ex-trophysiology that correlate with seizure behavior. In citability in epilepsy, because: (i) SRIF is preferentially particular, these animals also provide an opportunity to released from neurons during seizures [1,9,28]; (ii) Marked investigate self-recovery mechanisms after seizure on-set changes in the expression of SRIF mRNA, the levels of the
[3,13,17]. peptide and its receptors occur after experimentally
in-Recent studies [8,12,29] have demonstrated that seizure duced seizures and in human epileptic tissue; and (iii) activity in experimental models leads to dramatic changes intracerebral injections of SRIF, its analogues or SRIF-in the phenotypic capacities of different classes of neurons, specific antibodies affect seizures and epileptogenesis in particularly in the hippocampus. It is noteworthy that the rats (reviews [21,29]).
In the hilus of the dentate gyrus, distinct populations of peptidergic neurons are affected by seizures and
seizure-*Corresponding author. Fax:182-361-256-1614.
E-mail address: [email protected] (M.H. Won). induced cell damage. A subset of GABAergic interneurons
containing SRIF are highly vulnerable to damage induced removed, post-fixed in the same fixative for 4 h and rinsed by sustained stimulation of the perforant path or by in PB containing 30% sucrose at 48C for 2 days. Thereafter kainate-induced seizures in rats [24–26] and in human the tissues were frozen and sectioned with a cryostat at 30 temporal lobe epilepsy specimens [19]. The same neurons, mm and consecutive sections were collected in six-well however, show increased SRIF after repeated electro- plates containing phosphate buffered saline (PBS). These convulsive shocks and enhanced mRNA expression in free-floating sections were first incubated with 10% normal kindled rat [2,16,23]. Furthermore, in the Mongolian goat serum for 30 min at room temperature. They were gerbil, altered SRIF expression has not been definitively then incubated in the rabbit anti-SRIF antiserum (diluted determined; Wolf-Dieter et al. [31] reported that SRIF 1:2000, Peninsula, USA), which not cross-react with pro-levels elevated in a seizure sensitive group, though Buc- SRIF, in PBS containing 0.3% triton X-100 and 2% kmaster et al. [4] observed that the number of SRIF normal goat serum overnight at room temperature. After
1
immunoreactive (SRIF ) neurons in the dentate gyrus was washing three times for 10 min with PBS, sections were not significantly different in seizure resistant and seizure incubated sequentially, in goat anti-rabbit IgG (Vector, sensitive groups. Therefore, in the present study, we USA) and streptavidin (Vector, USA), diluted 1:200 in the investigated the immunohistochemical distribution of SRIF same solution as the primary antiserum. Between the in the hippocampal complex of the Mongolian gerbil ant incubations, the tissues were washed with PBS three times its association with different sequelae of spontaneous for 10 min each. The sections were visualized with DAB in seizure to identify the roles of SRIF in the recovery 0.1 M Tris buffer and mounted on the gelatin-coated
mechanisms in these animals. slides. The immunoreactions were observed under the
Axioscope microscope (Carl Zeiss, Germany). In order to establish the specificity of the immunostaining, a negative 2. Materials and methods control test was carried out with pre-immune serum instead of primary antibody. The negative control resulted in the
2.1. Experimental animals absence of immunoreactivity in any structures.
This study utilized the progeny of Mongolian gerbils 2.3. Quantitation of data and statistical analysis (Meriones unguiculatus) obtained from Experimental
Ani-mal Center, Hallym University, Chunchon, South Korea. Cell counting was performed on the sections incubated The animals were housed at constant temperature (238C) for immunohistochemistry to determine the relative
num-1 1
and relative humidity (60%) with a fixed 12 h light / dark ber of SRIF neurons per section. SRIF somata were cycle and free access to food and water. Procedures counted in each group of the hippocampal complex. Cell involving animals and their care were conducted in con- counts were carried out with a computerized image analy-formity with the institutional guidelines that are in com- sis system (Leica image scale). Sections were viewed pliance with international laws and policies (NIH Guide through a microscope connected via CCD camera to a PC for the Care and Use of Laboratory Animals, NIH Publi- monitor. At a maginification of 25–503, the region was
cation No. 85-23, 1985). outlined on the monitor and measured their area. Neurons
2
Each animal was tested a minimum of three times as per-mm were counted by clicking on their image on the described by Paul et al. [17]. Only animals with a monitor, at a magnification of 1003. Images of SRIF consistent stage four or five seizure score, according to the immunoreactivity in hippocampal complex were also seizure severity rating scale of Loskota et al. [15], were captured with an Applescanner. The brightness and con-included in the present study as SS gerbils. SR gerbils trast of each image file were uniformly enhanced by Adobe never demonstrated the seizure activity, thus they were Photoshop version 2.4.1, followed by analysis using NIH assigned seizure severity scores of zero. Image 1.59 software. Values of background staining were obtained and subtracted from the SRIF immunoreactive 2.2. Tissue processing and immunohistochemistry intensities.
All data obtained from the quantitative data were Fifty seizure sensitive (SS) and ten seizure resistant analyzed using one-way ANOVA test to determine statisti-(SR) gerbils (about 8-months-old) were used in the present cal significance. Bonferroni’s test was used for post-hoc experiment. To examine the temporal changes of SRIF comparisons. P-value below 0.01 or 0.05 was considered expression following seizure, SS gerbils were divided into statistically significant.
five groups; pre-seizure group (n510), post-seizure group I, II, III and IV (n510, respectively) that recovered
normally at 30 min, 12, 24 or 36 h after the on-set of 3. Results tonic–clonic generalized seizure, respectively [12]. The
gerbils were anesthetized with pentobarbital sodium, and 3.1. Hippocampus proper perfused via the ascending aorta with 200 ml of 4%
1
proper. Its immunoreactivity was highly localized within post-seizure groups, however, SRIF immunoreactivity was the cell bodies and extened throughout their short pro- weaker in post-seizure group I (P,0.01). In post-seizure
1 2
cesses. On the basis of their localization and morphology group II, the number of SRIF cells (4.362.11 per-mm ) these cells were identified as interneurons. Their mor- had significantly declined due to loss of SRIF immuno-phologies, population and immunodensities were similar in reactivity in the neurons (Figs. 1C, 4 and 5). The density
1
all groups (data not shown). of SRIF immunoreactivity and the number of SRIF cells
2
(7.463.23 per-mm ) in post-seizure group III were slightly
3.2. The dentate gyrus elevated compared to post-seizure group II (Fig. 1D).
Thirty-six hours after seizure on-set, the SRIF density
1
In the present study, SRIF cells in the dentate hilus recovered to the SS level (Figs. 4 and 5). were usually observed with multiple processes. In the
pre-seizure group of SS gerbils, neurons in the dentate 3.3. The subiculum hilus, were strongly stained for SRIF, than was the case in
1 1
SR group (Figs. 1A,B and 5). The populations of SRIF In the subiculum, SRIF cells were similar pattern to neurons were similar in these two groups, 17.966.08 and hippocampus proper, usually with short multiple processes.
2
18.264.07 per-mm , respectively (Fig. 4). The distribution There were no differences in the SRIF immunoreactivity of
1
pattern of SRIF neurons was similar in pre-seizure and the SR and pre-seizure group of SS gerbils (Fig. 2A). The
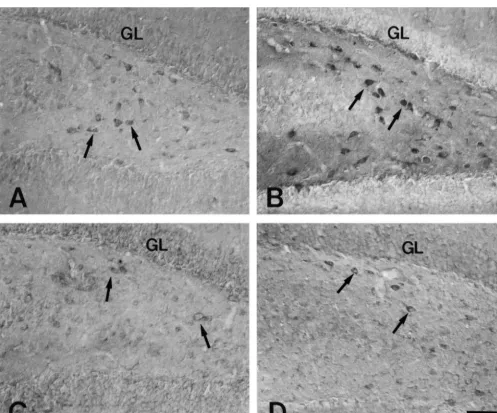
Fig. 1. Showing coronal sections of the dentate hilus of SR and SS gerbils. Compared to the case of SR (A), neurons in the dentate hilus, are strongly
1
1 2
population of SRIF cells per-mm were 11.164.01 and
1
12.264.91, respectively (Fig. 4). Interestingly, the SRIF immunoreactivities were markedly decreased in post-seizure groups I and II (Fig. 2B and 5). The population of
1
SRIF cells was also declined, 9.463.21 and 1.362.19 2
per-mm , respectively (Fig. 4). In these groups of SS gerbils, neurons in the subiculum were nearly devoid of SRIF immunoreactivity. In post-seizure group III, the distribution of SRIF immunoreactivity in the subiculum was slightly increased, thus the pattern of SRIF immuno-reactivity observed in the subiculum was similar to that of the dentate hilus (Figs. 2C, 4 and 5).
3.4. The entorhinal cortex
1
In the entorhinal cortex, SRIF neurons were densely localized in layers II–V with multiple processes. The
1
numbers of SRIF cells (15.263.99 and 14.264.24 per-2
mm , respectively) and their density in SR and pre-seizure groups were similar (Fig. 3A and B). In the post-seizure
2
group I, the population (36.267.87 per-mm ) and density
1
of SRIF cells were significantly elevated compared to pre-seizure group (Figs. 3C, 4 and 5). In post-seizure groups II and III of SS gerbils, however, the population of
1 2
SRIF cells (14.565.23 and 9.364.31 per-mm , respec-tively) in the EC had significantly decreased. Particularly,
1
in post-seizure group III the population of SRIF cells decreased by approximately 70–80% of post-seizure group I (Figs. 3D, 4 and 5). Thirty-six hours after seizure on-set, the level of SRIF density has recovered to the SS level.
4. Discussion
Many previous studies [18,20–22,29] in the hippocam-pal complex have indeed shown that SRIF has an inhib-itory action on the spontaneous activity of pyramidal cells, and that long-duration pressure ejection or bath application of the peptide induces dendritic hyperpolarization. The stimulation of SRIF receptors in the hippocampus sup-pressed chronic susceptibility to metrazole-induced convul-sions in kainic acid-treated rats. Furthermore, seizure-induced damage to SRIF neurons in the dentate hilus has been demonstrated in several experimental models of epilepsy [5,10,22,25–27,32].
In the present study, the similar distribution pattern of
1
SRIF neurons was observed, with the exception of the intensity of SRIF immunoreactivity in the pre-seizure group, and in the dentate gyrus and the subiculum of both SR and pre-seizure groups. These results are consistent with previous studies, which reported population
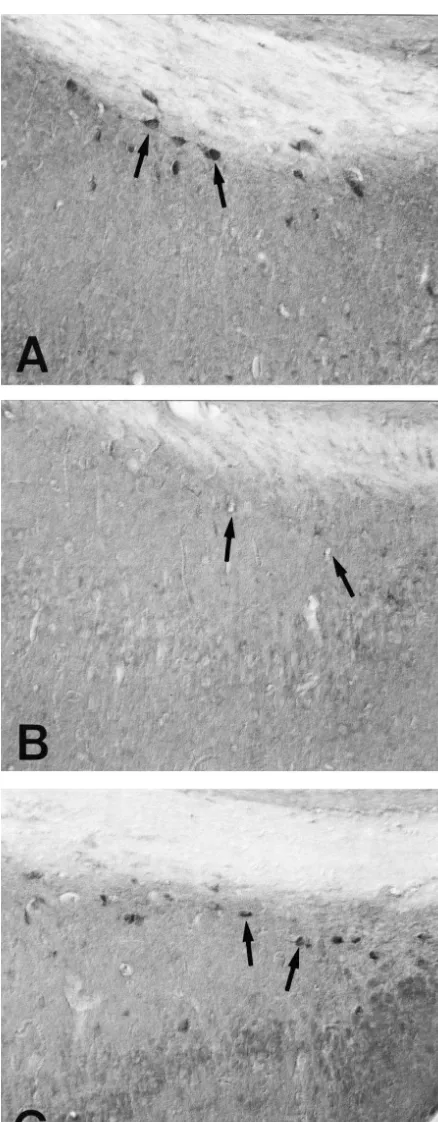
simi-Fig. 2. Showing coronal sections of the subiculum of SR (A) and SS larities in these regions in both SR and SS gerbils [4] and gerbils (B and C). Similar to the dentate gyrus, At 12 h postictal, the the enhancement of its expression in the surviving SRIF
1
number of SRIF cells (arrows) is significantly declined due to the loss of
neurons [26,27].
SRIF immunoreactivity in the neurons (B). At 36 h after the seizure
In our study a decline of SRIF immunoreactivity in the
on-set, the level of SRIF density recovers at the SR level (C). Bar530
1
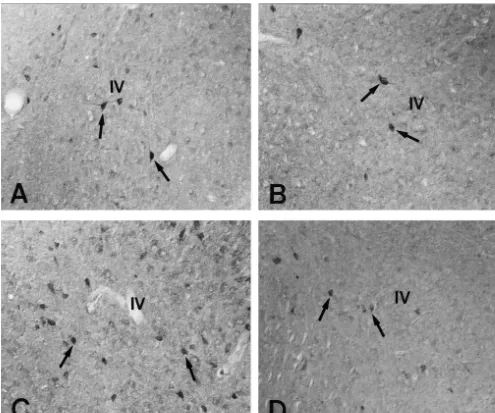
Fig. 3. Showing coronal sections of the entorhinal cortex of SR and SS gerbils. The numbers of SRIF cells and their density in SR and pre-seizure groups
1
are similar to each other (A and B). In the post-seizure group I, the population of SRIF cells is significantly elevated compared to pre-seizure group (C).
1
In the post-seizure groups III of SS gerbils, however, the population of SRIF cells in the entorhinal cortex is significantly decreased (D). IV: layer IV in the entorhinal cortex, respectively. Bar530mm.
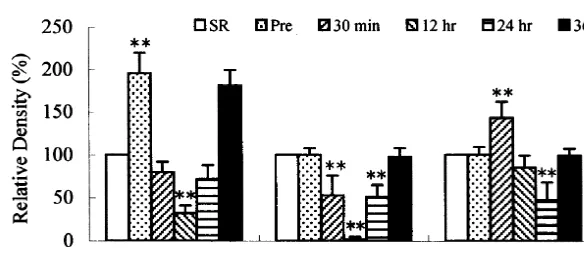
1 1
Fig. 4. The quantitative analysis of SRIF neurons in the hippocampal complex after the on-set of seizure. The increase of SRIF cells is statistically
1
significant in the entorhinal cortex of post-seizure group I, contrary to the decline of SRIF neurons in both dentate gyrus and subiculum. At 36 h after the
1
seizure on-set, the population of SRIF neurons recovers at the SR level (*P,0.05, **P,0.01, significant differences from seizure-resistant gerbils).
1 1
Fig. 5. The relative SRIF immunodesity in the hippocampal complex compared to SR gerbils. The increase of SRIF immunodensity is statistically
1
significant in the dentate gyrus of pre-seizure group. At 30 min postictal, SRIF immunodensity declines in both dentate gyrus and subiculum, but not entorhinal cortex. At 36 h after the seizure on-set, the density of SRIF recovers at the control level (*P,0.05, **P,0.01, significant differences from seizure-resistant gerbils).
hippocampus, is under strong local inhibitory control and Acknowledgements layer III projects into the subiculum and the CA1 neurons
where it evokes predominant inhibitory effects by pre- The authors would like to thank Mr. Suek Han and sumably stimulating GABA interneurons [7,11]. Kyung Jin Lee for their technical help on the illustrations. Therefore, these findings postulate a hypothesis that, in This work was supported by Korea Research Foundation the intrinsic and output connections of the hippocampal Grant (KRF-99-003-F00014).
complex, the rapid SRIF release serves a primary inhib-itory function which down-regulates the seizure activity and output signals from the hippocampus. Moreover, this
inhibitory control might provide an opportunity to enhance References SRIF synthesis in the entorhinal cortex, prior to inhibitory
modulation in the perforant path during epileptogenesis. [1] T. Bartfai, K. Iverfeldt, G. Fisone, P. Serfozo, Regulation of the release of coexisting neurotransmitters, Annu. Rev. Pharmacol.
Further research is needed to confirm this hypothesis.
Toxicol. 28 (1988) 285–310.
In conclusion, we suggest that the enhancement of SRIF
[2] C. Bendotti, A. Vezzani, G. Tarizzo, R. Samanin, Increased
expres-expression in gerbil entorhinal cortex may affect tissue sion of GAP-43, somatostatin and neuropeptide Y mRNA in the excitability and have a role in modulating recurrent hippocampus during development of hippocampal kindling in rats,
[3] J.R. Buchhalter, Animal models of inherited epilepsy, Epilepsia 34 [19] R.J. Robbins, M.L. Brines, J.H. Kim, T. Adrian, N. de Lanerolle, S. (Suppl. 3) (1993) 31–41. Welsh, D.D. Spencer, A selective loss of somatostatin in the [4] P.S. Buckmaster, E. Tam, P.A. Schwartzkroin, Electrophysiological hippocampus of patients with temporal lobe epilepsy, Ann. Neurol.
correlates of seizure sensitivity in the dentate gyrus of epileptic 29 (1991) 325–332.
juvenile and adult gerbils, J. Neurophysiol. 76 (1996) 2169–2180. [20] H.E. Scharfman, Presynaptic and postsynaptic actions of somato-[5] N.C. De Lanerolle, J.H. Kim, R.J. Robbins, D.D. Spencer, Hip- statin in area CA1 and the dentate gyrus of rat and rabbit pocampal interneuron loss and plasticity in human temporal lobe hippocampal slices, in: T.V. Dunwiddie (Ed.), Presynaptic Receptors epilepsy, Brain Res. 495 (1989) 387–395. in the Mammalian Brain, Birkhauser, Boston, 1993, pp. 42–70. [6] T. Deller, C. Leranth, Synaptic connections of neuropeptide Y [21] C. Schwarzer, G. Sperk, R. Samanin, M. Rizzi, M. Gariboldi, A.
(NPY) immunoreactive neurons in the hilar area of the rat hip- Vezzani, Neuropeptides-immunoreactivity and their mRNA expres-pocampus, J. Comp. Neurol. 300 (1990) 433–447. sion in kindling: functional implications for limbic epileptogenesis, [7] R. Empson, U. Heinemann, The perfornat path projection to Brain Res. Brain Res. Rev. 22 (1996) 27–50.
hippocampal area CA1 in the rat hippocampal–entorhinal cortex [22] C. Schwarzer, J.M. Williamson, E.W. Lothman, A. Vezzani, G. combined slice, J. Physiol. 4843 (1995) 707–720. Sperk, Somatostatin, neuropeptide Y, neurokinin B and [8] C. Gall, J. Lauterborn, P. Isackson, J. White, Seizures, neuropeptide cholecystokinin immunoreactivity in two chronic models of
tempo-regulation, and mRNA expression in the hippocampus, Prog. Brain ral lobe epilepsy, Neuroscience 69 (1995) 831–845.
Res. 83 (1990) 371–390. [23] H. Shinoda, N.S. Nadi, J.P. Schwartz, Alterations in somatostatin ¨
[9] T. Hokfelt, Neuropeptides in perspective: the last 10 years, Neuron 7 and proenkephalin mRNA in response to a single amygdaloid (1991) 867–879. stimulation versus kindling, Brain Res. Mol. Brain Res. 11 (1991) [10] J. Jolkkonen, E. Jolkkonen, A. Pitkanen, Seizure-induced damage to 221–226.
somatostatin–immunoreactive neurons in the rat hippocampus is [24] R.S. Sloviter, D.H. Lowenstein, Heat shock protein expression in regulated by fimbria–fornix transection, Exp. Neurol. 145 (1997) vulnerable cells of the rat hippocampus as an indicator of
excitation-141–153. induced neuronal stress, J. Neurosci. 12 (1992) 3004–3009.
[11] R.S.G. Jones, J.D.C. Lambert, Entorhinal–hippocampal connections: [25] R.S. Sloviter, Decreased hippocampal inhibition and a selective loss a speculative view of their function, Trend. Neurosci. 16 (1990) of interneurons in experimental epilepsy, Science 235 (1987) 73–76. 58–64. [26] G. Sperk, J. Marksteiner, B. Gruber, R. Bellmann, M. Mahata, M. [12] T.-C. Kang, S.H. Park, S.K. Park, J.C. Lee, S.M. Jo, S.G. Do, J.G. Ortler, Functional changes in neuropeptide Y- and somatostatin-Suh, Y.S. Oh, J.Y. Lee, M.H. Won, The temporal and spatial containing neurons induced by limbic seizures in the rat, Neuro-expression of nueropeptide Y induced by seizure in the hippocampal science 50 (1992) 831–846.
complex of gerbil, Brain Res. 870 (2000) 179–184. [27] G. Sperk, R. Wieser, R. Widmann, E.A. Singer, Kainic acid induced [13] P.R. Laming, R.W. Elwood, P.M. Best, Epileptic tendencies in seizures: changes in somatostatin, substance P and neurotensin,
relation to behavioral responses to a novel environment in the Neuroscience 17 (1986) 1117–1126.
Mongolian gerbil, Behav. Neural Biol. 51 (1989) 92–101. [28] A. Vezzani, R. Ruiz, A. Monno, M. Rizzi, N. Lindefors, R. Samanin, [14] C. Leranth, R. Nitsch, T. Deller, M. Frotscher, Synaptic connections E. Brodin, Extracellular somatostatin measured by microdialysis in of seizure-sensitive neurons in the dentate gyrus, Epilepsy Res. the hippocampus of freely moving rats: evidence for neuronal Suppl. 7 (1992) 49–64. release, J. Neurochem. 60 (1993) 671–677.
[15] W.J. Loskota, P. Lomax, S.T. Rich, The gerbil as a model for the [29] A. Vezzani, D. Hoyer, Brain somatostatin: a candidate inhibitory role study of the epilepsies. Seizure patterns and ontogenesis, Epilepsia in seizures and epileptogenesis, Eur. J. Neurosci. 11 (1999) 3767–
15 (1974) 109–119. 3776.
[16] J.D. Mikkelsen, D. Woldbye, J. Kragh, P.J. Larsen, T.G. Bolwig, [30] A. Vezzani, R. Monhemius, P. Tutka, R. Milani, R. Samanin, Electroconvulsive shocks increase the expression of neuropeptide Y Functional activation of somatostatin- and neuropeptide Y-contain-(NPY) mRNA in the piriform cortex and the dentate gyrus, Brain ing neurons in the entorhinal cortex of chronically epileptic rats, Res. Mol. Brain Res. 23 (1994) 317–322. Neuroscience 75 (1996) 551–557.
[17] L.A. Paul, I. Fried, K. Watanabe, A.B. Forsythe, A.B. Scheibel, [31] R. Wolf-Dieter, G. Heuschneider, G. Sperk, P. Riederer, Biochemical Structural correlates of seizure behavior in the Mongolian gerbil, events in spontaneous seizures in the Mongolian gerbil, Metab. Science 213 (1981) 924–926. Brain Dis. 4 (1989) 3–7.
´