TREE vol. 15, no. 9 September 2000 0169-5347/00/$ – see front matter © 2000 Elsevier Science Ltd. All rights reserved. PII: S0169-5347(00)01940-6 3 6 9
30 Waddell, P.J. et al. (1999) Using novel phylogenetic methods to evaluate mammalian mtDNA, including amino acid invariant sites LogDet plus site stripping, to detect internal conflicts in the data, with special reference to the positions of hedgehog, armadillo and elephant. Syst. Biol. 48, 31–53
31 Galtier, N. and Gouy, M. (1998) Inferring pattern and process: maximum likelihood implementation of a nonhomogeneous model of DNA sequence evolution for phylogenetic analysis.Mol. Biol. Evol. 15, 871–879
32 Galtier, N. and Mouchiroud, D. (1998) Isochore evolution in mammals: a human-like ancestral structure.Genetics 150, 1577–1584
33 Mouchiroud, D. et al. (1997) Impact of changes in GC content on the silent molecular clock in murids.Gene 205, 317–322
34 Galtier, N. et al. (1999) A nonhyperthermophilic common ancestor to extant life forms.Science 283, 220–221
35 Hillis, D.M. (1996) Inferring complex phylogenies.Nature 383, 130–131
36 Soltis, P.S. et al. (1999) Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology.Nature 402, 402–404
37 Karlin, S. and Mrazek, J. (1997) Compositional differences within and between eukaryotic genomes. Proc. Natl. Acad. Sci. U. S. A. 94, 10227–10232
38 Lockhart, P.J. et al. (1998) A covariate model explains apparent phylogenetic structure of oxygenic photosynthetic lineages. Mol. Biol. Evol. 15, 1183–1188
39 Steel, M. et al. (2000) Invariable site models and their use in phylogeny reconstruction. Syst. Biol. 49, 225–232
40 Chang, B.S.W. and Campbell, D.L. Bias in phylogenetic reconstruction of vertebrate rhodopsin sequences.Mol. Biol. Evol. (in press)
REVIEWS
T
here is an intriguing paradox in arbuscular mycorrhizal (AM) fungal ecology. Al-though there are numerous lab-oratory studies that have shown a variety of benefits to plants in forming a mycorrhizal association, there have been far fewer occa-sions when these benefits have also been demonstrated in natural situations1. Positive effects of AM fungi on plants include enhanced nutrient uptake, protection against pests and pathogens, and relief of drought stress2. Recently, it has been suggested that the response of any plant to AM colonization lies along a continuum from positive to negative3. Therefore, the question naturally arises as to whether lab-oratory studies at the positive end of this continuum are realisticmimics of field situations, which appear to lie in the null (no response) area. There is an urgent need to understand this problem, so that the ecology of mycorrhizas might be better described and future management of the symbiosis improved. In laboratory studies, many factors can be con-trolled, and some or all of these might be responsible for reducing the efficacy of AM fungi in field conditions. These include soil nutrient levels, plant stress factors (e.g. drought), plant diseases, and herbivorous and mycopha-gous animals4. An important group in the latter category is the Collembola (springtails).
Collembola are abundant microarthropods in virtually all soils, feeding on a range of materials, including fungi, bac-teria, lichens, decomposing vegetation and detritus. The feeding ecology of most species is poorly known5, but there appears to be a preference for fungal hyphae over other food types. By consuming dead vegetation and hyphae, these animals can play an important role in decomposition processes6. In many cases, Collembola can enhance the decomposition process because hyphal grazing stimulates growth and respiration of the fungi7. The fact that most of
the subterranean species feed (at least in part) on fungi has led to their being regarded as important regulators of the mycorrhizal sym-biosis. Reviews of AM–soil fauna interactions suggest that Collem-bola have the potential to restrict mycorrhizal functioning in the field8,9, but null and stimulative effects of their feeding have also been recorded. Other authors question their importance4, thus it is timely to ask whether Collem-bola are responsible for the dis-ruption of AM associations, and whether they are one reason for the failure of field experiments to match results obtained under con-trolled conditions.
Laboratory studies
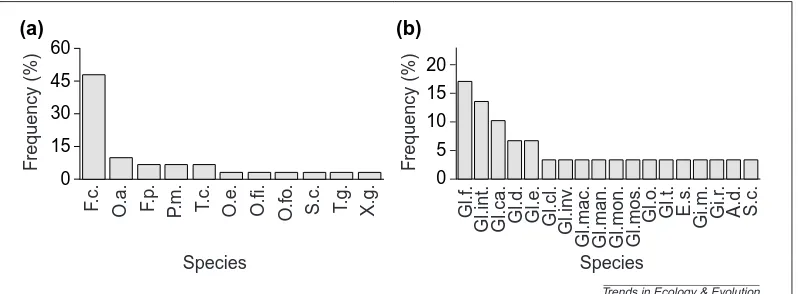
It is apparent that one species,
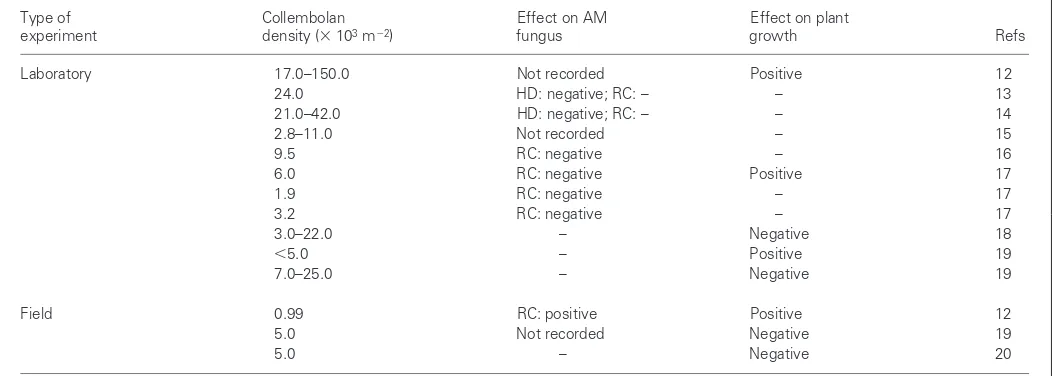
Folsomia candida, has been used in almost 50% of laboratory pot trials (Fig 1a). The reason is simple: F. candidais excep-tionally easy to culture, unlike many other species. However, to quote from a recent review10, using this species as repre-sentative of all Collembola ‘is about as ecologically sound as choosing a mole as a “typical” mammal’. It is interesting that the frequency distribution of AM fungal species used shows a less skewed distribution (Fig. 1b), but this also represents species that are generally amenable to pot culture. There have been remarkably few attempts to recreate a field situ-ation in the laboratory by using co-occurring species of Collembola and AM fungi from one field site (but see Ref. 11). Collembola densities in pot trials are usually given in numbers of individuals per dm3, but these have been con-verted to numbers per m2for ease of comparison with known field densities (Table 1). With few exceptions12, the density of animals used has been at, or below, that normally encoun-tered in comparable field situations21. A feature of this sum-mary is that only the two early studies recorded a negative effect on plant growth resulting from collembolan grazing on the mycorrhiza18,19. In these laboratory trials, there are three
Arbuscular mycorrhizal fungi,
Collembola and plant growth
Alan Gange
Arbuscular mycorrhizal fungi are ubiquitous in field soils, as are mycophagous animals such as Collembola. It has been suggested that these animals reduce the functioning of the mycorrhiza and are thus detrimental to plant growth. However, recent choice experiments suggest that Collembola preferentially feed on nonmycorrhizal
fungi in the rhizosphere. If these preferences also occur in field soils, then Collembola might indirectly benefit plants
through an enhancement of mycorrhizal functioning and indirect multitrophic links
to foliar-feeding insect herbivores.
Alan Gange is at the School of Biological Sciences, Royal Holloway University of London, Egham, Surrey,
instances of positive and two of negative effects. Six experi-ments produced no effect on plant growth, even though Collembola apparently reduced AM colonization in five of these13,14,16,17. Therefore, the notion that mycorrhizal grazing by Collembola disrupts the functioning of symbiosis is not entirely supported by the literature.
Perhaps the greatest problem with most pot trials is that they are set up with a single plant–fungus–collembolan com-bination – a situation that is most unrealistic of field condi-tions. Of crucial importance is the fact that the soil used would not have contained as diverse an array of nonmycor-rhizal fungi as would be found in the field. Although none of the trials in Table 1 was performed in sterile conditions, it is probable that the most abundant fungal hyphae would have been mycorrhizal because these were inoculated in each case. When fungal preference trials have been per-formed22,23, it has been shown that Collembola consistently graze on other soil fungi, in preference to AM species. Fur-thermore, if different Collembola species are offered a selec-tion of AM fungal species, distinct preferences are seen by each Collembolan species; however, these are not consist-ent between Collembola24,25. Therefore, Collembola will graze on AM fungi but not through choice. The conclusion is that future pot trials involving Collembola, AM fungi and plants should also include a known nonmycorrhizal fungal complement. If a situation is set up in which the animals have little choice but to feed on the mycorrhizal species offered, then at reasonably high density we would expect to see a detrimental effect on the functioning of the symbiosis. However, if other fungal species are preferentially grazed, then the outcome of the experiment could be positive for the plant (see next section).
Field experiments
To understand the role of Collembola in AM associations, we need to perform studies in field situations. However, manipu-lation of soil communities is extremely difficult because there is no biocide specific to either Collembola or AM fungi. In the few situations where insecticide application has been used to reduce numbers of Collembola19,20(Table 1), there was some evidence that reduction in grazing resulted in increased phosphorus (P) uptake and plant growth. This
might be circumstantial evi-dence that the Collembola were grazing on the AM fungi, thus reducing plant P uptake. It is also possible that the death of many soil animals resulted in a flush of nutrients for plants, thus resulting in higher P content in the bio-cide treatment. Furthermore, the insecticide used in both studies was broad-spectrum and the observed effects could have resulted from the removal of other larger rhi-zophagous insects, which feed on roots and also disrupt the mycorrhizal mycelium.
Instead of attempting to reduce numbers of a target group, one can take the al-ternative approach and aug-ment a community with a particular species. Techni-cally, this is much easier than reduction, but is open to criticism in that the resulting densi-ties might be unrealistically high. One experiment has taken this approach, with the addition of Collembola to micro-cosms surrounding soybean plants12. In this case, the aug-mented densities were not excessive (a 26% increase), possi-bly owing to predation of the Collembola. However, what is interesting is that mycorrhizal colonization of the plants was enhanced in Collembola addition treatments, although no effect on plant growth was recorded. It is plausible that this study provides the first field evidence to prove that, at mod-erate densities, Collembola are actually beneficial to mycor-rhizas rather than detrimental. These effects could have been caused by enhanced grazing on fungi, which compete with, or are antagonistic to, AM species – similar to the laboratory experiments described in the next section.
Positive effects on plants
Several controlled studies have shown a positive effect on plant growth of collembolan feeding on mycorrhiza12,17,19. In each case, the response of the plant at increasing collem-bolan densities has been bell-shaped, remarkably similar to that seen in situations where Collembola graze on non-AM fungi10. For the plant–AM fungal association, stimulation of plant growth at intermediate densities is thought to result from either an increase in hyphal growth or mineralization of nitrogen (N) and P. At low collembolan densities, the hyphae are not stimulated to grow, but at high densities the grazing is detrimental. At intermediate densities, the preferential re-moval of small diameter hyphae towards the exterior of the mycelium22 might result in proliferation and a consequent increase in benefit to the plant through mineral uptake. Al-ternatively, the release of N and P from Collembola faeces26, and subsequent plant uptake, might be sufficient to more than compensate for hyphal loss at intermediate animal densities.
These studies12,17,19also suffer from oversimplification, because Collembola were presented with only one mycor-rhizal species as a food source. A much more realistic experiment11examined the effects of feeding by a variety of microarthropods on AM fungi and growth of sugar maple (Acer saccharum). Here, the simultaneous addition of three mite and three collembolan species, at densities similar to those found in the field, had no effect on maple growth.
REVIEWS
3 7 0 TREE vol. 15, no. 9 September 2000
Fig. 1. The frequency that Collembola and arbuscular mycorrhizal (AM) species are used in laboratory experiments reported in the ISI database. (a) The frequency histogram of Collembola is heavily skewed, being dominated by the eas-ily cultured Folsomia candida. Key: F.c., Folsomia candida; O.a., Onychiurus ambulans; F.p., Folsomia penicula; P.m., Proisotoma minuta; T.c., Tullbergia clavata; O.e., Onychiurus encarpatus; O.fi., Onychiurus fimatus; O.fo., Onychiurus folsomi; S.c., Sinella coeca; T.g., Tullbergia granulata; and X.g., Xenylla grisea. (b) The frequency histogram of AM fungi is also heavily skewed and dominated by species widely available in culture. Key: Gl.f., Glomus fasciculatum; Gl.int., Glomus intraradices; Gl.ca., Glomus caledonium; Gl.d., Glomus deserticolum; Gl.e., Glomus etunicatum; Gl.cl., Glomus clarum; Gl.inv., Glomus invermaium; Gl.mac., Glomus macrocarpum; Gl.man., Glomus manihotis; Gl.mon., Glomus monosporum; Gl.mos., Glomus mosseae; Gl.o., Glomus occultum; Gl.t., Glomus tenue; E.s., Entrophosphora schenkii; Gi.m., Gigaspora margarita; Gi.r., Gigaspora rosea; A.d., Acaulospora denticulata; and S.c., Scutellospora calospora.
Trends in Ecology & Evolution
TREE vol. 15, no. 9 September 2000 3 7 1
However, addition of the microarthropods and some de-caying maple leaf litter resulted in a 59% increase in arbus-cular colonization and a 32% increase in shoot biomass. Because the microarthropods preferred to feed on the non-AM fungi in the experiment, it appears that providing them with an alternative food source allowed mycorrhizal growth. It was suggested that by feeding on fungi that might compete with the mycorrhiza for root space and by the release of minerals from the ingested hyphae, greater AM colonization of roots and nutrient uptake occurred, with a benefit to plant growth. Therefore, this study11(like the pre-viously mentioned field experiment12) raises the intriguing possibility that Collembola might be beneficial, rather than detrimental, to mycorrhizal functioning.
Mechanisms
There is no doubt that Collembola are capable of grazing on mycorrhizal hyphae, but these are probably not their pre-ferred food23. Choice experiments in which Collembola are fed AM and non-AM fungi are extremely limited22,27. As a result, it is unknown why AM fungi appear to be relatively unpalatable to these animals, compared with saprophytic fungi. One reason, based on the optimal foraging model28, has been put forward27. According to this model, one would expect Collembola to preferentially feed on the food source that is most energetically rewarding and that maximizes reproductive success. It is interesting that the one study to address this question has found that reproductive success was indeed greater on non-AM fungi27when consumed in preference to AM fungi. However, the actual mechanism determining palatability remains unknown. It might be due to hyphal thickness and architecture, because most of the intri-cate AM mycelium is composed of hyphae .10 mm (Ref. 29) and Collembola preferentially attack thin hyphae ,0.5 mm in diameter22. Alternatively, AM hyphae might be low in nutrients or high in antifeedants compared with saprophytic fungal hyphae, but these possibilities have yet to be examined.
At some of the extremely high field densities that have been reported for these animals21, it is possible that pre-ferred fungal food resources could be depleted to the extent that AM fungi become heavily grazed. However, we
have yet to see a field experiment with Collembola and AM fungi in which the animal densities reach their recorded upper range21of 1 3105 m22. If, at these extraordinary den-sities, AM fungi are eaten in the field, then the nature of the grazing interaction becomes important. If the ends of hyphal elements are severed, then regeneration might occur and the chance for proliferation of the hyphae exists. However, a mycorrhiza has an internal hyphal element in a root and an external one in the soil. If the hyphae are sev-ered at the root surface, then this could have serious con-sequences for the plant. The internal mycelium would still receive carbon compounds from the host but the recipro-cal transaction, namely the provision of mineral nutrients, is lost. Because the internal mycelium might represent as much as 20% of the biomass of a root8, it would become para-sitic rather than mutualistic. The limited evidence we have is that even at high densities Collembola do not sever the larger hyphae at the root surface, but instead attack thin walled hyphae away from the root22,23. Such a pattern of attack might limit the ability of the fungus to forage for nutrients, but the effects on the plant will be far less severe than if the hyphae were severed at the root surface. There-fore, it is possible that in most field situations, Collembola do not have a sufficiently negative effect on mycorrhizal functioning to be manifest in directly reduced plant growth. Instead, they are more likely to have positive effects on plant growth and there are several mechanisms by which this might occur. Grazing on non-AM fungi might result in increased N mineralization; however, this might not always result in an increased uptake of nitrate (NO32) by the
mycorrhiza26. This is because other microbes in the rhizo-sphere might take up the N and immobilize it. Grazing on non-AM fungi, which compete with the mycorrhizal fungus for root space, might allow greater colonization of the root by AM fungi. There is little evidence for this (Table 1), but if it did occur, it might be of indirect benefit to plants through enhanced nutrient uptake or protection against root pathogenic fungi30.
A fascinating possibility is that Collembola might have indirect effects on plant growth by causing changes in the performance of foliar-feeding insects on the same plant.
REVIEWS
Table 1. Investigation of grazing by Collembola on AM fungi and the consequences for plant growtha,b
Type of Collembolan Effect on AM Effect on plant
experiment density (3103m22) fungus growth Refs
Laboratory 17.0–150.0 Not recorded Positive 12 24.0 HD: negative; RC: – – 13 21.0–42.0 HD: negative; RC: – – 14 2.8–11.0 Not recorded – 15 9.5 RC: negative – 16 6.0 RC: negative Positive 17 1.9 RC: negative – 17 3.2 RC: negative – 17 3.0–22.0 – Negative 18
,5.0 – Positive 19 7.0–25.0 – Negative 19
Field 0.99 RC: positive Positive 12 5.0 Not recorded Negative 19 5.0 – Negative 20
aKey: AM, arbuscular mycorrhizal fungi; HD, hyphal density; RC, density of root colonization; –, no effect.
bReferences obtained from the ISI database. To put the collembolan densities used into perspective, an approximate average value for the temperate ecosystems
REVIEWS
3 7 2 TREE vol. 15, no. 9 September 2000
Although not a mycorrhizal experiment, it has recently been shown that the presence of Collembola in soil can lead to a decrease in reproduction of the aphid Myzus persicae when feeding on Trifolium repens31. The causal mechanism was unclear. If, at moderate densities, Collem-bola can enhance mycorrhizal colonization, this might lead to improved plant growth because AM fungi increase the resistance of foliar tissues to chewing insects32. The mechanism is thought to be one in which the C:N ratio of the plant is increased by the mycorrhiza, leading to an increase in carbon-based defence compounds that are active against generalist chewing insects33. Meanwhile, if high densities of Collembola do reduce AM colonization, then the performance of foliar-sucking insects might also be reduced because AM fungi have recently been shown to increase aphid performance34.
A final mechanism by which Collembola might positively affect AM fungi is through their dispersal. Several studies have shown that AM fungal spores can be present in the guts of Collembola8and the first demonstration of AM dispersal in soil by Collembola has recently been presented35. The effect depends on the species of mycorrhiza involved.
Prospects
Evidence for the disruption of the AM mutualism by Collem-bola is equivocal. Indeed, the opposite might be true; Collembola might allow enhanced mycorrhizal growth and thereby be of indirect benefit to plants. However, there is an urgent need for the design of microcosm experiments that are ecologically realistic, so as to better understand this fascinating interaction. In particular, densities of Collembola need to mimic those found in the field, with eco-logically realistic combinations of AM and non-AM fungi. The mechanism determining fungal palatability needs to be established and experiments conducted to determine whether preferential feeding is caused by chemical or mor-phological differences in AM and non-AM fungi. Finally, to determine if Collembola do reduce mycorrhizal functioning in the field, technologically difficult experiments need to be done, in which populations of animals and fungi are manipu-lated, although other soil organisms are unaffected. Given that AM fungi can affect the structure of plant communities, enhancing diversity and productivity36, an understanding of the interactions between Collembola, AM fungi and her-bivorous insects will be an important step forward in our knowledge of community-structuring forces.
Acknowledgements
I am grateful to A. Fitter and J. Klironomos for their helpful comments on this article.
References
1 Klironomos, J.N. and Kendrick, W.B. (1993) Research on mycorrhizas –
trends in the past 40 years as expressed in the MYCOLIT database. New Phytol. 125, 595–600
2 Dodd, J.C. (2000) The role of arbuscular mycorrhizal fungi in agro- and natural ecosystems. Outlook Agric. 29, 5–62
3 Gange, A.C. and Ayres, R.L. (1999) On the relation between arbuscular mycorrhizal colonization and plant ‘benefit’. Oikos 87, 615–621
4 Smith, S.E. and Read, D.J. (1997) Mycorrhizal Symbiosis, Academic Press
5 Rusek, J. (1998) Biodiversity of Collembola and their functional role in the ecosystem. Biodiv. Conserv. 7, 1207–1219
6 Hedlund, K. and Ohrn, M.S. (2000) Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88, 585–591
7 Lussenhop, J. (1992) Mechanisms of microarthropod–microbial interactions in soil. Adv. Ecol. Res. 23, 1–33
8 Fitter, A.H. and Sanders, I.R. (1992) Interactions with the soil fauna. In Mycorrhizal Functioning, An Integrative Plant–Fungal Process (Allen, M.F., ed.), pp. 333–354, Chapman & Hall
9 Fitter, A.H. and Garbaye, J. (1994) Interactions between mycorrhizal
fungi and other soil organisms. Plant Soil 159, 123–132
10 Hopkin, S.P. (1997) Biology of the Springtails (Insecta: Collembola),
Oxford University Press
11 Klironomos, J.N. and Kendrick, W.B. (1995) Stimulative effects of
arthropods on endomycorrhizas of sugar maple in the presence of decaying litter. Funct. Ecol. 9, 528–536
12 Lussenhop, J. (1996) Collembola as mediators of microbial symbiont
effects upon soybean. Soil Biol. Biochem. 28, 363–369
13 Larsen, J. and Jakobsen, I. (1996) Interactions between a
mycophagous Collembola, dry yeast and the external mycelium of an arbuscular mycorrhizal fungus.Mycorrhiza 6, 259–264
14 Larsen, J. and Jakobsen, I. (1996) Effects of a mycophagous Collembola on the symbioses between Trifolium subterraneum and three arbuscular mycorrhizal fungi. New Phytol. 133, 295–302
15 Boerner, R.E.J. and Harris, K.K. (1991) Effects of collembola
(Arthropoda) and relative germination date on competition between mycorrhizal Panicum virgatum (Poaceae) and non-mycorrhizal Brassica nigra (Brassicaceae). Plant Soil 136, 121–129
16 Kaiser, P.A. and Lussenhop, J. (1991) Collembolan effects on establishment of vesicular–arbuscular mycorrhizae in soybean (Glycine max). Soil Biol. Biochem. 23, 307–308
17 Harris, K.K. and Boerner, R.E.J. (1990) Effects of belowground grazing
by collembola on growth, mycorrhizal infection, and P uptake of Geranium robertianum. Plant Soil 129, 203–210
18 Warnock, A.J. et al. (1982) The influence of a springtail Folsomia candida (Insecta, Collembola) on the mycorrhizal association of leek Allium porrum and the vesicular–arbuscular mycorrhizal endophyte Glomus fasciculatus. New Phytol. 90, 285–292
19 Finlay, R.D. (1985) Interactions between soil micro-arthropods and
endomycorrhizal associations of higher plants. In Ecological Interactions in Soil (Fitter, A.H. et al., eds), pp. 319–331, Blackwell
20 McGonigle, T.P. and Fitter, A.H. (1988) Ecological consequences of arthropod grazing on VA mycorrhizal fungi. Proc. R. Soc. Edinburgh Sect. B 94B, 25–32
21 Petersen, H. and Luxton, M. (1982) A comparative analysis of soil faunal
populations and their role in decomposition processes. Oikos 39, 287–388
22 Klironomos, J.N. and Kendrick, W.B. (1996) Palatability of microfungi
to soil arthropods in relation to the functioning of arbuscular mycorrhizae, Biol. Fertil. Soils 21, 43–52
23 Klironomos, J.N. and Ursic, M. (1998) Density-dependent grazing on
the extraradical hyphal network of the arbuscular mycorrhizal fungus, Glomus intraradices, by the collembolan, Folsomia candida. Biol. Fertil. Soils 26, 250–253
24 Moore, J.C.et al. (1985) Ingestion of vesicular–arbuscular mycorrhizal
hyphae and spores by soil microarthropods. Ecology 66, 1979–1981
25 Thimm, T. and Larink, O. (1995) Grazing preferences of some
collembola for endomycorrhizal fungi. Biol. Fertil. Soils 19, 266–268
26 McGonigle, T.P. (1995) The significance of grazing on fungi in nutrient
cycling. Can. J. Bot. 73 (Suppl.), S1370–S1376
27 Klironomos, J.N. et al. (1999) Reproductive significance of feeding on saprobic and arbuscular mycorrhizal fungi by the collembolan, Folsomia candida. Funct. Ecol. 13, 756–761
28 Stephens, D.W. and Krebs, J.R. (1986) Foraging Theory, Princeton
University Press
29 Friese, C.F. and Allen, M.F. (1991) The spread of VA mycorrhizal fungal
hyphae in soil – inoculum types and external hyphal architecture. Mycologia 83, 409–418
30 Newsham, K.K. et al. (1995) Multifunctionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10, 407–411
31 Scheu, S. et al. (1999) Links between the detritivore and herbivore
system: effects of earthworms and Collembola on plant growth and aphid development. Oecologia 119, 541–551
32 Gange, A.C. and Bower, E. (1997) Interactions between insects and mycorrhizal fungi. In Multitrophic Interactions in Terrestrial Systems (Gange, A.C. and Brown, V.K., eds), pp. 115–132, Blackwell Science
33 Gange, A.C. and West, H.M. (1994) Interactions between arbuscular
mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 128, 79–87
34 Gange, A.C. et al. (1999) Positive effects of mycorrhizal fungi on aphid life history traits. Oecologia, 120, 123–131
35 Klironomos, J.N. and Moutoglis, P. (1999) Colonization of
nonmycorrhizal neighbours as influenced by the Collembolan, Folsomia candida. Biol. Fertil. Soils 29, 277–281
36 van der Heijden, M.G.A. et al. (1998) Mycorrhizal fungal diversity determines