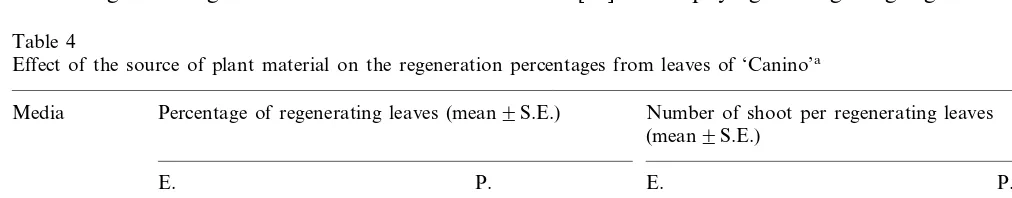Assessment of factors affecting adventitious shoot regeneration
from in vitro cultured leaves of apricot
Olaya Pe´rez-Tornero, Jose´ Egea, Alicia Vanoostende, Lorenzo Burgos*
Departamento de Mejora y Patologı´a Vegetal,Centro de Edafologı´a y Biologı´a,Aplicada del Segura,CSIC,P.O.Box4195,
30080Murcia,Spain
Received 20 April 2000; accepted 2 June 2000
Abstract
Relatively high percentages of adventitious shoot regeneration have consistently been obtained from leaves of some apricot cultivars. For the cultivar ‘Helena’, explants from the proliferation medium were more reactive than those from the elongation medium. The best results were obtained with thidiazuron (TDZ). When 6-benzylamino-purine (BAP) was used instead of TDZ, the regeneration percentages were very low. Higha-naphthaleneacetic acid (NAA) concentration had an important effect upon the
decrease of the secretion of phenolic substances. Young expanding leaves with the adaxial side touching the culture medium, maintained for 2 or 3 weeks in darkness, produced the best results. There was a significant genotypic variability in adventitious bud formation. Several caulinar meristems arose from very small areas of the leaf but only one meristem developed to form a shoot when those buds were transferred to elongation medium. However, the fact that several caulinar meristems exist in early steps could be an advantage when genetically transforming these leaves, since a high number of cells would have the possibility of being transformed and producing adventitious transformed buds. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Prunus armeniaca; Leaf age; Tissue culture; Thidiazuron; Organogenesis; Woody plant
www.elsevier.com/locate/plantsci
1. Introduction
Adventitious regeneration is a key step in the application of genetic engineering techniques to the breeding of plants. Fruit trees are among the most recalcitrant to produce adventitious shoots, although reports on the successful regeneration and transformation of different species have ap-peared in recent years [1 – 3]. Reports of in vitro adventitious shoot regeneration from mature ex-plants ofPrunusare few. Adventitious shoots have been obtained from leaves ofPrunus domestica [4],
Prunus canescens [5], P. dulcis [6], P. persica [7] and P. serotina and P. a6ium [8]. In apricot
(Prunus armeniaca L.), shoot regeneration through adventitious induction has been achieved fre-quently from juvenile explants or endosperm [9 –
11]. Because apricot is a highly heterozygous species, the use of seed-derived material should be avoided whenever elite clones have already been selected. Thus, it is important to establish effective protocols for induction of shoot regeneration from mature apricot explants. Only one paper about regeneration in apricot from adult tissue has been published to date [12]. The authors of that report found the results to be poorly reproducible.
The present study was designed to explore the conditions required for induction of adventitious buds and regeneration of shoots from explants of mature apricot trees propagated in vitro.
2. Materials and methods
2.1. Maintenance of shoot cultures
This study has been carried out with the
Ameri-* Corresponding author. Tel.: +34-968-215717; fax: + 34-968-266613.
E-mail address:[email protected] (L. Burgos).
can cultivar ‘Helena’ obtained in the apricot-breeding program at the Horticultural Crops Research Laboratory in Fresno, California and kindly provided by Dr Craig A. Ledbetter. In vitro shoots were maintained by sub-culturing at 3-week intervals onto a shoot multiplication medium. This medium consisted of QL macronutrients [13] and micronutrients, vitamins and organic compounds as described previously [14], 3% sucrose and 0.6% agar (Hispanlab, S.A.), and was supplemented with 3.1 mM
N6-benzylamino-purine (BAP) and 0.2
mM
indole-3-butyric acid (IBA). The pH of the medium was adjusted to 5.7 prior to autoclaving at 121°C for 20 min. Cultures were maintained at 2291°C under cool white fluorescent tubes (55
mmol m−2 s−1) with a 16-h photoperiod.
2.2. General strategy for regeneration
The first four apical expanding leaves were harvested in sterile water from 3-week-old prolif-eration shoots. Each leaf was transversely cut three or four times across the midrib without fully separating the segments. Leaves were cul-tured with the adaxial side in contact with the regeneration medium, which consisted of a basal medium with the same composition as the multi-plication medium, with the exception of the growth regulators, unless stated otherwise. All media were autoclaved for 20 min at 121°C and then dispensed aseptically, 25 ml per sterile plas-tic Petri dish (8.5 cm diameter×1.5 cm depth). For each treatment at least five Petri dishes were prepared, each containing seven leaves. Experi-ments, where regeneration was achieved, were re-peated at least twice. After explants were positioned on the medium, the dishes were sealed with parafilm. All explants were incubated in the dark at 2291°C for 3 weeks before expo-sure to light with a 16-h photoperiod unless stated otherwise. Following regeneration, shoots were transferred from the Petri plates to the me-dia used for micropropagation.
2.3. Effect of leaf origin, type of material, type and concentration of growth regulators
In this experiment, leaves from proliferated shoots and leaves and internodes from elongated shoots were used. Shoots were elongated in a
basal medium with the same composition as the multiplication medium but supplemented with 0.9 mM BAP and 0.2 mM IBA. For the
experi-ments with internodes, two Petri dishes were pre-pared with ten internodes per dish. The regeneration medium was supplemented with cy-tokinin, as BAP (4.4, 13.2 or 22.0 mM) or
1-Phenyl-3-(1,2,3-thiadiazol-5-yl) urea (Thidiazuron, TDZ) (2.3, 4.5, 9.0, 11.3 or 13.5
mM), and auxin, as a-Naphthaleneacetic acid
(NAA; 0.5, 1.3, 2.7, 4.0 or 5.4 mM; Table 1).
2.4. Effect of leaf age and position on the culture medium
Young leaves, corresponding to the first four apical expanded leaves and also the last four older leaves, from the proliferating shoots were cultured onto the RM12, RM18 and RM21 me-dia (Table 1), with the adaxial or abaxial side touching the culture medium.
2.5. Control of the secretion of phenolic substances
Young expanding leaves were harvested in sterile water with 0.1% ascorbic acid and cul-tured in different regeneration media. Also, leaves were cultured in regeneration media sup-plemented with 0.01% citric acid and 0.01% ascorbic acid or 0.1% polyvinylpyrrolidone (PVP, molecular weight 10 000). RM10, RM12 and RM13 (Table 1) were used in these experiments. Also, media RM19, RM20, RM21 and RM22 were tried, in which the NAA concentration was increased to 4.0 or 5.4 mM (Table 1).
2.6. Effect of light intensity
Young expanding leaves were cultured in the media RM12, RM15 and RM18 (Table 1). After the culture in the dark, leaves were transferred to 55 or 110 mmol m−2 s−1 light intensity.
2.7. Effect of the dark period
2.8. Effect of using an induction medium followed by an expression medium
Young expanding leaves were cultured in the media RM12, RM18 and RM21 (Table 1). After 3 weeks in the dark, the explants were transferred to auxin-free media (expression medium) with 1.1
mM or 2.3 mM TDZ, when the induction medium
was RM12, and 2.3, 4.5 or 9.0 mM TDZ when the
induction medium was RM18 or RM21. Also, when RM18 was used as induction medium, ex-pression media with 2.2 or 4.4 mM BAP, and
without TDZ or NAA, were tried (Table 1).
2.9. Effect of different gelling agents
Young expanding leaves were harvested from
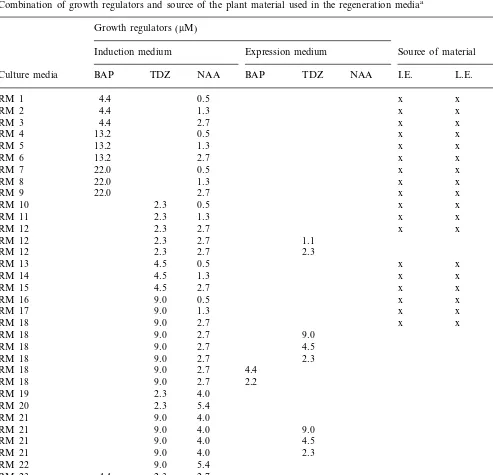
Table 1
Combination of growth regulators and source of the plant material used in the regeneration mediaa
Growth regulators (mM)
Induction medium Expression medium Source of material
BAP TDZ NAA I.E. L.E. L.P.
Culture media BAP TDZ NAA
x
aI.E., internodes from explants in elongation; L.E., leaves from explants in elongation; L.P., leaves from explants in
explants in proliferation and cultured in a regener-ation medium with 0.6% agar (Hispanlab, S.A.), 0.45% agargel or 0.25% gelrite (Sigma). The rest of the medium components and culture conditions were the same as for the above experiments, and the growth regulators of the media RM12, RM18 and RM21 (Table 1) were used.
2.10. Effect of the genotype
Young expanding leaves were harvested from explants in proliferation of the cultivars ‘Helena’, ‘Bu´lida’, ‘Canino’ and ‘Lorna’. Also, young ex-panding leaves from explants in elongation of the cultivar ‘Canino’ were used. Growth regulators used were those in the media RM12, RM18 and RM21 (Table 1). The macronutrients used for the last three cultivars were a WPM modification, as described previously [14]. The rest of the medium components and culture conditions were the same as for the above experiments, except that 0.45% agargel was used as gelling agent.
2.11. E6aluation criteria and statistical analysis
For up to 8 weeks after the dark period the number of leaves forming adventitious buds and the number of buds formed per leaf were recorded. Data were analysed by a maximum likelihood analysis of variance and, when necessary, specific contrasts of maximum likelihood were designed.
2.12. Light microscopy
Sections of young expanding leaves that stayed for 0, 1, 2, 3 or 4 weeks in the regeneration medium were fixed in FAA (90% of 70% ethanol, 5% of 40% formaldehyde, 5% glacial acetic acid). After this, the leaves were placed in 70% ethanol to remove the fixer, dehydrated using a tertiary-butyl alcohol series (TBA) and then embedded in Paraplast. Serial sections 5 or 10 mm deep were
mounted on slides impregnated with an adhesive of gelatine, glycerine and 3% formaldehyde. To stain the samples, the Paraplast was eliminated with xylene. The leaves were stained with a mix-ture of 0.71% Na2HPO4, 0.48% citric acid and 0.025% toluidine blue for 30 s, then they were rinsed in water, dried and mounted with a syn-thetic mounting medium.
2.13. Scanning electron microscopy
Sections of young expanding leaves that were kept for 4 or 5 weeks in the regeneration medium were fixed for 4 h at room temperature in a 0.1 M-phosphate buffer, pH 7.2, containing 3% glu-taraldehyde. Afterwards, samples were postfixed with 1% osmium tetroxide in the same buffer, and dehydrated in an acetone series. Subsequently, the samples were transferred into amyl acetate and processed using the critical point method (BASES UNION CT20). The specimens were sputtered with gold and observed using a Jeol-T300 scan-ning electron microscope [15].
3. Results
The first change, observed within 7 days on regeneration medium, was the enlargement of ex-plants to twice their original size. Morphogenesis occurred mostly on the cut edges and midribs or in association with vascular tissue. Most of the buds developed from calli, but sometimes appeared to arise directly from petiole tissue. The segments closer to the petiole were more regenerable than distal leaf segments. The first adventitious buds were observed 3 or 4 weeks after the beginning of the experiment. Most regeneration occurred from the calli in contact with the medium. Generally, the number of buds per regenerating explant was one although up to 3 or 4 buds per leaf have been found. Frequently, the buds developed leaf rosettes.
In general, explants from the proliferation medium were more reactive than those from the elongation medium. The regeneration percentages were very low or zero when leaves or internodes from explants in elongation were used.
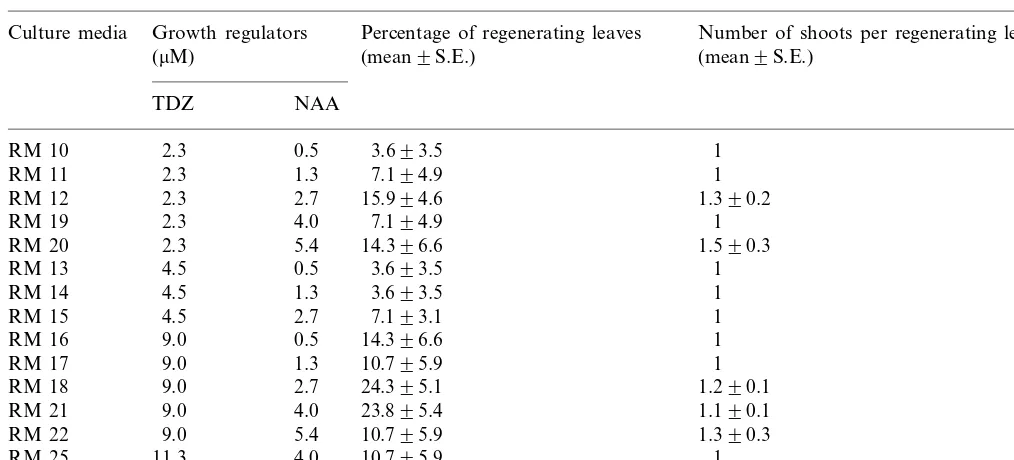
The proportion of leaves that produced adventi-tious buds was significantly greater with TDZ than with BAP. When BAP was used the average regen-eration percentage was 0.8%, with a maximum of 3.6% of regenerating explants. When the results of using different concentrations of TDZ or NAA were analysed, the regeneration percentages were significantly affected by the TDZ concentration (PB0.05) but not by NAA concentration. The best results were obtained with a TDZ concentra-tion of 9.0 mM (Table 2). The NAA concentration
Table 2
Regeneration percentages obtained in media with different concentrations of TDZ and NAAa
Growth regulators
Culture media Percentage of regenerating leaves Number of shoots per regenerating leaf
(mean9S.E.)
aLeaves were excised from proliferating shoots.
Table 3
Effect of leaf age and position in the culture medium on the percentage of regenerating leaves
Culture media Age Positiona Percentage of regenerating leaves Number of shoots per regenerating leaf (mean9S.E.)
(mean9S.E.)
Adaxial 14.396.6
Young 1
RM 12
Young
RM 12 Abaxial 2.992.8 1
RM 12 Old Adaxial 8.694.7 1.390.3
Abaxial 2.992.9
Old 1
Adaxial 11.495.4
Old 1
RM 18
RM 18 Old Abaxial 0 –
Adaxial 23.895.4
Young 1.190.1
aAbaxial and adaxial leaf side touching the culture medium.
phenolic substances. The best results were ob-tained when the NAA concentration was above 2.7 mM.
Young expanding leaves produced better results than adult leaves. Regeneration percentages in-creased at least two-fold when young leaves were used (Table 3). Also, the leaf position in the culture medium was an important factor. The best results were obtained when the leaf adaxial side was touching the culture medium, in this position the regeneration obtained was four- or five-fold
higher than when the leaf abaxial side was in contact with the medium (Table 3).
The treatments that decreased the secretion of phenolic substances, and did not affect the regen-eration percentages, were PVP and the increased NAA concentration in the regeneration medium. The rest of the treatments either did not have any effect on the phenolic secretion or diminished re-generation percentages.
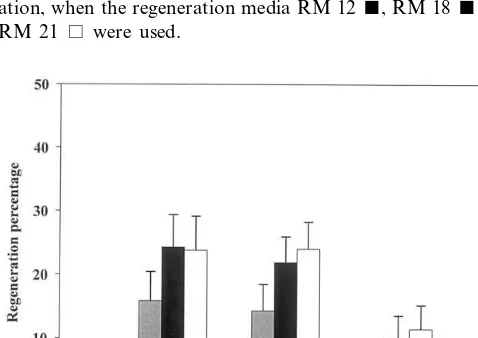
The dark period had a significant effect on the regeneration percentage (PB0.001). The regenera-tion was zero or very low with a dark period of 1 or 4 weeks and the best results were obtained with 2 or 3 weeks (Fig. 1), without significant differ-ences between these two periods in darkness.
The use of an expression medium different to the induction medium had no significant effect on the regeneration percentages. Moreover, a consid-erable increase in the secretion of phenolic sub-stances was observed (data not shown).
When different types of gelling agents were used, the regeneration percentages were signifi-cantly affected (PB0.01). The highest
regenera-Fig. 3. Regeneration percentages from leaves of the cultivars ‘Helena’, ‘Bu´lida’, ‘Canino’ and ‘Lorna’ cultured in the regen-eration media RM 12 , RM 18 and RM 21.
Fig. 1. Effect of the dark period on the regeneration percent-ages from ‘Helena’ leaves, obtained from explants in prolifer-ation, when the regeneration media RM 12, RM 18and RM 21 were used.
tion percentages were obtained with agar and agargel as gelling agents (Fig. 2). When agargel was used, this clearer gel allowed a better visibility of the adventitious buds.
The genotype significantly affected (PB0.001) the regeneration percentages. While in ‘Canino’ the regeneration obtained was significantly higher than in ‘Helena’ (PB0.001), in ‘Lorna’ and ‘Bu´l-ida’ zero or very low regeneration percentages were obtained (Fig. 3). Leaves of ‘Bu´lida’ and ‘Lorna’ formed very few calli and died. In ‘Canino’, when leaves from explant in prolifera-tion or elongaprolifera-tion were used, there were no signifi-cant differences in the regeneration percentages, although slightly better results were obtained with leaves from explants in elongation (Table 4).
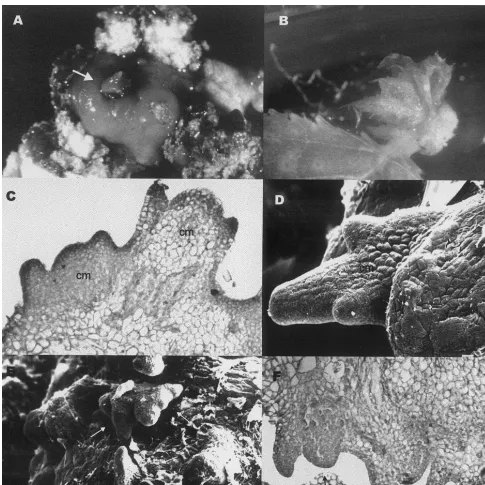
When examined with the light or scanning elec-tron microscope, it was seen that leaf rosettes regenerated from the cultured leaves were formed by a group of several adventitious buds (Fig. 4). In the region where adventitious buds were produced several caulinar meristems alongside the surface were observed. However, when the leaf rosettes were transferred to a culture medium for their elongation and proliferation, only one shoot developed.
4. Discussion
Many authors have observed that the majority of adventitious buds develop from calli in the basal cuts of leaves or from the petiole directly [12,16,17], which is in agreement with our results. Fig. 2. Effect of the utilisation of agar, agargel and gelrite as
Usually, the regeneration is associated with the cut side and the vascular tissue [18 – 20]. Sarwar and Skirvin [21] proposed that the cut edges provided a way for nutrients and growth regulators to be absorbed efficiently from the medium. Although most regeneration occurred from the calli exposed to air in P. canescens [5], in our study most adventitious buds originated from the tissue in contact with the medium.
Many authors use leaves from explants in elon-gation, cultured with low or zero cytokinins [5,6], however the success of this depends on the genotype.
Thidiazuron was required for efficient regenera-tion of adventitious shoots, which is consistent with the results of many authors working with woody plants [12,22 – 24]. This cytokinin-mimick-ing compound has been more effective than the true cytokinin BAP for inducing shoot organogen-esis in apricot (see our results and [12]) and also in other woody species [8,21,24].
Young expanding leaves with the adaxial side touching the culture medium have frequently pro-duced the best results in the regeneration of shoots from leaves of apple [17,20,25]. Famiani et al. [26] proposed that the higher leaf regeneration rates of apical leaves might be because the youngest leaves, still developing, have less differentiated and more metabolically active cells, with a more suitable hormonal and nutritional situation that could im-prove organogenesis. In spite of this, Antonelli and Druart [5] obtained the best results in apple using older leaves.
Probably the better regeneration obtained in the adaxial position is due to increased oxygen ex-change, since stomata are located abaxially in the leaf [27], and/or the ability of the palisade par-enchyma on the adaxial surface to transport nutri-ents and growth regulators from the medium into
the explant more efficiently [28]. However, other authors have obtained the best results when the leaf abaxial side was touching the culture medium [1,16,22].
An increase in NAA concentration was the best treatment to eliminate the secretion of phenolic substances. This effect might be due to the oxida-tion of phenols by auxin oxidase and therefore, the high NAA concentrations in the culture medium could compete for the active sites of the enzyme. We have been unable to find other reports where this effect of the NAA is described.
Although the decrease of light intensity from 110 to 55 mmol m−2 s−1 had no significant effect
on the regeneration percentage, Druart [16] ob-served a greater regeneration percentage in P.
canescens when light intensity was decreased. Many authors have observed that the optimum dark period to obtain adventitious buds changes according to the species, varying between 1 and 4 weeks [6,22,23,25]. In our study dark periods of 2 or 3 weeks produced the best results.
Leblay et al. [22] and Chevreau et al. [29], in pear, and Miguel et al. [6], in almond, obtained good results using an expression medium with the same components as the induction medium but eliminating the NAA. None of the combinations of the induction and expression media had a sig-nificant effect on the regeneration percentages in apricot.
Welander and Maheswaran [17] observed that in apple rootstocks gelrite was clearly superior to agar in promoting adventitious shoot development and hyperhydricity was not encountered. Explants on gelrite usually remained green and healthy, irrespective of the number of shoots produced, while explants in agar media frequently turned brown and deteriorated. In pear, Chevreau et al. [29] used phytagel as gelling agent with good
Table 4
Effect of the source of plant material on the regeneration percentages from leaves of ‘Canino’a
Media Percentage of regenerating leaves (mean9S.E.) Number of shoot per regenerating leaves (mean9S.E.)
Fig. 4. Microscopic examination of adventitious buds, (A), adventitious bud (arrowed) arising from callus (×20); (B), bud developing from callus formed on the petiole (×8); (C) and (D), light (×200) and scanning (×450) micrographs showing details of caulinar meristems (cm) forming on the leaf calli; (E) and (F), scanning (×140) and light (×100) micrographs showing a group of caulinar meristems (arrowed) arising very close together within the same leaf area.
results, however the authors had important prob-lems of hyperhydricity.
Genotype variability in adventitious bud forma-tion was evident. While with ‘Canino’ almost 60% of explants regenerated, in ‘Lorna’ or ‘Bu´lida’ the regeneration was zero or very low. These impor-tant differences between genotypes have been ob-served previously in apple [1,23] or peach [7], and
make necessary the individualised study of each cultivar.
to discriminate when they developed. In spite of the low number of regenerating shoots per leaf, the existence of several caulinar meristems arising in the same leaf may be beneficial when genetically transforming this material, since a high number of cells would have the possibility of being trans-formed and producing adventitious buds, espe-cially if competition is occurring at early stages of caulinar meristem development.
The present study is the first report on adventi-tious regeneration from mature explants in apricot where results have been consistently reproduced when the experiments were repeated. Only one paper on regeneration from adult tissue has been published in this species [12] previously where lower percentages of regeneration than those re-ported here, as well as a great variability in the responses, were obtained.
Currently, experiments are underway to im-prove the conditions, in order to achieve higher regeneration percentages and a higher number of regeneration sites per leaf. Also, factors affecting transformation are under study.
References
[1] I.V. Bartish, V.I. Korkhovoi, The composition of nutrient medium and the efficiency of shoot induction in vitro from apple leaf explants, Russ. J. Plant Physiol. 44 (1997) 381 – 385.
[2] S. Sriskandarajah, P. Goodwin, Conditioning promotes regeneration and transformation in apple leaf explants, Plant Cell Tissue Org. Cult. 53 (1998) 1 – 11.
[3] A.S. Trifonova, A.I. Atanassov, Studies on genetic trans-formation of apple, Acta Hort. 484 (1999) 591 – 594. [4] G. Bassi, F. Cossio, In vitro shoot regeneration on
‘Bluefre’ and ‘Susina di Dro’ prune cultivars (Prunus domesticaL.), Acta Hort. 289 (1991) 81 – 82.
[5] M. Antonelli, P. Druart, The use of a brief 2,4-D treatment to induce leaf regeneration onPrunus canescens Bois, Acta Hort. 280 (1990) 45 – 50.
[6] C.M. Miguel, P. Druart, M.M. Oliveira, Shoot regenera-tion from adventitious buds induced on juvenile and adult almond (Prunus dulcisMill.) explants, In Vitro Cell. Dev. Biol. Plant 32 (1996) 148 – 153.
[7] V. Declerck, S.S. Korban, Influence of growth regulators and carbon sources on callus induction, growth and morphogenesis from leaf tissues of peach (Prunus persica L Batsch), J. Hort. Sci. 71 (1996) 49 – 55.
[8] N. Hammatt, N.J. Grant, Shoot regeneration from leaves ofPrunus serotinaEhrh. (black cherry) andP.a6iumL.
(wild cherry), Plant Cell Rep. 17 (1998) 526 – 530. [9] W.D. Lane, F. Cossio, Adventitious shoots from
cotyle-dons of immature cherry and apricot embryos, Can. J. Plant Sci. 66 (1986) 953 – 959.
[10] R.E. Pieterse, Regeneration of plants from callus and embryos of ‘Royal’ apricot, Plant Cell Tissue Org. Cult. 19 (1989) 175 – 179.
[11] J.C. Goffreda, A.L. Scopel, J.A. Fiola, Indole butyric acid induces regeneration of phenotypically normal apricot (Prunus armeniaca L) plants from immature embryos, Plant Growth Regul. 17 (1995) 41 – 46.
[12] V. Escalettes, F. Dosba, In vitro adventitious shoot regeneration from leaves of Prunus spp., Plant Sci. 90 (1993) 201 – 209.
[13] M. Quoirin, P. Lepoivre, Etude de milieux adaptes aux cultures in vitro de prunus, Acta Hort. 78 (1977) 437 – 442. [14] O. Pe´rez-Tornero, F. Ortı´n-Pa´rraga, J. Egea, L. Burgos, Medium-term storage of apricot shoot tips in vitro by minimal growth method, Hort. Sci. 34 (1999) 1277 – 1278. [15] E. Olmos, E. Hellı´n, Cellular adaptation from a salt-tol-erant cell line of Pisum sati6um, J. Plant Physiol. 148
(1996) 727 – 734.
[16] P. Druart, Effect of culture conditions and leaf selection on organogenesis ofMalus domesticacv. McIntosh ‘Wi-jcik’ and Prunus canescensBois GM79, Acta Hort. 280 (1990) 117 – 124.
[17] M. Welander, G. Maheswaran, Shoot regeneration from leaf explants of dwarfing apple rootstocks, J. Plant Physiol. 140 (1992) 223 – 228.
[18] D.J. James, A.J. Passey, E. Rugini, Factors affecting high frequency plant regeneration from apple leaf tissues cultured in vitro, J. Plant Physiol. 132 (1988) 148 – 154. [19] M. Laimer da Caˆmara Machado, A. da Caˆmara Machado, V. Hanzer, D. Mattanovich, G. Himmler, H.W.D. Katinger, Regeneration of shoots from leaf discs and stem microcuttings of fruit trees as a tool for transformation, Acta Hort. 235 (1988) 85 – 92.
[20] L.M. Yepes, H.S. Aldwinckle, Factors that affect leaf regeneration efficiency in apple, and effect of antibiotics in morphogenesis, Plant Cell Tissue Org. Cult. 37 (1994) 257 – 269.
[21] M. Sarwar, R.M. Skirvin, Effect of thidiazuron and 6-benzylaminopurine on adventitious shoot regeneration from leaves of three strains of ‘McIntosh’ apple (Malus x domestica Borkh) in vitro, Sci. Hort. Amsterdam 68 (1997) 95 – 100.
[22] C. Leblay, E. Chevreau, L.M. Raboin, Adventitious shoot regeneration from in vitro leaves of several pear cultivars (Pyrus communis L.), Plant Cell Tissue Org. Cult. 25 (1990) 99 – 105.
[23] S.S. Korban, P.A. O’Connor, A. Elobeidy, Effects of thidiazuron, naphthaleneacetic acid, dark incubation and genotype on shoot organogenesis from Malusleaves, J. Hort. Sci. 67 (1992) 341 – 349.
[24] A. De Bondt, K. Eggermont, I. Penninckx, I. Goderis, W.F. Broekaert,Agrobacterium-mediated transformation of apple (Malus x domestica Borkh): an assessment of factors affecting regeneration of transgenic plants, Plant Cell Rep. 15 (1996) 549 – 554.
[26] F. Famiani, N. Ferradini, P. Staffolani, A. Standardi, Effect of leaf excision time and age, BA concentration and dark treatments on in vitro shoot regeneration of M.26 apple rootstock, J. Hort. Sci. 69 (1994) 679 – 685. [27] M.M. Blancke, A.R. Belcher, Stomata of apple leaves cultured in vitro, Plant Cell Tissue Org. Cult. 19 (1989) 85 – 89.
[28] M. Welander, Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees, J. Plant Physiol. 132 (1988) 738 – 744.
[29] E. Chevreau, F. Mourgues, M. Neveu, M. Chevalier, Effect of gelling agents and antibiotics on adventitious bud regeneration from in vitro leaves of pear, In Vitro Cell. Dev. Biol. Plant 33 (1997) 173 – 179.