Journal of Life Sciences
Volume 7, Number 9, September 2013 (Serial Number 65)
David Publishing Company www.davidpublishing.com
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 16710 East Johnson Drive, City of Industry, CA 91745, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Prof. Dr. Fadel Djamel (Algeria), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Prof. Dr. Ismail Salih Kakey (Iraq), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.org.
Editorial Office
16710 East Johnson Drive, City of Industry, CA 91745, USA Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374
E-mail:[email protected], [email protected]
Copyright©2011 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA
Database of Cambridge Science Abstracts (CSA), USA Database of Hein Online, New York, USA
Ulrich’s Periodicals Directory, USA Universe Digital Library S/B, Proquest
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA China National Knowledge Infrastructure, CNKI, China
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Index Copernicus, Index Copernicus International S.A., Poland
Google Scholar (scholar.google.com)
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560.
David Publishing Company
16710 East Johnson Drive, City of Industry, CA 91745, USA Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374 E-mail: [email protected]
David Publishing Company www.davidpublishing.org
DAV ID P UBL ISH IN G
J LS
Journal of Life Sciences
Volume 7, Number 9, September 2013 (Serial Number 65)
Contents
Biochemistry and Molecular Biology
913 Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in
Paracoccidioides brasiliensis Infection
Elis Araujo Morais, Estefânia Mara do Nascimento Martins, Viviane Cristina Fernandes, Íria Gabriela Dias dos Santos, Gerluza Aparecida Borges Silva, Dawidson Assis Gomes and Alfredo Miranda de Goes
928 Induction of Apoptosis in HeLa Cells by Methanolic Extract of Litsea cubeba Fruit Residue from Essential Oil Extraction
Trisonthi Piyapat, Miyagawa Kana and Tamura Hirotoshi
935 Pros and Cons in Therapeutic Evaluation of Paraoxonase 1 in Nerve Agent Toxicity
Manojkumar Valiyaveettil, Yonas Alamneh, Bhupendra P. Doctor, Alfred M. Sciuto and Madhusoodana P. Nambiar
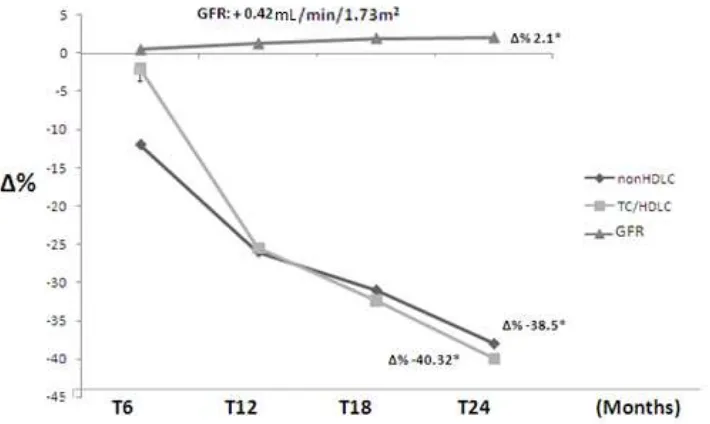
941 Efficacy and Safety of Dif1stat® for the Treatment of Secondary Dyslipidemia in Chronic Kidney Disease
Fabio Mazza and Claudia Stefanutti
950 Mesenchymal Stem Cells Express Low Levels of Cardiomyogenic Genes and Show Limited Plasticity towards Cardiomyogenic Phenotype
Juliana Lott de Carvalho, Danilo Roman Campos, Maira Souza Oliveira, Jader Santos Cruz, Nathalia Martins Breyner, Dawidson Assis Gomes and Alfredo Miranda de Goes
965 Morphological Evaluation of Adhesion and Proliferation of Osteoblast Like Cells Grown on Gelatin/Genipin Scaffold
971 Genetic Diversity among Some Sheep Breeds in Sulaimani Governorate Using RAPD-PCR Technique
Yousif Muhammad Salih Al-Barzinj and Muqdad Kamal Ali
Agricultural Science and Ecology
980 Contribution of Different Agronomic Practices and the Fungus Beauveria bassiana on the Coffee Berry Borer
Alberto Fernández Turro, Ernesto Castañeda Hidalgo, Francisco Simón Ricardo, Everton Kort Kamp Fernandes, Juana Iris Durán Cos and Odisa Baqué Fuentes
993 Effect of Water Stress in Grape Berries Cabernet Sauvignon (Mendoza, Argentina) during Four Years Consecutives
Leonor Deis and Juan Bruno Cavagnaro
1002 Long-Term H2O and CO2 Trends in Conifer Disc Tree Rings and Meteorological Parameters
Ageev Boris Grigor’evich, Gruzdev Aleksandr Nikolaevich, Bondarenko Svetlana Leonidovna and Sapozhnikova Valeria Aleksandrovna
1009 Qualitative and Environmental Aspects Study of Water Distributed in West Algeria: The Case of Sidi Bel Abbés City
September 2013, Vol. 7, No. 9, pp. 913-927 Journal of Life Sciences, ISSN 1934-7391, USA
Immunization with rPb27 Protects Mice from the
Disruption of VEGF Signaling in
Paracoccidioides
brasiliensis
Infection
Elis Araujo Morais1, Estefânia Mara do Nascimento Martins2, Viviane Cristina Fernandes1, Íria Gabriela Dias dos Santos3, Gerluza Aparecida Borges Silva3, Dawidson Assis Gomes1 and Alfredo Miranda de Goes1
1. Department of Biochemistry and Immunology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte 31270-901, Brazil
2. Albert Einstein College of Medicine, Yeshiva University, New York, NY 10033, USA
3. Department of Morphology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte 31270-901, Brazil
Received: March 14, 2013 / Accepted: June 14, 2013 / Published: September 30, 2013.
Abstract: VEGF (Vascular endothelial growth factor) signaling is critical for endothelial cell survival and maintenance of the vasculature. Deregulation of VEGF signaling contributes to the physiopathology of many diseases. However, the ways in which infection with Paracoccidioides brasiliensis affects VEGF signaling and the influence of immunization with rPb27 (recombinant protein Pb27) on VEGF signaling have not yet been studied. Animals were immunized with rPb27 and subsequently infected with a virulent strain of P. brasiliensis. The fungal load was evaluated by measuring CFU (colony-forming unit) and histology was performed to evaluate the inflammatory reaction. At the two time points analyzed, the PC (positive control) and TREAT (treated) animals had decreased levels of pulmonary VEGF compared to basal levels. However, in the immunized (Pb27) and treated mice (Pb27 + TREAT), VEGF expression remained unchanged after infection. In the case of VEGFR-2, the Pb27 and Pb27 + TREAT groups showed increased levels of expression. Regarding the levels of the eNOS enzyme, only the Pb27 group did not reduce the expression levels relative to baseline. The immunization with rPb27 kept VEGF signaling, NO production and increased VEGFR-2 expression, after infection with P. brasiliensis. Thus, the authors infer that immunization with rPb27 protects mice from the disruption of VEGF signaling in Paracoccidioides brasiliensis infection.
Key words: rPb27, VEGF, VEGFR, eNOS, Paracoccidioides brasiliensis, paracoccidioidomycosis, inflammatory infiltrate.
1. Introduction
PCM (Paracoccidioidomycosis) is a systemic mycosis caused by the dimorphic fungus
Paracoccidioides brasiliensis. The disease is considered to be the most prevalent systemic fungal infection in Brazil and is present in many Latin American countries [1-4]. P. brasiliensis infection is acquired upon the inhalation of airborne spores derived from the saprophytic mycelium form of the
Corresponding author: Alfredo Miranda de Goes, Ph.D., associate professor, research fields: biochemistry, immunology, biomaterials and tissue engineering. E-mail: [email protected].
fungus [5, 6]. The resulting systemic disorder primarily involves the lungs and disseminates to other organs and systems, but the lung is commonly the most affected organ [7, 8]. In the lung, P. brasiliensis
converts to its parasitic yeast form and may cause destruction of the alveoli and bronchial tree. The infection may also lead to chronic pulmonary insufficiency resulting from the development of fibrosis. This framework changes over time and can be consolidated into a number of chronic granulomatous processes, resulting in hypoxemia and ventilatory dysfunction [9, 10].
D
in Infection
Antifungal therapy is required to control the Paracoccidioidomycosis. Extended periods of therapy are usually required for a good clinical response [11]. The conventional treatment of PCM relies on sulfonamides, amphotericin B and azole derivatives. However, new and more effective alternatives for PCM treatment have been studied, and antigens used for immunization showed protection against P.
brasiliensis [12]. Among the antigens of P. brasiliensis, the recombinant protein rPb27, which has been studied, is especially remarkable because it has shown significant potential for the induction of a protective immune response against PCM in previous reports [12-18].
The maintenance of the microvasculature in the lung is critical for gas exchange, integrity of the alveolar structure and tissue repair [19]. VEGF (vascular endothelial growth factor) is an important regulator of vascular development [20-22], and lung microvascular endothelial cells produce significant amounts of VEGF, which contributes to endothelial maintenance and homeostasis within the lungs in an autocrine manner [23, 24]. VEGF is a highly vascular-specific signaling molecule and its biological activity is dependent on its interaction with specific receptors (VEGF-R1, 2, and 3), which are expressed not only by endothelial cells but also by activated macrophages and alveolar type II epithelial cells. Endothelial survival is mediated via VEGFR-2/kinase insert KDR (domain-containing receptor) [25-27]. Several lines of investigation have revealed that the inhibition of the VEGF-VEGFR-2 signaling pathway results in decreased lung alveolarization and arterial density [20].
eNOS (endothelial nitric oxide synthase) plays a central role in maintaining vascular integrity [20] and studies have demonstrated that eNOS/NO plays an important role in many VEGF-induced processes [28]. NO (nitric oxide) is also known to be an essential mediator of endothelial cell migration and VEGF-induced angiogenesis [29]. VEGF and
VEGFR2-mediated downstream signaling activate eNOS, resulting in the release NO [30], which is considered responsible for maintaining the homeostasis of the airways. The mechanism by which NO promotes cell survival has been directly linked to increased neovascularization and cell migration [21, 28, 31, 32].
Deregulated VEGF signaling is found in the physiopathology of diseases such as asthma, emphysema, pulmonary hypertension and acute respiratory distress syndrome [24, 33]. However, the effect of P. brasiliensis on the expression of VEGF and VEGFR-2 has not been studied. Therefore, in this study, authors evaluated the effect of P. brasiliensis infection and the influence of rPb27 immunization with or without antifungal therapy on the expression of VEGF, VEGFR-2 and eNOS after infection.
2. Materials and Methods
2.1 Animals
Adult male BALB/c mice (6-8 weeks old) were purchased from the vivarium of ICB-UFMG (Belo Horizonte, MG, Brazil) and divided into six groups of five animals each: NC (negative control): group without any intervention; PC (positive control): infected only; ADJ (adjuvant): injected with
Corynebacteriumparvum (R.V Special Manipulations, Rio de Janeiro, RJ, Brazil) and Al(OH)3
(Pesamar-Sanosi-synthelado, RJ, Brazil) as adjuvant; immunized (Pb27): immunized with rPb27; treated and immunized (Pb27 + TREAT): immunized with rPb27 and treated with fluconazole; and TREAT (treated): infected and treated with fluconazole. The specifications of the groups and the interventions made in the animals during the experiment are shown in Table 1. The experiments were performed three times to determine the repetition of the results.
2.2 P. brasiliensis Strain
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
915
Table 1 Experimental groups.
Groups Immunization Infection Treatment
NC - - -
yeast extract, 0.5% peptone, 1.5% D-glucose, 1.5% agar, pH 7.0] at 36 °C. The viability of fungal suspensions was determined by staining with Janus Green B vital dye method (Merck. Darmstadt, Germany) and was always higher than 90%. The virulence of the human isolate was checked in each experiment by infecting intratracheally BALB/c mice and recovering the yeast cells from their organs [12].
2.3 Immunization
Groups of male BALB/c mice were immunized by the subcutaneous injection of 50 µg of rPb27 (GenBanK-NCBI accession number AAC49615) in the presence of 100 µg of C. parvum (R.V Special Manipulations) and 1 mg Al(OH)3 as adjuvant. The
animals were boosted three times at fifteen-day intervals with the same amount of antigen, seven days after last immunization the challenge infection was performed [12, 13, 15].
2.4 Intratracheal Infection
Male BALB/c mice were inoculated intratracheally with 3 105 viable yeast cells of virulent P. brasiliensis, which was grown on YPD-agar and suspended in sterile PBS as described by Fernandes et al. [12]. Briefly, mice were anesthetized intramuscularly with 40 µL of a solution containing 57% ketamine (Dopalen, Vetbrands, Brazil) and 43% xylazine (Dopaser, Laboratório Calier do Brazil LTDA, Brazil). After approximately 10 min, their necks were extended, the trachea was exposed at the level of the thyroid, and yeast cells were injected in 50 µL of PBS using a 30-gauge needle. The incisions were sutured with 4-0 silk [13].
2.5 Antifungal Therapy
After 30 days of infection, animals were treated every day for one month. The group of mice received doses of 10 mg/kg of fluconazole (Mantena laboratories limited) every 24 h. All drug administration was intraperitoneally.
2.6 Lung CFU Measurement from Intratracheally Infected BALB/c Mice Immunized with rPb27 and/or Treated with Fluconazole
The analysis of organ CFUs was performed 30 and 90 days post infection (dpi). The organs were removed, weighed, homogenized in PBS (pH 7.2) and plated on solid brain heart infusion medium (BHI, Difco Laboratories, Detroit, MI, USA) supplemented with 4% fetal calf serum and 5% P. brasiliensis spent culture medium (Pb18) as growth factors. Gentamicin was added at a concentration of 40 mg/L. The plates were incubated at 36 °C, and the CFUs were counted after 20 days of incubation. The results are expressed as the log10 of the number of CFUs of viable yeast
cells of P. brasiliensis per gram of tissue per mouse in each experimental group (log10/g).
2.7 Lung Fixation, Histology and IHC (Immunohistochemistry)
in Infection
mouse were analyzed using a microscope with the 2 magnification; sections were then scanned using JVC TK-1270/RGB micro camera. Using a digital pad, in each section, the total lung area and the area of intense inflammatory infiltrate were measured (areas with increased cellularity but without obstruction of alveoli were not measured), and the results were expressed in square millimeters (mm2). From these values, the percentage of area with inflammatory infiltrate was calculated.
With regard to the IHC, briefly, the lung sections were deparaffinized in xylene and rehydrated in alcohol, and then the endogenous peroxidase was inhibited with 10% hydrogen peroxide. The sections were stained with a primary antibody overnight at 4 °C. Immunodetection was performed using a goat anti-rabbit IgG conjugated to peroxidase (Sigma- Aldrich) and 3,3’- diaminobenzidine (DAB) (K3468- DAKO, Glostrup, Denmark). The nuclei were counterstained with Harris hematoxylin solution. For negative control of the each section, the primary antibody was omitted from one section of each of the samples.
2.8 Lung Tissue Homogenate and WB (Western Blot)
Lung tissue (0.1 g) was homogenized in 1 mL of lysis buffer (100 mM NaCl, 50 mM Tris base, 5 mM EDTA, 50 mM sodium pyrophosphate decahydrate, 1% NP-40, 0.3% TRITON, sodium deoxycholate 0.5%, 20 mM sodium fluoride and a complete protease cocktail inhibitor (Sigma-Aldrich)). The homogenate was kept on ice for 45 min and then centrifuged at 16.500 ×g for 15 min at 4 °C and the supernatant was stored at -20 °C until further use. The protein concentrations were determined using the Bradford method [34]. The protein samples (30 µg) were separated on a 6-15% gradient SDS-PAGE gel by electrophoresis and transferred onto a PVDF (polyvinylidene fluoride) membrane (Millipore, MA, USA). The membranes were blocked with 5% nonfat milk and incubated with the relevant primary antibody. The primary antibodies used were rabbit
anti-VEGF (SC152- Santa Cruz Biotechnology), rabbit anti-VEGFR-2 (Ab39638-Abcam, Cambridge, MA, USA) and rabbit anti-GAPDH (SC 25778-Santa Cruz Biotechnology, Inc, CA, USA). After washing, the bound antibody was detected using an anti-rabbit antibody (1:10.000 dilutions) conjugated to peroxidase (Sigma-Aldrich, St Louis, MO, USA). Blots were visualized by enhanced chemiluminescence and quantitatively analyzed using Image software.
2.9 cDNA Synthesis and Quantitative Real Time PCR (qPCR) Analysis
Total RNA was extracted using TRIzol (Life Technologies, Grand Island, NY, USA). The RNA was extracted from the samples according to the manufacturers. The samples were treated with RNase-free DNase (Promega, Madison, WI, USA) and the synthesis of cDNA was performed with RevertAidTM H Minus First Strand cDNA Synthesis Kit (Fermentas Life Sciences Inc, Ontario, Canada) using an oligo(dT) adapter primer.
The reactions were performed in triplicate using SYBRGreen PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. The reactions were carried out in a 96-well plate using an ABIPrism 7900 (Applied Biosystems, Carlsbad, CA, USA). Gene expression was quantified using the comparative Ct (2-ΔΔCt) method and β-actin were used as endogenous control genes for data normalization, and the results represent the relative quantification of gene expression normalized to the constitutively expressed β-actin gene. The NC (negative control) was used to obtain the basal levels of expression [35].
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
917
Table 2 Primer sequences used for quantitative qPCR.
Primer Sequence 5′-3′ (Gene ID)
eNOS Forward CAA CGC TAC CAC GAG GAC ATT (18127)
Reverse CTC CTG CAA AGA AAA GCT CTG G
VEGF Forward GGC AGC TTG AGT TAA ACG AAC (22339)
Reverse TGG TGA CAT GGT TAA TCG GTC
VEGFR-2 Forward ATA GAA GGT GCC CAG GAA AAG (16542)
Reverse TCT TCA GTT CCC CTT CAT TGG
β-ACTIN Forward GGA TGC AGA AGG AGA TTA CTA CTG (11461) Reverse CGA TCC AGA GAG AGA GTA CTT G
2.10 Statistical Analysis
The statistical analysis about determine the significance of the data was performed using Prism 5.0 software (GraphPad, CA, USA). The data were normally distributed, and the values obtained from the different groups of mice were compared using a one-way ANOVA (analysis of variance) with Bonferroni post- tests. Data were considered statistically significant at P < 0.05.
2.11 Ethics Statement
This work was approved by the CETEA/UFMG (Committee on the Ethics of Animal Experimentation of Federal University of Minas Gerais) under permit number: 203/2010 and was carried out in strict accordance with the regiment of ethics commission on the use of Animals. All surgery was performed with use of anesthesia, and all efforts were made to minimize suffering.
3. Results
3.1 Fungal Loads in Lungs of Mice after Challenge Infection
The CFU analysis performed 30 and 90 dpi showed significantly fewer fungi which recovered from the lungs of animals (CFU) immunized with rPb27. In these animals, there was a 75% reduction in the CFUs 30 days post infection compared to the PC and ADJ groups (Fig. 1A).
After 90 days of infection, all groups showed reduction of the fungal load in comparison with the results of 30 dpi. However the ADJ, Pb27 and Pb27 +
TREAT groups showed a significant reduction in the CFU recovered from the lungs compared to the PC and TREAT groups. No fungal colonies were recovered from the ADJ and Pb27 groups, 90 days after infection, and the mice from Pb27 + TREAT group had a 90% reduction in lung CFUs in comparison to the PC and TREAT groups (Fig. 1B).
in Infection
3.2 Lung Histology
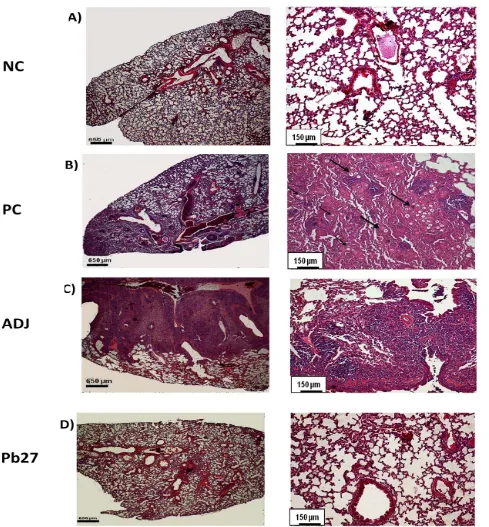
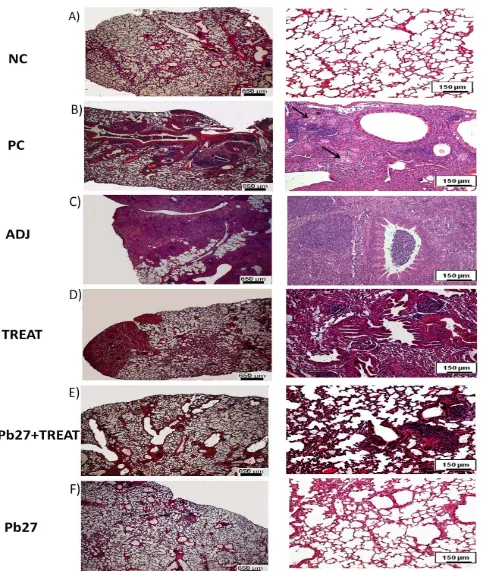
The histology of the lungs was performed qualitatively for the demonstration of inflammatory reactions. At the two time points investigated, the lungs of animals of PC and TREAT showed multiple foci of inflammation, containing yeast cells of P. brasiliensis. The mice of Pb27 + TREAT group presented compact infiltrates and showed a considerable reduction in the size of lesions after 90 days of infection in comparison with PC (Figs. 2 and 3). The Pb27 group, at the two time points investigated, showed limited areas with a compact cellular infiltration that did not form into a considerable lesion (Figs. 2 and 3).
To investigate the protective activity induced by vaccination with rPb27, the authors measured the extent of the inflammatory infiltrate, 90 days after infection, using Image J software. Analysis of the PC and TREAT groups showed that approximately 40% of the area of the lungs had intense inflammatory infiltrate. Among the groups the ADJ had greater area of inflammatory infiltrate. The immunized groups (Pb27 and Pb27 + TREAT) had smaller areas of inflammatory infiltrate even the Pb27 group showed that only 10% of the field of lungs had intense inflammatory infiltrate and with no significant differences with the NC. All other groups showed significant differences in the area of inflammatory infiltrate compared with the NC. Comparison with the PC showed that the immunized groups (Pb27 and Pb27 + TREAT) had significant lower areas of inflammatory infiltrate. In contrast, the TREAT group did not show statistic differences with the PC group. These results confirm the efficiency of Pb27 in modulating the inflammatory response.
3.3 VEGF Expression Levels in Mouse PCM Model Lungs
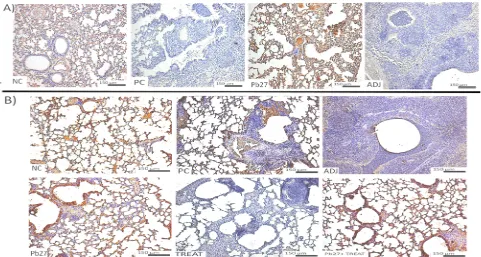
To investigate the expression of VEGF in mouse lungs infected with P. brasiliensis, WB and IHC were performed. The results obtained by the WB showed a
significant reduction in VEGF expression after 30 days of infection with P. brasiliensis in PC and ADJ animals compared with NC. However, there was no decrease in VEGF expression in the Pb27 group (Fig. 4).
After 90 days of infection, animals in the PC, TREAT and ADJ groups had reduced VEGF expression, and again, there was no decrease in VEGF expression in the immunized mice (Pb27 and Pb27 + TREAT) (Fig. 4B). The IHC experiments confirmed qualitatively the results obtained with the WB, showing lower levels of VEGF expression in PC, ADJ and TREAT animals compared to the NC and Pb27 (Fig. 5A and 5B).
3.4 VEGFR-2 Expression Levels in Mouse PCM Model Lungs
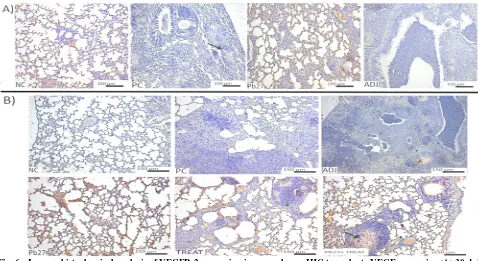
The authors also examined the effects of P.
brasiliensis infection on VEGFR-2 expression in mouse lungs IHC. After 30 days of infection, the NC and the Pb27 groups showed more marked areas of the VEGFR-2 expression than the PC group (Fig. 6A). After 90 days of infection, Pb27 and Pb27 + TREAT groups showed higher levels of VEGFR-2 expression in the lungs (Fig. 6B).
Additionally, the authors also observed that the relative mRNA expression of VEGFR-2 by qPCR after 30 and 90 dpi was significantly increased in the immunized groups (Pb27 and Pb27 + TREAT) compared to the other groups and basal levels. The Pb27 group had higher levels of VEGFR-2 mRNA expression compared to the other groups (Fig. 7A and B).
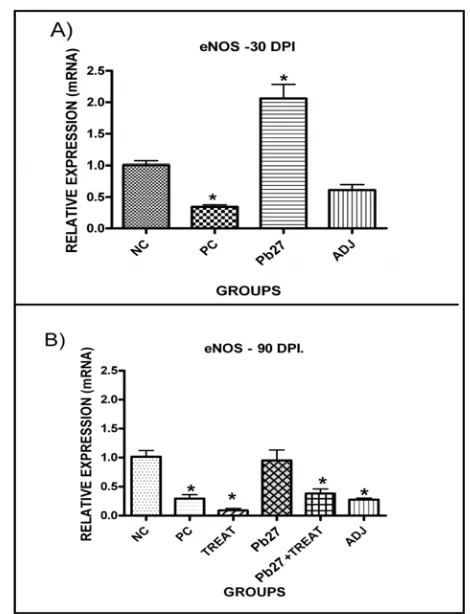
3.5 The eNOS Expression Levels in the Lungs of Mice Infected with P. brasiliensis
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
919
Fig. 2 Histological analysis of the lungs of mice subjected to 30 days of P. brasiliensis infection. After 30 days of infection with P. brasiliensis,mouse lungs were stained with HE and analyzed for histology. The representative figure of each group is shown with low magnification (4) in the left panel and higher magnification (10) in the right of the panel. The groups are as follows: (NC) negative control (A), (PC) positive control (B), (ADJ) adjuvant (C), and (Pb27) immunized (D). The black arrows indicate the location of fungal cells in the tissue.
reduced compared to basal levels and to those found in the Pb27 group (Fig. 8A). However, 90 days after infection, only the immunized group had expression
in Infection
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
921
Fig. 4 Western blot of VEGF expression. Representative WB of the lung tissue homogenate shows VEGF expression, (A) 30 dpi and (B) 90 dpi. The histograms represent the mean ± SE (pool of five animals per group and repeated three times (n = 15) of VEGF expression. The * indicates that P < 0.05 compared to the positive control animals. The # indicates thatP < 0.001 compared to the negative control and Pb27 animals.
in Infection
Fig. 6 Immunohistochemical analysis of VEGFR-2 expression in mouse lungs. IHC to evaluate VEGF expression (A) 30 dpi and (B) 90 dpi. Microscopy was performed at 10x magnification. The representative figure of each group and the microscopy was performed at 4x magnification. The groups are as follows: (NC) negative control; (PC) positive control; (ADJ) adjuvant; (Pb27) immunized; (TREAT) treated; and (Pb27 + TREAT) immunized and treated.
4. Discussion
The rPb27 was first described by McEwen, et al. [36] and has demonstrated great potential in the immunodiagnostics of PCM [13, 16, 36-38]. Previous immunization experiments in mice showed that Pb27 was able to provide protection against a challenge infection in both prophylactic and therapeutic protocols and it has therefore become a strong vaccine candidate for PCM [12-15, 36]. In this work, the authors confirmed efficacy of the protective effect of rPb27 immunization in prophylactic protocol against PCM and reported the effect of the immunization on the expression of VEGF, VEGFR and eNOS.
The fungal load was examined in the lungs of infected mice and the quantification of CFUs in the lungs showed a protective effect of rPb27 immunization against infection. It was observed that immunized mice had a significant decrease in lung CFUs when compared with controls, 30 dpi, the immunization reduced the CFU number by 75% in comparison to control groups (PC and ADJ). At the
second time point (90 dpi), the immunization reduced the fungal load to undetectable levels. These results are consistent with the results obtained by Reis et al. [15] and confirm the ability of the rPb27 protein to protect mice against PCM. The combined administration of rPb27 and fluconazole also resulted in a significant reduction in the fungal load, which was 90% compared to the PC. However, the fungal load was not reduced in the TREAT group, probably due to the short treatment time.
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
923
Fig. 7 Relative expression of VEGFR-2 mRNA in mouse lungs infected with P. brasiliensis as assessed by qPCR. VEGFR-2 mRNA expression (A) 30 dpi and (B) 90 dpi. The histograms represent the mean ± SE of the VEGFR-2 expression (of five animals per group and repeated three times (n = 15). The * indicates that P < 0.05 compared to the negative control animals. The NC (negative control) was used to obtain the basal levels of expression. The groups are as follows: (NC) negative control; (PC) positive control; (ADJ) adjuvant; (Pb27) immunized; (TREAT) treated; and (Pb27 + TREAT) immunized and treated.
immune response to the antigen by augmenting the activity of dendritic cells and macrophages, mimicking a natural infection.
Thus, the adjuvant used in this work by itself may have induced an inflammatory infiltrate, however, in association with Pb27, it helps this protein to establish the protective effect and down modulation of the inflammatory response showing lungs without fungal cells and unobstructed airways with a preserved lung parenchyma as well few inflammatory infiltrates. Based on that, we can infer that others adjuvant by itself does not induce inflammatory reactions which must be investigated.
Fig. 8 Relative expression of eNOS mRNA in mouse lungs infected with P. brasiliensis as assessed by qPCR. eNOS mRNA expression (A) 30 dpi and (B) 90 dpi. The histograms represent the mean ± SE of the eNOS expression (of five animals per group and repeated three times (n = 15). The * indicates that P < 0.05 compared to the negative control animals. The NC (negative control) was used to obtain the basal levels of expression. The groups are as follows: (NC) negative control; (PC) positive control; (ADJ) adjuvant; (Pb27) immunized; (TREAT) treated; and (Pb27 + TREAT) immunized and treated.
In this context the authors infer that in addition to decreasing the CFUs in the lung, the immunization reduce obstructions from airways after infection (with lower intensity of inflammatory infiltrates and tissue damage) and thus they confirm the protective capacity of Pb27 showed by Reis et al. [15].
in Infection
/fluconazole) the group with the largest lesions was the TREAT group.
The fluconazole antifungal therapy has significant activity in human PCM and is used most commonly in cases of central nervous system involvement by the fungus, nevertheless its use is not the most recommended form of treatment against paracoccidioidomycosis, and the most recommended therapy has been mainly itraconazole. Though, recently fluconazole was associated with rPb27 protein and had proven additive efficiency in therapeutic treatment of mice infected with P.
brasiliensis [12], in this work the authors showed that the association of this drug with the rPb27 on the prophylactic protocol do not have the same additional effect. Other antifungal agents should be tested in association with the protein to obtain better results.
VEGF is a signaling protein that stimulates angiogenesis and vascularization and is part of the system that restores oxygen to the tissues where blood circulation is inadequate [39-43]. Many lung diseases are characterized by changes in the expression of this molecule [36, 42, 44], including damage caused by cigarette smoke [20-22, 45], pulmonary infection by
Pseudomonas aeruginosa [46], asthma [47], emphysema, pulmonary hypertension and acute respiratory distress syndrome [24, 33]. Furthermore, recently VEGFR-2 was considered to be valuable predictive biomarkers in the development of ALI (acute lung injury) and ARDS (acute respiratory distress syndrome) [48]. In these diseases, the expression of VEGF/VEGFR is reduced and lung function is impaired.
In this study, the authors show that, as in the diseases mentioned above, PCM disrupted VEGF expression in mouse lungs. This reduction was showed in all tests made in PC mouse 30 and 90 dpi. The immunized (Pb27 and Pb27 + TREAT) group showed that the immunization increases the VEGF expression if compared with the other groups. When compared with the NC the levels of the expression
was greater 30 dpi and similar 90 dpi. The TREAT group was similar to the PC at the two time points.
The biological effects of VEGF are mediated through its binding to specific receptors expressed on the cell surface [19]. The protein-tyrosine kinase receptor FLk-1(VEGFR-2) is an important factor in endothelial survival and the maintenance of the vasculature [21, 22, 49]. In this work, the immunized animals showed a significant increase in the expression levels of VEGFR-2 in comparison with PC animals.
Studies have shown that VEGF-VEGFR-2 signaling enhances the expression of eNOS, and eNOS inhibitors disrupt NO production and significantly change the effects of VEGF. Thus, the improvement in endothelial function by VEGF expression is associated with increased eNOS and NO release [28, 44]. It is also known that NO produced by eNOS is an essential mediator of endothelial cell migration and VEGF-induced angiogenesis [31, 50]. Because this enzyme plays a central role in maintaining vascular integrity and is activated by VEGF [20, 44], authors determined the levels of lung eNOS after infection with P. brasiliensis. The results allow us to suggest that P. brasiliensis infection alters or negatively modulates the expression of this enzyme and that immunization prevents changes in its expression after infection, whereas, among the groups studied only the Pb27 not showed reduced levels of eNOS 30 dpi and 90 dpi.
5. Conclusion
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
925
basal levels, increases VEGFR-2 expression, and consequently, maintains eNOS expression at basal levels 90 days after infection. Thus, we showed that immunization maintains key functions that are normally inhibited by infection with P. brasiliensis, and the expression of the VEGF, VEGFR-2 and eNOS possibly can be related to the maintenance of the structure of the lung, proliferation and migration of endothelial cells, pulmonary regeneration and homeostasis [20, 23, 24, 51].
Acknowledgments
This research was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-Reitoria de Pesquisa da UFMG (PRPQ). Thank Laboratory of Cellular and Molecular Immunology (LICM).
References
[1] R. Martinez, Paracoccidioidomycosis: The dimension of the problem of a neglected disease, Rev. Soc. Bras. Med. Trop. 43 (4) (2010) 480.
[2] M.R. Fortes, H.A. Miot, C.S. Kurokawa, M.E. Marques, S.A. Marques, Immunology of paracoccidioidomycosis, An. Bras. Dermatol. 86 (3) (2011) 516-524.
[3] V.L. Calich, C.A. Vaz, E. Burger, Immunity to Paracoccidioides brasiliensis infection, Res. Immunol. 149 (4-5) (1998) 407-417.
[4] M.A. Shikanai-Yasuda, F.F. de Q. Telles, R.P. Mendes, A.L. Colombo, M.L. Moretti, Guidelines in paracoccidioidomycosis, Rev. Soc. Bras. Med. Trop. 39 (3) (2006) 297-310.
[5] A. Pina, P.H. Saldiva, L.E. Restrepo, V.L. Calich, Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts, J. Leukoc Biol. 79 (6) (2006) 1202-1213.
[6] A.H. Tavares, L.S. Derengowski, K.S. Ferreira, S.S. Silva, C. Macedo, A.L. Bocca, et al., Murine dendritic cells transcriptional modulation upon Paracoccidioides brasiliensis infection, PLoS Negl. Trop. Dis. 6 (1) (2012) 1459.
[7] E. Brummer, E. Castaneda, A. Restrepo, Paracoccidioidomycosis: An update, Clin Microbiol. Rev.
6 (2) (1993) 89-117.
[8] M. Armas, C. Ruivo, R. Alves, M. Goncalves, L. Teixeira, Pulmonary paracoccidioidomycosis: A case report with high-resolution computed tomography findings, Rev. Port. Pneumol. 18 (4) (Jul-Aug 2012) 190-193.
[9] S.H.M. Silva, A.L. Colombo, M.H.S.L. Blotta, F. Queiroz-Telles, A.B. Balthazar, J.D. Lopes, et al., Diagnosis of paracoccidioidomycosis by detection of antigen and antibody in bronchoalveolar lavage fluids, American Society for Microbiology 13 (12) (2006) 1363-1366.
[10] F.C.D. Silva, T.I.E. Svidzinski, E.V. Patussi, C.P. Cardoso, M.M.D.O. Dalalio, L. Hernandes, Morphologic organization of pulmonary granulomas in mice infected with Paracoccidioidesbrasiliensis, Am. J. Trop. Med. 80 (5) (2009) 798-804.
[11] G. Visbal, G. San-Blas, J. Murgich, H. Franco, Paracoccidioides brasiliensis, paracoccidioidomycosis, and antifungal antibiotics, Curr. Drug Targets Infect Disord 5 (3) (2005) 211-226.
[12] V.C. Fernandes, E.M. Martins, J.N. Boeloni, J.B. Coitinho, R. Serakides, A.M. Goes, Additive effect of rPb27 immunization and antifungal therapy in experimental paracoccidioidomycosis, PLoS One 6 (3) (2011) 17885.
[13] V.C. Fernandes, J.B. Coitinho, J.M. Veloso, S.A. Araujo, E.P. Pedroso, A.M. Goes, Combined use of Paracoccidioides brasiliensis recombinant rPb27 and rPb40 antigens in an enzyme-linked immunosorbent assay for immunodiagnosis of paracoccidioidomycosis, J. Immunol Methods 367 (1-2) (2011) 78-84.
[14] V.C. Fernandes, E.M. Martins, J.N. Boeloni, J.B. Coitinho, R. Serakides, A.M. Goes, The combined use of Paracoccidioides brasiliensis Pb40 and Pb27 recombinant proteins enhances antifungal therapy effects in experimental paracoccidioidomycosis, Microbes Infect 13 (12-13) (2011) 1062-1072.
[15] B.S. Reis, V.C. Fernandes, E.M. Martins, R. Serakides, A.M. Goes, Protective immunity induced by rPb27 of Paracoccidioides brasiliensis, Vaccine 26 (43) (2008) 5461-5469.
[16] L.S. Santos, V.C. Fernandes, S.G. Cruz, W.C. Siqueira, A.M. Goes, E.R Pedroso, Profile of total IgG, IgG1, IgG2, IgG3 and IgG4 levels in sera of patients with paracoccidioidomycosis: Treatment follow-up using Mexo and rPb27 as antigens in an ELISA, Mem Inst Oswaldo Cruz 107 (1) (2012) 1-10.
in Infection
Chemother 50 (8) (2006) 2814-2819.
[18] A.F. Marques, M.B. da Silva, M.A. Juliano, J.E. Munhõz, L.R. Travassos, C.P. Taborda, Additive effect of P10 immunization and chemotherapy in anergic mice challenged intratracheally with virulent yeast of Paracoccidioides brasiliensis, Microbes Infect. 10 (12-13) (2010) 1251-1258.
[19] N. Ferrara, T. Davis-Smyth, The biology of vascular endothelial growth factor, Endocr. Rev. 18 (1) (1997) 4-25.
[20] I. Edirisinghe, S.R. Yang, H. Yao, S. Rajendrasozhan, S. Caito, D. Adenuga, et al., VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction, Faseb J 22 (7) (2008) 2297-2310.
[21] J.A. Marwick, I. Edirisinghe, G. Arunachalam, C.S. Stevenson, W. Macnee, P.A Kirkham, et al., Cigarette smoke regulates VEGFR2-mediated survival signaling in rat lungs, J Inflamm (Lond) 7 (1) (2010) 11.
[22] J.A. Marwick, C.S. Stevenson, J. Giddings, W. MacNee, K. Butler, I. Rahman, et al., Cigarette smoke disrupts VEGF165-VEGFR-2 receptor signaling complex in rat lungs and patients with COPD: Morphological impact of VEGFR-2 inhibition, Am J Physiol Lung Cell Mol Physiol 290 (5) (2006) 897-908.
[23] N.F. Voelkel, R.W. Vandivier, R.M. Tuder, Vascular endothelial growth factor in the lung, Am J Physiol Lung Cell Mol Physiol 290 (2) (2006) 209-221.
[24] N.F. Voelkel, C. Cool, L. Taraceviene-Stewart, M.W. Geraci, M. Yeager, T. Bull, et al., Janus face of vascular endothelial growth factor: The obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension, Crit Care Med 30 (5 Suppl) (2002) 251-256.
[25] Y. Abadie, F. Bregeon, L. Papazian, F. Lange, B. Chailley-Heu, P. Thomas, et al., Decreased VEGF concentration in lung tissue and vascular injury during ARDS, Eur Respir J 25 (1) (2005) 139-146.
[26] M.J. Cross, Claesson-Welsh. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition, Pharmacological Sciences 22 (4) (2001) 201-207.
[27] N.C. Rivron, E.J. Vrij, J. Rouwkema, S.L. Gac, A. Van den Berg, R.K. Truckenmüller, et al., Tissue deformation spatially modulates VEGF signaling and angiogenesis, Proc Natl Acad Sci USA 109(18) (2012) 6886-6891. [28] S. Ben-Quan, D.Y. Lee, H.F. Zioncheck, Vascular
endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway, The Journal of Biological Chemistry 274 (46) (1999) 33057-33063.
[29] S.E. Michaud, S. Dussault, J. Groleau, P. Haddad, A. Rivard, Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: Role of NO and reactive oxygen species, J Mol Cell Cardiol 41 (2) (2006) 275-284.
[30] T. Tanimoto, Z.G. Jin, B.C. Berk, Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS), J Biol Chem 277 (45) (2002) 42997-43001.
[31] R.F. Filho, B. Zilberstain, Óxido nítrico: o simples mensageiro percorrendo a complexidade, Metabolismo, síntese e funções, Revista da Associação Medica Brasileira 46 (3) (2000) 265-271.
[32] N. Kang-Decker, S. Cao, S. Chatterjee, J. Yao, L.J. Egan, D. Semela, et al., Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2, J Cell Sci 120 (Pt 3) (2007) 492-501. [33] G. Korpanty, E. Smyth, L.A. Sullivan, R.A. Brekken,
D.N. Carney, Antiangiogenic therapy in lung cancer: Focus on vascular endothelial growth factor pathway, Exp Biol Med (Maywood) 235 (1) (2010) 3-9.
[34] D.A.M. Zaia, C.T.B.V. Zaia, J. Lichtig, Determination of total protein by spectrophotometry: Advantages and disadvantages of proposed methods, Quím Nova 21 (6) (1998) 787-793.
[35] A. Giulietti, L. Overbergh, D. Valckx, B. Decallonne, R. Bouillon, C. Mathieu, An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression, Methods 25 (4) (2001) 386-401.
[36] J. McEwen, B.L Ortiz, A. García, A. Florez, S. Botero, A. Restrepo, Molecular cloning, nucleotide sequencing, and characterization of a 27-kDa antigenic protein from Paracoccidioides brasiliensis, Fungal Genet Biol 20 (2) (1996) 125-131.
[37] M.M. Correa, A.M. Bedoya, M.P. Guerrero, J. Mendez, A. Restrepo, J.G. McKeen, Diagnosis of paracoccidioidomycosis by a dot blot assay using a recombinant Paracoccidioides brasiliensis p27 protein, Mycoses 50 (1) (2006) 41-47.
[38] B.L. Ortiz, S. Diez, M.E. Uran, J.M. Rivas, M. Romero, V. Caicedo, et al., Use of the 27-kilodalton recombinant protein from Paracoccidioides brasiliensis in serodiagnosis of paracoccidioidomycosis, Clin Diagn Lab Immunol 5 (6) (1998) 826-830.
[39] H.F. Dvorak, L.F. Brown, M. Detmar, A.M. Dvorak, Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis, American Journal of Pathology 237 (1999) 97-132.
Immunization with rPb27 Protects Mice from the Disruption of VEGF Signaling in Paracoccidioides brasiliensis Infection
927
Bioscience; MCBDIL Database [Internet], Landes Bioscience, Madame Curie, Bioscience Austin (TX), 2000.
[41] H.J.H. Marti, M. Bernaudin, A. Bellail, H. Schoch, M. Euler, E. Petit, et al., Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia, American Journal of Pathology 156 (3) (2000) 965-976.
[42] G. Neufeld, T. Cohen, S. Gengrinovitch, Z. Poltorak, Vascular endothelial growth factor (VEGF) and its receptors, The FASEB Journal 13 (1999) 9-22.
[43] S.A. Marques, Paracoccidioidomycosis: Epidemiological, clinical and treatment up-date, Anais Brasileiros de Dermatologia 78 (2) (2003) 135-150.
[44] N.F. Cerqueira, W.B. Yoshida, Óxido Nítrico, Revisão, Acta Cirúrgica Brasileira 17 (6) (2002) 417-423.
[45] X.J. Guan, L. Song, F.F. Han, Z.L. Cui, X. Chen, X.J. Guo, et al., Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary funtion partly via VEGF-VEGF receptors, J Cell Biochem 114 (2) (2013) 323-335.
[46] E.C. Breen, J.L. Malloy, K. Tang, F. Xia, Z. Fu, R.E. Hancock, et al., Impaired pulmonary defense against Pseudomonas aeruginosa in VEGF gene inactivated
mouse lung, J Cell Physiol. (2012), doi: 10.1002/jcp.24140.
[47] M. Balantic, M. Rijavec, M. Skerbinjek Kavalar, S. Suskovic, M. Silar, M. Kosnik, et al., Asthma treatment outcome in children is associated with vascular endothelial growth factor A (VEGFA) polymorphisms, Mol Diagn Ther 16 (3) (Jun 1, 2012) 173-180.
[48] T. Wada, S. Jesmin, S. Gando, Y. Yanagida, A. Mizugaki, S.N Sultana, et al., The role of angiogenic factors and their soluble receptors in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) associated with critical illness, Journal of Inflammation (2013) doi: 10.1186/1476-9255-10-6.
[49] Y. Kasahara, R.M. Tuder, L. Taraseviciene-Stewart, T.D. Le Cras, S. Abman, P.K Hirth, et al., Inhibition of VEGF receptors causes lung cell apoptosis and emphysema, J Clin Invest 106 (11) (2000) 1311-1319.
[50] I.M. Bird, Endothelial nitric oxide synthase activation and nitric oxide function: New light through old windows, Journal of Endocrinology 210 (3) (2011) 239-241. [51] M. Jakkula, T.D. Le Cras, S. Gebb, K.P. Hirth, R.M.
September 2013, Vol. 7, No. 9, pp. 928-934 Journal of Life Sciences, ISSN 1934-7391, USA
Induction of Apoptosis in HeLa Cells by Methanolic
Extract of
Litsea cubeba
Fruit Residue from Essential
Oil Extraction
Trisonthi Piyapat1, Miyagawa Kana2 and Tamura Hirotoshi2
1. United Graduate School of Agricultural Sciences, Ehime University, Ehime 790-8566, Japan
2. Department of Applied Biological Science Faculty of Agriculture, Kagawa University, Kagawa 761-0795, Japan
Received: May 31, 2013 / Accepted: August 01, 2013 / Published: September 30, 2013.
Abstract: Anticancer activity in vitro of Litsea cubeba fruit extracts was investigated, focusing on the fruit residue from essential oil extraction. The methanol extract was fractionated by an Amberlite® XAD-7 column. Cell viability, cell proliferation and cell death were determined using conversion of WST-8, BrdU incorporation and measurement of released LDH, respectively. Activation of caspase-3/-7 was detected using Z-DEVD-R substrate and morphological characteristics of apoptotic cells were revealed by DAPI staining. It was found that 80-100% methanol fractions (RME-4B, -5A, -5B and -5C) were effective against HeLa cell viability and also promoted cell death. RME-5A and -5B were highly effective in suppressing DNA replication (IC50 4.89 and 3.26 g/mL at 48 h)
and also in activation of caspase-3/-7 (9 and 17 times of untreated population at 12 h). The presence of apoptotic bodies was clearly observed. The results of this study suggested that L. cubeba fruit residue has remarkable apoptosis induction potential for further use in cancer drug research and for waste management in the essential oil industry.
Key words:Litsea cubeba, lauraceae, apoptosis, caspase-3/-7, HeLa cell.
1. Introduction
More than 50% of cancer in human is caused by mutation of tumor suppressor p53 gene, which is a transcription factor that regulates cell cycle arrest and an upstream molecule of apoptotic caspases [1-3]. Presently, chemotherapeutic methods still rely on administration of non-selective cytotoxins. Treatment failure could occur from resistance to chemotherapy and radioactive exposure [4-6]. Therefore, development of cancer drugs that target on restoration of cell cycle function in cancer cells is crucial for cancer therapeutic research.
Plant secondary metabolites have been proven as a reliable source of anticancer agents. Various researches
First author: Trisonthi Piyapat, master, research field: applied biological sciences. E-mail: [email protected].
Corresponding author: Tamura Hirotoshi, Ph.D., professor, research field: applied biological sciences. E-mail: [email protected].
revealed about active compounds from plants that promote cell cycle arrest and activate apoptotic caspases in cancer cells, for example, Vinca alkaloids from Catharanthus roseus, Taxanes from Taxus
brevifolia, Podophyllotoxin from Podophyllum
peltatum and sesquiterpene lactones from Smallanthus sonchifolius and berberine from herbal plants in China [7-9]. Many studies also reported about the anticancer potential of plant extracts, such as, methanol extract of
Pterocarpus santalinus, ether extract from
Cremanthodium humile and hot water extract of a Korean herb formula [10-12]. The results from these studies are valuable as pioneer research for the discovery of new cancer drugs. In this study, the potential of Litsea cubeba was investigated.
L. cubeba (Lauraceae) is an important essential oil plant that is cultivated for essential oil production in China, Japan and Taiwan. Approximately 2,000 tons
D
Induction of Apoptosis in HeLa Cells by Methanolic Extract of Litsea cubeba
Fruit Residue from Essential Oil Extraction
929
of the oil is produced yearly in China. Fruit essential oil of L. cubeba is a material for production of citral and ionones, a modifier for lemon and lime flavor in food industry and a substitute of verbena and lemongrass oil in perfume industry. L. cubeba fruit is also a traditional medicine for treatment of inflammation [13, 14]. As oxidative stress, inflammation and carcinogenesis are linked in the molecular pathway [15], the authors assumed the presence of apoptosis promoting compounds in L. cubeba fruits which could be useful for further cancer drug research and utilization of the organic waste from essential oil production.
2. Material and Method
2.1 General
EMEM (Eagle’s minimum essential medium) with Earle’s salt and all solvents were purchased from Wako Pure Chemical Industry (Tokyo, Japan). Amberlite® XAD-7, Etoposide and DAPI were purchased from Sigma-Aldrich (MO, USA). FBS (Fetal bovine serum) was purchased from Equitech-bio (TX, USA). PBS and Antibiotics were purchased from Invitrogen (NY, USA). HEPES was purchased from Dojindo Molecular Technologies (Tokyo, Japan). Colorimetric measurement was carried out using a MTP-450 Lab microplate reader (Corona electric, Ibaraki, Japan). Transparent 96-well plates were purchased from TPP (Trasadingen, Switzerland). All reagents in assay kits were prepared and used according to the manufacturers’ instructions.
2.2 Extraction and Fractionation
Litsea cubeba fruits were extracted with hexane to obtain EO (essential oil). The residue was re-extracted with methanol and double distilled water to obtain RME (methanol extract) and RWE (water extract). RME was fractionated in a glass column packed with Amberlite® XAD-7 (400 50 mm). RME (50 g) was fractionated to 8 fractions using 20-100% methanol (1 L). Fraction 4 (80% methanol) was collected twice as
RME-4A and -4B. Fraction 5 (100% methanol) was collected three times as RME-5A, -5B and -5C.
2.3 Cell Culture
The human cervical carcinoma cell line (HeLa) was purchased from Cell Bank, RIKEN BioResource Center (Ibaraki, Japan). The cell culture medium was EMEM, supplemented with 4.2 mM HEPES, 10% FBS, 0.1 mg/mL Penicillin and Streptomycin. Incubating condition was set at 37 ºC, humidified and 5% carbon dioxide in atmosphere. Exponentially growing cells were harvested for cell based assays and grown for 24 h prior to samples administration. The control population was exposed to 0.1% DMSO.
2.4 Cell Viability Assay
Cell counting kit 8 was purchase from Dojindo Molecular Technologies (Tokyo, Japan). Treated HeLa cells (2.5 103 cells/well) were exposed to WST-8 reagent (0.1% v/v) for 180 min (37 °C). Absorbance was measured at 450 nm. The cell viability percentage was calculated compared to that of control treatment.
2.5 Cell Proliferation Assay (BrdU Incorporation)
The cell proliferation ELISA BrdU (colorimetric) kit was purchased from Roche Applied Science (Mannheim, Germany). Treated HeLa cells (1 104 cells/well) were exposed to BrdU labeling solution for 120 min (37 °C) and then fixed by a FixDenat solution. The fixed cells were exposed to an anti-BrdU peroxidase solution for 90 min at RT (room temperature). The BrdU-Anti BrdU peroxidase complex was revealed by exposure to TMB (tetramethylbenzidine) for 10 min (RT), followed by aqueous sulfuric acid (0.2 M). Absorbance was measured at 450 nm. The cell proliferation percentage was calculated compared to that of the control population.
2.6 LDH Determination Assay
Fruit Residue from Essential Oil Extraction
Cytotoxicity Detection Kitplus (Roche Applied Science, Mannheim, Germany). The assay medium was replaced with EMEM supplemented with 1% FBS prior to sample administration. The initial cell concentration was 5 103 cells/well. The high control population was treated with Triton-X-100 (5%) for 15 min (37 °C). The amount of LDH was determined by exposing to mixture of tetrazolium salt INT, sodium lactate, NAD+ and diaphorase for 4 min (RT). Absorbance was measured at 492 nm. The LDH releasing percentage was calculated compared to that of the high control.
2.7 Caspase-3/-7 Determination Assay
Determination of caspase-3/-7 was carried out using Apo-ONE® Homogeneous caspase-3/-7 assay kit (Promega Corp., WI, USA). HeLa cells (1 104 cells/well) were treated with each sample (50 g/mL) in black-solid 96-well assay plates (BD-Bioscience, CA, USA). Treated cells were exposed to Z-DEVD-R substrate for 60 min (RT). Measurement was done using a fluorescent microplate reader (CYTOFLUOR series 4000, Applied Biosystem, TX, USA) at 485 ± 20 nm excitation and 530 ± 25 nm emission wavelength.
2.8 DAPI Staining
Treated HeLa cells (1 104 cells/well) in 12-well chamber slide (Thermo Fisher Scientific, FL, USA) were washed with ice-cold PBS and fixed with 10% formaldehyde for 24 h (4 °C). The fixed cells were exposed to the DAPI solution (1 g/mL in PBS) for 30 min (RT) then washed with PBS. Mounting solution used was ProLong® Antifade kit (Invitrogen, NY, USA). The stained cells were observed under fluorescent microscope (Olympus BX51, Olympus Optical, Tokyo, Japan) with 20 magnification power.
2.9 IC50 Value and Statistical Analysis
The results were presented as the mean and SD of 4 replications. IC50 value, indicating concentration of
sample that promoted 50% activity in each assay, was calculated by Graphpad Prism 5.0. Statistical analysis was carried out by SPSS (20.0 trial version). Data were analyzed using one way ANOVA ( = 0.05), followed by Duncan’s multiple range test. The significant difference is represented in alphabetical order.
3. Results and Discussion
3.1 Screening Test: Cell Viability
In this study, cell viability was determined by measurement of formazan, converted from WST-8 tetrazolium salt by activity of dehydrogenases in living cells [16]. As shown in Table 1, the amount of viable HeLa cell was decreased in EO (essential oil) and fruit RME (residue methanol extract) treated population. RME was more effective than EO, while water extract did not exhibit the activity. As methanol is a high polar solvent that can dissolve wide range of compounds, RME was fractionated by reverse phase chromatography, using Amberlite® XAD-7 column, to narrow down the effective compound groups by polarity and hydrophilic-hydrophobic property [17]. At 48 h, only fraction RME-4B, -5A, -5B and -5C, eluded by 80-100% methanol, were effective against HeLa cell viability (Table 1). Table 2 shows effect of these samples compared with that of etoposide.
Table 1 Yield percentage of each test sample, compared to weight of fresh fruits, and their activity to decrease HeLa cell viability after 48h of exposure period.
Induction of Apoptosis in HeLa Cells by Methanolic Extract of Litsea cubeba
Fruit Residue from Essential Oil Extraction
931
Table 2 Activity of EO (essential oil), RME-4B, 5A, 5B, 5C and etoposide to decrease cell viability, cell proliferation and promote cell death in HeLa cells after 24 and 48 h of sample exposure period.
Sample
IC50 (g/mL)
Cell viability Cell proliferation LDH release
24 h 48 h 24 h 48 h 24 h 48 h
Crude EO 70.91 42.02 N/A N/A 103.2 98.62
RME-4B 50.22 23.73 10.90 6.47 65.50 80.05
RME-5A 13.98 8.62 7.44 4.89 22.40 50.91
RME-5B 33.69 8.395 3.83 3.26 18.85 36.67
RME-5C 63.70 23.39 20.48 13.90 206.7 112.9
Etoposide 68.53 4.17 N/A 2.09 3718 118.4
*N/A = no activity.
3.2 Suppression of Cell Proliferation
BrdU (bromodeoxyuridine) was used as a marker to indicate the cell cycle status. BrdU is an analog of thymidine which can incorporate into newly synthesized DNA during the S phase of cell cycle by replacing thymidine in binding to adenine [18]. The amount of BrdU detected was proportional to the number of replicating cells which indicates the S-phase fraction in each treated population [18, 19].
As shown in Table 2, RME-5B treatment at 24 h (IC50 = 3.83 g/mL) exhibits almost as high activity as
that of etoposide at 48 h. RME-5B might triggered cell cycle arrest in the S phase like etoposide [20]. A decrease in number of replicating cells was also observed at 24 h in RME-5A (IC50 = 7.44), -4B (IC50
= 10.90) and -5C (IC50 = 20.48) treatment. On the
other hand, cell populations treated with essential oil and etoposide showed no decrease in cell proliferation at 24 h. Etoposide had improved activity at 48 h (IC50
= 2.09 g/mL), while treatment with essential oil still exhibited no activity. This result suggested that the effective fractions could halt cell cycle progression in treated HeLa cells during G1/S phase and these
samples expressed the activity faster than etoposide.
3.3 Release of Lactate Dehydrogenase
LDH (Lactate dehydrogenase) is a common enzyme in living cells. In in vitro experiments, release of LDH occurs during either late apoptosis or early necrosis [8, 21]. The amount of dead cells was indicated by
absorbance of red color formazan, converted from Tetrazolium salt INT which was reduced by activity of the released LDH to convert lactate to pyruvate [22]. The activity of each sample was compared with that of 5% triton-X-100, which indicated maximum LDH release in the same cell population size. As shown in Table 2, at 24 h, RME-5B was the most effective sample followed by RME-5A. The amount of LDH in these treatments decreased at 48 h. This was caused by degradation of earlier leaked LDH, as half-life of LDH at 37 °C is 9 h [22]. LDH released was also detected in essential oil, RME-4B and -5C treatments. This result suggested that these samples could promote HeLa cell death.
3.4 Caspase-3/-7 Activation and Nuclear Morphological Changes
Fruit Residue from Essential Oil Extraction
Fig. 1 shows that the amount of caspase-3/-7 in each treatment was compared with that of the negative control population, which indicated spontaneous caspase-3/-7 activation. A significantly high amount of caspase-3/-7 was detected in treatment with RME-5B. At 6 h, this sample promoted caspase-3/-7 activation about 12 times more than that of the negative control and increased sharply to around 17 times at 12 hours. RME-5A exhibited the second highest activity. The amount of detected caspase-3/-7 was about 6 and 9 times of negative control at 6 and 12 hours, respectively. The activity of RME-5A and -5B were significantly higher than that of etoposide at this exposure time range. For other samples, RME-4B and -5C induced a lower amount of caspase-3/-7 which was still slightly higher than of etoposide.
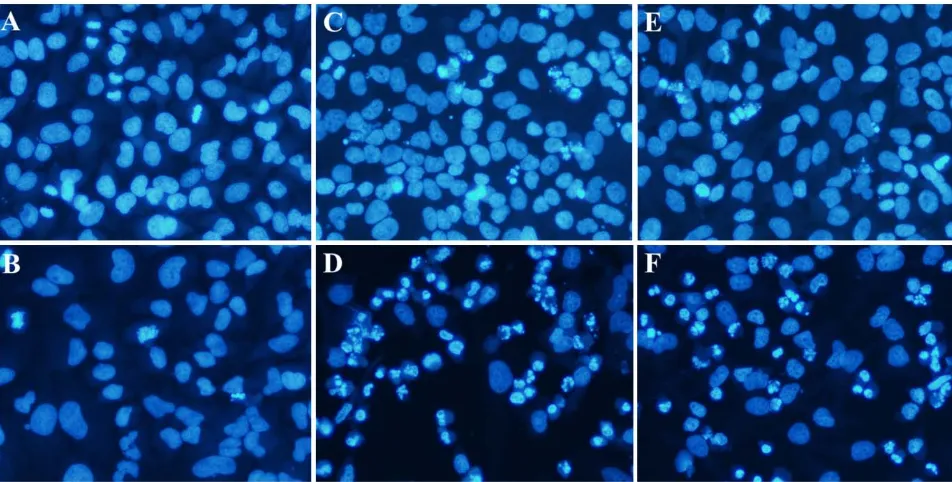
Apoptosis promoting potential of RME-5A and -5B was confirmed by presence of apoptotic bodies. HeLa cells treated with RME-5A and -5B (10 g/mL) for 48 h clearly exhibited cell shrinkage, chromatin condensation and margination, and fragmentation of nuclei (Fig. 2D and 2F), which are markers indicating cells undergoing apoptosis [24, 28]. These characteristics were also slightly observed in 24 h treatment (Fig. 2C and 2E). Fig. 2A shows the untreated population exhibited normal morphological characteristics. Chromatin condensation was that of
metaphase and anaphase, and after 48 h (Fig. 2B) a few spontaneous apoptosis were observed.
Despite the result on cell viability and LDH release, the amount of caspase-3/-7 detected in essential oil treatment (6-18 h) was not different from that of the untreated population. This result can be related to its lack of anti-proliferative effect (Table 2). It could be suggested that the fruit essential oil might cause another type of cell death such as necrosis, or it might require more sample concentration and exposure time to induce apoptosis in HeLa cells.
Several papers also reported about the anticancer activity of Litseacubeba. The fruit oil obtained from hydro-distillation exhibited cytotoxicity to the human lung, liver and oral cancer cells [29]. Recently, it was revealed that volatile compounds from L. cubeba seed, at 2,000 times dilution, could induce apoptosis and cell cycle arrest in an A549 human lung cancer cell line [30]. In this study, fraction RME-5A and 5B from methanol extract of L. cubeba fruit residue effectively induced apoptosis in human cervical cancer cells. Furthermore, these fractions were not yet purified but they were highly effective at low administrated concentration (≤ 10 g/mL, cell proliferation was inhibited and apoptotic cells were observed). Therefore, the purified active compound(s) might possess even stronger activity. At this point, the result
Induction of Apoptosis in HeLa Cells by Methanolic Extract of Litsea cubeba
Fruit Residue from Essential Oil Extraction
933
Fig. 2 Nuclear morphological changes in HeLa cells after being treated with DMSO 0.1% for 24 h (A) and 48 h (B), RME-5A 10 g/mL for 24 h (C) and 48 h (D) and RME-5B 10 g/mL for 24 h (E) and 48 h (F). Nuclei condensation and fragmentation patterns resembled to that of apoptotic body were clearly observed in treatment with RME-5A and 5B.
of this study suggested that L. cubeba fruit residue from essential oil extraction has high potential to be a new source for a plant derived anticancer drug and merit studies in active compound purification, in vivo study and clinical trials. Furthermore, it could prove beneficial as an effective tool for waste management in L. cubeba fruit essential oil industry.
4. Conclusion
Apoptosis induction activity in vitro of Litsea cubeba fruit was investigated, focusing on the fruit residue from essential oil extraction. Fractions RME-5A and -5B from L. cubeba fruit residue effectively inhibited HeLa cell replication and the activity might take place between the G1/S phases of
cell cycle. These fractions also activated executioner caspase-3/-7, which leads to apoptotic cell death and the activity was significantly higher and more acute than the standard cancer drug (etoposide). Although the active compounds have not been identified, the result of this study demonstrated that the fruit residue from L. cubeba essential oil extraction has potential to be a new source for development of plant derived
cancer drugs and merits study.
Acknowledgment
This research was supported by Japanese Ministry of Education, Culture, Sport, Science and Technology (Monbukagakusho scholarship).
References
[1] T. Ozaki, A. Nakagawara, Role of p53 in dell death and human cancers, Cancers 3 (2011) 994-1013.
[2] H. Hamada, Y. Tashima, Y. Kisaka, K. Iwamoto, T. Hanai, Y. Eguchi, et al., Sophisticated framework between cell cycle arrest and apoptosis induction base of p53 dynamics, PLoS ONE 4 (3) (2009) e4795.
[3] W.S. El-Deiry, Insight into cancer therapeutic design based on p53 and TRAIL receptor signaling, Cell Death and Differentiation 8 (2001) 1066-1075.
[4] H. Chang, X. Yang, Protease for cell suicide: Functions and regulation of caspases, Microbiology and Molecular Biology Reviews 64 (2000) 821-846.
[5] Q.P. Peterson, D.C. Hsu, D.R. Goode, C.J. Novotny, R.K. Totten, P.J. Hergenrother, Procaspase-3 activation as an anti-cancer strategy: Structure-activity relationship of procaspase-activating compound 1 (PAC-1) and its cellular co-localization with caspase-3, Journal of Medicinal Chemistry 52 (2009) 5721-5731.
Fruit Residue from Essential Oil Extraction
sensitizes p53-deficient human non-small cell lung cancer cells to caspase-7 mediated apoptosis, Apoptosis 10 (2005) 643-650.
[7] M.J. Nirmala, A. Samundeeswari, P.D. Sankar, Natural plant resources in anti-cancer therapy-A review, Research in Plant Biology 1 (3) (2011) 1-14.
[8] D. Siriwan, T. Naruse, H. Tamura, Effect of epoxides and
-methylene--lactone skeleton of sesquiterpenes from yakon (Smallanthus sonchifolius) leaves on caspase-dependent apoptosis and NFB inhibition in human cervical cancer cells, Fitoterapia 82 (2011) 1093-1101.
[9] B. Lu, M. Hu, K. Liu, J. Peng, Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction, Toxicology in vitro 24 (2010) 1482-1490.
[10] H.J. Kwon, Y.K. Hong, K.H. Kim, C.H. Han, S.H. Cho, J.S. Choi, et al., Methanolic extract of Pterocarpus santalinus induces apoptosis in HeLa cells, Journal of Ethnopharmacology 105 (2009) 229-234.
[11] H. Li, L. Wang, G. Qiu, J. Yu, S. Liang, X. Hu, Apoptosis of HeLa cells induced by extract from Cremanthodium humile, Food and Chemical Toxicology 45 (2007) 2040-2046.
[12] H. Chae, S. Yang, D. Kim, H.M. Kim, S.W. Chae, K.S. Keum, et al., Ge-Jee-Bok-Ryung-Hwan induces apoptosis in human cervical carcinoma HeLa cells-an endoplasmic reticulum stress pathway, Life Science 75 (2004) 2997-3016.
[13] M.A. Nor Azah, S. Susiarti, Litsea cubeba (Lour.) Persoon, in: Plant Resources of South-East Asia 19, Essential Oil Plant Backhuys Publishers, Netherland, 1999, pp. 123-126.
[14] L. Hu, Y. Wang, M. Du, J. Zhang, Characterization of the volatiles and active components in ethanol extracts of fruits of Litsea cubeba (Lour.) by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O), Journal of Medicinal Plants Research 5 (14) (2011) 3298-3303. [15] S. Reuter, S.C. Gupta, M.M. Chaturvedi, B.B. Aggarwal,
Oxidative stress, inflammation, and cancer: How are they linked?, Free Radical Biology & Medicine 49 (2010) 1603-1616.
[16] Dojindo Molecular Technologies Inc., Cell counting kit 8 technical manual, available online at: http://www.dojindo.com/TechnicalManual/Manual_CK0 4.pdf, 2013.
[17] Rohm and Haas Company, Amberlite® XAD7HP
product datasheet, available online at: http://www. rohmhaas.com/ionexchange/pharmaceuticals/Bioprocessi ng_doc/english/xad7hp.PDF, 2013.
[18] K.A. Schafer, The cell cycle: A review, Veterinary Pathology 35 (1998) 461-478.
[19] Roche Applied Science, Cell proliferation ELISA BrdU (Colorimetric) version 14.0, available online at: https://www.roche- applied-science.com, 2013.
[20] N.O. Karpinich, M. Tafani, R.J. Rothman, M.A. Russo, J.L. Farber, The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c, The Journal of Biological Chemistry 277 (19) (2002) 16547-16552. [21] G. Denecker, D. Vercammen, M. Steemans, T. Vanden
Berghe, G. Brouckaert, G. Van Loo, et al., Death receptor-induced apoptotic and necrotic cell death: Differential role of caspases and mitochondria, Cell Death and Differentiation 8 (2001) 829-840.
[22] Roche Applied Science, Cytotoxicity detection kit PLUS
(LDH) version 5.0, available online at: https://www.roche-applied- science.com, 2013.
[23] B. Pucci, M. Kasten, A. Giordano, Cell cycle and apoptosis, Neoplasia 2 (4) (2000) 291-299.
[24] S. Elmore, Apoptosis: A review of programmed cell death, Toxicologic Pathology 35 (2007) 495-516.
[25] W.C. Earnshaw, L.M. Martins, S.H. Kaufmann, Mammalian caspases: Structure, activation, substrates and functions during apoptosis, Annual Review of Biochemistry 68 (1999) 383-424.
[26] Promega Corporation, Apo-ONE® homogeneous caspase-3/7 assay, available online at: http://www.promega.com, 2013.
[27] E. Trotta, M. Paci, Solution structure of DAPI selectively bound in the minor groove of a DNA T-T mismatch-containing site: NMR and molecular dynamics studies, Nucleic Acids Research 26 (20) (1998) 4706-4713.
[28] D.V. Krysko, T.V. Berghe, K. D’Herde, P. Vandenabeele, Apoptosis and necrosis: Detection, discrimination and phagocytosis, Methods 44 (2008) 205-221.
[29] C.L. Ho, O. Jie-Pinge, Y.C. Liu, C.P. Hung, M.C. Tsai, P.C. Liao, et al., Compositions and in vitro anticancer activities of leaf and fruit oils of Litsea cubeba from Taiwan, Natural Products Communication 5 (4) (2010) 617-620.