L
Journal of Experimental Marine Biology and Ecology 247 (2000) 233–242
www.elsevier.nl / locate / jembe
Effects of copper and zinc on growth, feeding and oxygen
consumption of Farfantepenaeus paulensis postlarvae
(Decapoda: Penaeidae)
* ´
Marcos Henrique Silva Santos, Nerile Troca da Cunha, Adalto Bianchini
˜
´ ˆ ´
Laboratorio de Zoofisiologia, Departamento de Ciencias Fisiologicas, Fundac¸ao Universidade Federal do
Rio Grande, Caixa Postal 474, CEP 96201-900 Rio Grande, RS, Brazil
Received 17 August 1999; received in revised form 1 November 1999; accepted 31 December 1999
Abstract
The effect of chronic exposure (35 days) to sub-lethal concentrations of copper (17–212 ppb) and zinc (41–525 ppb) on growth of Farfantepenaeus paulensis postlarvae 17 days old (PL )17 was analysed. The effects of acute exposure of PL17 to the same metal on food ingestion and oxygen consumption were also evaluated. Studies were performed using copper and zinc singly, and in a mixture of equipotent concentrations (1:2.5). Chronic exposure to copper (85 and 212 ppb) and zinc (106, 212 and 525 ppb) reduced PL17 growth. Acute exposure to copper (212 ppb) and zinc (525 ppb) reduced the number of Artemia sp. predated during 30 min and the positive feeding response induced by L-isoleucine. Despite of the lower positive feeding response when PL17were exposed to zinc, a significant difference from control condition was not seen. Oxygen consumption was reduced by all copper and zinc concentrations tested. The mean reduction was approximately 32%. The copper zinc-mixture did not modify food consumption and feeding response, or the oxygen consumption of the PL . The inhibition of food and oxygen consumption17 induced by copper and zinc could explain, at least in part, the long-term reduction of growth observed in chronically exposed PL . Our results also suggest that the inhibition of food17 consumption induced by copper is possibly due to an effect on chemosensory mechanisms. Finally, an antagonism between copper and zinc was observed, when were employed to analyse feeding behaviour and aerobic metabolism after acute exposure. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Shrimp; Copper; Zinc; Antagonism; Food consumption; Oxygen consumption
*Corresponding author. Tel.:155-532-31-1900 (ext. 149); fax:155-532-338655.
E-mail address: [email protected] (A. Bianchini)
1. Introduction
Industrial activity along coastal regions has significantly contributed to increases of natural levels of heavy metals in aquatic systems. In the Patos Lagoon estuary (Southern Brazil), areas in which copper and zinc concentrations exceed the estimated natural levels have been reported (Baisch et al., 1988; Niencheski et al., 1994). However, this region is a natural hatchery for the pink shrimp Farfantepenaeus paulensis, which is the most important fishing resource in Southern Brazil (Valentini et al., 1991). The life cycle includes both, marine and estuarine phases. They penetrate the Patos Lagoon estuary as postlarvae (6th substage) at approximately 3 weeks of age (Iwai, 1978), and develop until a pre-adult stage (7–8 cm total length) when they return to sea (D’Incao, 1991). During their residence in the estuary, postlarvae are susceptible to all contaminants available in the environment, and as mentioned below, the effects of excessive copper and zinc concentrations on postlarvae physiology must be considered.
Copper and zinc are essential metals to normal physiology of crustaceans. However, they become toxic if high environmental concentrations are attained. Some general effects of heavy metals on crustacean physiology have been reported (Thurberg et al., 1973; Spicer and Weber, 1991; Wong et al., 1993). These effects also include growth and reproduction disturbances (Nimmo and Hamaker, 1982). Some of these effects may be due to an energetic imbalance carried out by the action of heavy metals on enzymatic systems, which in turn lead to metabolic changes. Considering food ingestion as the major input of energy in crustaceans, and oxygen consumption as a measure of metabolism, the effects of sub-lethal copper and zinc concentrations on these parameters were analysed in F. paulensis postlarvae. Their effects on feeding response were also considered. Chronic effects of sub-lethal concentrations of copper and zinc on PL17
growth was studied for 35 days. All studies were performed using copper and zinc singly, and in a mixture of equipotent concentrations.
2. Material and methods
Farfantepenaeus paulensis postlarvae (PL) were reared in the Marine Aquaculture
˜
Station of the ‘‘Fundac¸ao Universidade Federal do Rio Grande’’ (Rio Grande, RS, Southern Brazil) following the method described by Marchiori (1996). Two day old PL (PL ) were previously acclimated to aerated saline water at 252 61.5‰; 2561.08C and photoperiod 12L:12D, for 15 days. Saline water employed was pumped from Cassino Beach (Rio Grande, RS, Southern Brazil) and filtered (1 mm mesh). The acclimated PL17 (total length59.860.4 mm; wet weight53.760.4 mg) were then employed for the experiments. Before tests, they were starved for 24 h in plastic flasks containing 500 ml of saline water at the same conditions of acclimation.
In order to determine the 96h-LC , twenty PL were exposed to each of the following50
nominal concentrations of copper or zinc: 0 (control); 0.5; 1.0; 2.0; 4.0 and 8.0 ppm of
21 21
Based on the 96h-LC50 values, PL were exposed to the following nominal
con-21
centrations of copper and zinc: 0 (control); 17; 43; 85 and 212 ppb of Cu as
21
CuSO .5H O; and 0 (control); 41; 106; 212 and 525 ppb of Zn4 2 as ZnSO .7H O. It4 2
must be pointed out that both concentration sequences present the same lethal toxicity and correspond to approximately 0; 1.2; 3; 6 and 15% of the 96h-LC50 (see Results). Cooper and zinc were tested singly or in mixture. Copper–zinc mixtures (approximately 1:2.5) were obtained adding the equipotent concentrations of these metals, e.g. 17 ppb
21 21
Cu 141 ppb of Zn . All experimental media were prepared employing the same water used for postlarvae acclimation. Experiments were performed under the same conditions (temperature, salinity and photoperiod).
2.1. Growth experiments
Acclimated PL17 were exposed to the different concentrations of copper and zinc, singly or in mixture, in plastic trays containing 10 l of the experimental medium, for 35 days. Every 2 days, the experimental media were totally renewed. During the exposure period, PL were fed ad libitum with nauplii (first 15 days) or adults of Artemia sp. At the beginning of the experiment (N543) and after 35 days of exposure (N513–129; Table 1), PL were captured, measured (total length) and weighed (wet weight and dry weight). Experiments were performed in duplicate. Results were submitted to Kruskal-Wallis analysis followed by a posteriori test described in Conover (1980).
2.2. Feeding experiments
Acclimated PL17 (N510–13; Table 2) non pre-exposed to the metals were in-dividually exposed to the different concentrations of copper and zinc, singly or in mixture, for 30 min. They were exposed in separated plastic flasks containing 10 ml of experimental medium and 10 nauplii of Artemia sp., in darkness because F. paulensis PL is more active and feeds at night (Iwai, 1978). PL was then isolated and weighed (wet weight). The nauplii remaining in the flask were counted and the results were expressed in terms of number of nauplii predated / 30 min. In the control group, a possible effect of PL weight on the food consumption was analysed by means of linear regression. Data of food consumption were submitted to Kruskal-Wallis analysis followed by a posteriori test described in Conover (1980).
In order to study the effect of copper and zinc on the postlarvae feeding response, we
o
]
employed the method described by Santos F (1983). Acclimated PL17 (N510) non pre-exposed to the metals were individually exposed to the highest concentrations of copper and zinc tested (singly or in mixture), for 4 min. They were exposed in separated flasks containing 10 ml of experimental medium and a piece of filter paper (Whatman No. 1; 2.532.5 mm), previously immersed in a saturated sea water solution of L-isoleucine and dried at 378C. This aminoacid was used since it was demonstrated to be
o
]
of filter paper in their mouths within 2 min after presentation and swallowing it or, rejecting it only after, at least, 4 min in their mouths’’. Data were submitted to the Kolmogorov-Smirnov two-sample test.
2.3. Oxygen consumption experiments
Acclimated PL17 (N55–8; Table 3) non pre-exposed to the metals were exposed to the different concentrations of copper and zinc, singly or in mixture, in a static respirometer containing 7.5 ml of experimental medium, for 15 min. In this case, saline water was previously filtered (1 mm mesh) and autoclaved (20 min at 1208C). The respirometer was mounted on a magnetic stirrer and provided with an O2 electrode connected to an oxymeter (Digimed, Brazil). Each 5 min, dissolved O was measured2
and the oxygen consumption calculated, taking into account the respirometer volume. Previous experiments indicated that by 15 min constant consumption rates were obtained. So, after 15 min the PL17 oxygen consumption was measured, in duplicate. PL17 were then isolated and weighed (wet weight). Results were expressed in mg
21 21
O .g2 .h and submitted to ANOVA followed by Duncan’s multiple range test. In the control group, a possible effect of PL weight on the oxygen consumption was analysed by means of linear regression.
All statistical analysis were performed with ‘‘Statistica for Windows’’ (StatSoft, Inc., 1995) and the significance level adopted was 5% (a 50.05).
3. Results
The 96h-CL50 and the corresponding 95% intervals for copper and zinc were
21
estimated as 1.4 (0.9–1.9) and 3.5 (3.0–4.6) ppm (mg.l ). Chronic exposure (35 days) to sub-lethal concentrations of copper and zinc, singly or in mixture, caused important and significant reductions in PL17 growth. These reductions were observed on both total length and wet or dry weight (Table 1). Some mortality was also registered after 35 days of experiment, but only in the highest concentrations of metals. At 212 ppb of copper or zinc and in the mixture of 851212 ppb of copper1zinc, mortality was about 50%. At 525 ppb of zinc and in the mixture of 2121525 ppb of copper1zinc, it was about 85%. In the food consumption experiments, results from the control group showed no significant correlation between food consumption and wet weight (P.0.05; r50.04;
N513). However, acute exposure to copper or zinc significantly reduced the number of nauplii predated by PL . Concentrations of 43 and 212 ppb of copper caused reductions17
of 28.3 and 27.1% in food consumption, respectively. For zinc, only the highest concentration tested (525 ppb) affected food consumption. In this case, 25.0% inhibition was observed. In the other hand, no significant effect on food consumption was observed when copper–zinc mixtures were tested (P.0.05) (Table 2).
Table 1
Total length and weight (wet and dry) of Farfantepenaeus paulensis postlarvae 17 days old before (T group)0
and after (copper, zinc and copper1zinc groups) exposure to sub-lethal concentrations of copper
a
(CuSO .5H O) and zinc (ZnSO .7H O), either singly or in mixture (1:2.5), for 35 days4 2 4 2
Group Concentration Total length Wet weight Dry weight N
(ppb) (cm) (mg) (mg)
T0 0.9260.01 2.960.2 0.9060.04 43
Copper Control 2.0060.02 (a) 59.662.4 (a) 11.460.5 (a) 100
17 2.0160.03 (a) 61.162.5 (a) 11.260.5 (a) 108
43 1.9660.03 (a) 54.862.2 (b) 10.360.4 (a) 103
85 1.7860.02 (b) 42.261.6 (c) 7.760.3 (b) 129
212 1.4860.02 (b) 22.561.1 (d) 4.360.2 (c) 61
Zinc Control 2.0060.02 (a) 59.662.4 (a) 11.460.5 (a) 100
41 1.9860.03 (a) 58.262.6 (a) 11.060.5 (ac) 104
106 1.8760.03 (b) 49.562.4 (b) 9.360.5 (b) 108
212 1.8760.03 (b) 48.362.5 (b) 9.160.5 (c) 49
525 1.5960.09 (c) 34.166.4 (b) 5.761.2 (d) 13
Copper Control 2.0060.02 (a) 59.662.4 (a) 11.460.5 (a) 100
1 17141 1.8860.02 (b) 47.261.9 (b) 8.860.4 (b) 116
Zinc 431106 1.7560.02 (c) 38.261.6 (c) 7.360.3 (c) 106
851212 1.6460.03 (d) 32.662.4 (d) 6.560.5 (c) 52 2121525 1.2760.04 (e) 15.061.9 (e) 2.460.3 (d) 18
a
Data are means6S.E. Same letters indicate equal means (P.0.05) in the same group for each parameter.
Table 2
Mean number (6S.E.) of Artemia sp. nauplii predated by F. paulensis postlarvae 17 days old during 30 min. Postlarvae were acutely exposed to different concentrations of copper (CuSO .5H O) and zinc (ZnSO .7H O)4 2 4 2
a
either isolated or in mixture (1:2.5)
Metal Concentration Food consumption Inhibition N
(ppb) (number of nauplii) (%)
Copper Control 8.2360.43 (a) – 13
17 7.9060.84 (a) 4.0 10
43 5.9060.92 (b) 28.3 10
85 7.2060.53 (ab) 12.5 10
212 6.0060.91 (b) 27.1 10
Zinc Control 8.2360.43 (a) – 13
41 8.6760.31 (a) – 12
106 8.4260.50 (a) – 12
212 8.0960.31 (ab) 1.7 11
525 6.1760.71 (b) 25.0 12
Copper Control 8.2360.43 (a) – 13
1 17141 7.7060.62 (a) 6.4 10
Zinc 431106 8.0060.58 (a) 2.8 10
851212 7.4060.43 (a) 10.1 10
2121525 7.6060.69 (a) 7.7 10
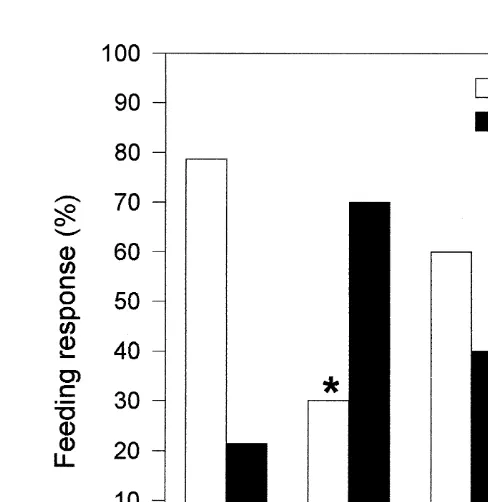
a
Fig. 1. Feeding response of Farfantepenaeus paulensis postlarvae 17 days old acutely exposed to copper (212 ppb) as CuSO .5H O and zinc (525 ppb) as ZnSO .7H O, either singly or in mixture. * Indicates group4 2 4 2
significantly different from control group (P,0.05).
Table 3
Oxygen consumption of F. paulensis postlarvae 17 days old acutely exposed to different concentrations of
a
copper as CuSO .5H O and zinc as ZnSO .7H O, either singly or in mixture4 2 4 2
Metal Concentration Oxygen consumption Inhibition N 21 21
(ppb) (mg O .g2 .h ) (%)
Copper Control 1.7760.10 (a) – 8
17 1.1860.25 (b) 33.5 5
43 1.1760.17 (b) 34.0 5
85 1.1460.11 (b) 35.6 5
212 1.3460.10 (b) 24.2 7
Zinc Control 1.7760.10 (a) – 8
41 1.2760.29 (ab) 28.0 5
106 1.2360.19 (b) 30.5 5
212 1.0660.13 (b) 40.3 5
525 1.2460.15 (b) 30.0 5
Copper Control 1.7760.10 (a) – 8
1 17141 1.7860.28 (a) – 5
Zinc 431106 1.5260.18 (a) 14.4 5
851212 1.6260.17 (a) 8.4 5
2121525 1.1660.24 (a) 34.7 5
a
PL17 oxygen consumption was significantly reduced by all copper and zinc con-centrations tested, except 41 ppb of zinc. For both metals, the effect was not dose dependent, and the mean reduction registered was of approximately 32%. As observed above for feeding experiments, the copper–zinc mixture did not significantly modify the PL17 oxygen consumption, despite the fact that a reduction of 34.7% was observed in the higher concentration tested (Table 3). It is important to note that in the control group, no significant correlation could be established between oxygen consumption rate and PL17 wet weight (P.0.05; r50.15; N58).
4. Discussion
Acute exposure to copper and zinc reduced food consumption by Farfantepenaeus
paulensis PL . Wong et al. (1993) also observed a reduction in the rate of Artemia sp.17
nauplii consumption by PL of Metapenaeus ensis, when PL were exposed to copper (23
ppm), chromium (8 ppm) and nickel (1 ppm). In the crab Cancer irroratus, Johns and Miller (1982), reported that larvae food ingestion was reduced by exposure to copper (10 ppb) and cadmium (50 ppb). In the same species, Johns and Pechenik (1980), demonstrated that larvae exposure to crude oil also reduced food ingestion. The authors suggested that this response was due to narcosis caused by the oil on the sense organs of the larvae, as previously proposed by Blumer et al. (1973) for other marine organisms. In the case of metals, the physiological basis of pollutant action on the feeding behaviour is not well established. However, it is known that metals inhibit the chemoreception in aquatic animals (Sutterlin, 1974). Further, it was demonstrated that the metal effects on the nervous system could induce impairment of prey capture and manipulation, as well as, of locomotory activity (Bryan et al., 1995). Despite the fact that our results can not discard this hypothesis, they strongly suggest that the inhibition of food ingestion induced by copper is, at least in part, due to an inhibition of the mechanisms involved in chemoreception. This assumption is based on the fact that the feeding response induced by L-isoleucine was significantly reduced after acute exposure to copper (212 ppb) and tended to be lower after acute exposure to zinc (525 ppb).
In addition to the inhibitory effect of copper and zinc on feeding behaviour, a significant reduction of PL17oxygen consumption was observed. This effect was noticed in all concentrations tested, except in the lowest concentration of zinc (41 ppb). A decrease in metabolic rate induced by heavy metals has been also described for other crustacean species. Exposure to cooper and zinc, for example, significantly reduced the metabolic rate in the shrimp Caridina rajadhari (Chinnaya, 1971). A similar effect was observed when the shrimp Palaemon serratus was exposed to cadmium and mercury (Papathanassiou, 1983) and the mysid Leptomysis lingvura was contaminated with sub-lethal concentrations of cadmium (Gaudy et al., 1991). According to Bryan (1971) and Mehrle and Mayer (1985), alterations in oxygen consumption induced by metals could be due to their effects on oxydizing enzymes.
maintenance and growth under this situation would be reduced. So, if the PL did not reduce the energy requirement to maintain other physiological processes than growth, a reduction in the growth rate would be expected under this situation. In fact, it was observed that chronic exposure to sub-lethal concentrations of copper and zinc, singly or in mixture, significantly reduced both the total length and weight (wet and dry) of F.
paulensis PL . Inhibition of growth after copper and zinc exposure has also been17
described for other crustaceans species (Saliba and Krzyz, 1976; McKeenney and Neff, 1979; White and Rainbow, 1982).
In the present study, acute exposure of F. paulensis PL17 to mixtures of sub-lethal and equipotent concentrations of copper and zinc did not change the feeding behaviour and the aerobic metabolism. Thus, our results, suggest an antagonism between these metals under the experimental conditions employed here. Antagonism between metals has also been widely described in aquatic toxicology experiments using several groups of living organisms. For example, nickel toxicity to growth of filamentous bacteria could be reduced by addition of zinc to culture medium (Shuttleworth and Unz, 1991). Antagonism between cadmium and zinc was observed in metal accumulation in the macroalga Enteromorpha prolifera (Haritonidis et al., 1994). In the marine prosobranch
Monodonta turbinata, a mixture of copper and chromium caused less pronounced effects
on the survival, oxygen consumption and bioaccumulation than copper and chromium acting alone (Catsiki et al., 1993). Alonso and MartinMateo (1996) have established a competition between zinc and copper for the production of metallothionein in the oyster
Ostrea edulis. Moulder (1980) observed a reduction of the lethal toxicity induced by
inorganic mercury in Gammarus duebeni when in presence of sub-lethal concentrations of copper. In fathead minnows Pimephales promelas, Parrott and Sprague (1993) also observed antagonism for some combinations of toxicants. In the other hand, according to Klaassen and Eaton (1991) the most common result of exposure to a metal mixture is an additive effect. In fact, reciprocal effect of metals (additive, synergism and antagonism) depends on the metal ion speciation and the comparative concentrations of each metal (Parrott and Sprague, 1993; Wang et al., 1995). For example, in the marine algae
Phaeosactylum antagonism occurs if Cd / Cu is in the range of 0.4 to 4, but synergism
appeared beyond this concentration range. Zinc and copper presented antagonism when the ratio of Zn / Cu was between 1 and 20, synergism appeared out of this range (Wang et al., 1995). So, the metal interaction observed in the present study is consistent with the data presented above, since antagonism between zinc and copper was observed when Zn / Cu52.5 was tested on feeding behaviour and oxygen consumption of F. paulensis PL . The physiological basis of this antagonism cannot be explained from our results.17
However, it seems that this interaction is consistent with the physicochemical similarity between zinc and copper, which implies in similar potential effects on metal ion speciation, competitive interactions affecting membrane transport, or competitive interactions at sites of toxic lesions (Taylor et al., 1992; Sandstead, 1995).
5. Conclusion
significantly reduced the food and oxygen consumption of PL17 of F. paulensis. These effects could explain, at least in part, the long-term reduction of growth observed in F.
paulensis PL17chronically exposed to the same concentrations of these metals. Also, our results suggest that the inhibition of food consumption induced by copper is possibly due to an effect on the postlarvae chemosensory mechanisms. Finally, a marked antagonism between copper and zinc was observed when mixtures of sub-lethal and equipotent concentrations were employed to analyse feeding behaviour and aerobic metabolism.
Acknowledgements
We thank E.A. Santos for careful reading of the manuscript and FAPERGS for financial support (Proc. 97 / 0535-8). A. Bianchini is a research fellow from Brazilian CNPq (Proc. 300536 / 90-9). [SS]
References
Alonso, J.I., MartinMateo, M.C., 1996. Induction and characterization of metallothionein in different organs of
Ostrea edulis L. Biol. Trace Element Res. 53, 85–94.
Baisch, P.R., Niencheski, L.F.H., Lacerda, L.D., 1988. Trace metal distribution in sediments of the Patos Lagoon estuary, Brazil. In: Seeliger, U., de Lacerda, L.D., Patchineelam, S.R. (Eds.), Coastal Environments of Latin America, Springer Verlag, Berlin, pp. 59–64.
Blumer, M., Hunt, J.M., Atema, J., Stein, L., 1973. Interaction between marine organisms and oil pollution. Ecol. Res. Ser. No. EPA-R3-73-042, US Environmental Protection Agency, Washington, DC.
Bryan, G.W., 1971. The effects of heavy metals (other than mercury) in marine and estuarine organisms. Proc. R. Soc. (ser B) 117, 389–410.
Bryan, M.D., Atchison, G.J., Sandheinrich, M.D., 1995. Effects of cadmium on the foraging behavior and growth of juvenile bluegill, Lepomis macrochirus. Can. J. Fish. Aquat. Sci. 52, 1630–1638.
Catsiki, V.A., Vakalopoulou, C., Moraitouapostolopoulou, M., Verriopoulos, G., 1993. Monodonta turbinata (Born) – toxicity and bioaccumulation of Cu and Cu1Cr mixtures. Toxicol. Environm. Chem. 37, 173–184.
Chinnaya, B., 1971. Effect of heavy metals on the oxygen consumption by the shrimp Caridina rajadhari. Bouvier. Indian J. Exp. Biol. 9, 277–278.
Conover, W.J., 1980. Practical Nonparametric Statistics, John Wiley & Sons, New York. ˆ
D’Incao, F., 1991. Pesca e Biologia de Penaeus paulensis na Lagoa dos Patos, RS. Atlantica 13 (1), 159–169. ´
Gaudy, R., Guerin, J.P., Kerambrun, P., 1991. Sublethal effects of cadmium on respiratory metabolism, nutrition, excretion and hydrolase activity in Leptomysis lingvura (Crustacea: Mysidacea). Mar. Biol. 109, 493–501.
Haritonidis, S., Rijstenbil, J.W., Malea, P., Vandrie, J., Wijnholds, J.A., 1994. Trace-metal interactions in the macroalga Enteromorpha prolifera (of Muller) I. Grown in water of the Scheldt estuary (Belgium and The Netherlands), in response to cadmium exposure. Biometals 7, 61–66.
´ ´
Iwai, M., 1978. Desenvolvimento larval e pos-larval de Penaeus (Melicertus) paulensis Perez-Farfante, 1967
˜ ˆ ˜
(Crustacea, Decapoda) e o ciclo de vida dos camaroes do genero Penaeus da regiao centro-sul do Brasil.
˜ ˜
PhD. Thesis, Universidade de Sao Paulo, Sao Paulo, Brazil, 138 p.
Johns, D.M., Miller, D.C., 1982. The use of bionergetics to investigate the mechanisms of pollutant toxicity in crustacean larvae. In: Vernberg, W.B., Calabrese, A., Thurberg, F.B., Vernberg, F.J. (Eds.), Physiological Mechanisms of Marine Pollutant Toxicity, Academic Press, New York, pp. 261–288.
Klaassen, C.D., Eaton, D.L., 1991. Principles of Toxicology. In: Amdur, M.O., Doull, J., Klaassen, C.D. (Eds.), Casaret and Doull’s Toxicology – The Basic Science of Poisons, 4th edn., Pergamon Press, New York, pp. 12–49.
˜ ˜
Marchiori, M.A., 1996. Guia Ilustrado de Maturac¸ao e Larvicultura do Camarao-Rosa Penaeus paulensis ´
Perez-Farfante, 1967. EdFURG, Rio Grande-RS, Brazil.
McKeenney, C.L., Neff, J.M., 1979. Individual effects and interactions of salinity, temperature and zinc on larval development of the grass shrimp Palaemonetes pugio. I. Survival and developmental duration through metamorphosis. Mar. Biol. 52, 177–188.
Mehrle, P.M., Mayer, F.L., 1985. Biochemistry / Physiology. In: Rand, G.M., Petrocelli, S.R. (Eds.), Fundamentals of Aquatic Toxicology: Methods and Applications, Taylor & Francis, USA, pp. 264–282. Moulder, M.S., 1980. Combined effect of the chlorides of mercury and copper in sea water on the euryhaline
amphipod Gammarus duebeni. Mar. Biol. 59, 193–200.
Niencheski, L.F., Windom, H.L., Smith, R., 1994. Distribution of particulate trace metal in Patos Lagoon estuary (Brazil). Mar. Poll. Bull. 28, 96–102.
Nimmo, D.R., Hamaker, T.L., 1982. Mysids in toxicity testing – a review. Hydrobiologia 93, 171–178. Papathanassiou, E., 1983. Effects of cadmium and mercury ions on respiration and survival of the common
prawn Palaemon serratus (Pennant). Rev. Int. Oceanogr. Med. 72, 21–35.
Parrott, J.L., Sprague, J.B., 1993. Patterns in toxicity of sublethal mixtures of metals and organic-chemicals
determined by microtox and by DNA, RNA, and protein-content of fathead minnows (Pimephales
promelas). Can. J. Fish. Aquat. Sci. 50, 2245–2253.
Saliba, J., Krzyz, R.M., 1976. Acclimation and tolerance to copper salts. Mar. Biol. 38, 231–238. Sandstead, H.H., 1995. Requirements and toxicity of essential trace-elements, illustrated by zinc and copper.
Am. J. Clin. Nutr. 61, S621–S624.
o ]
Santos F , E.A. dos, 1983. The inducer of the feeding response in Penaeus paulensis (Crustacea-Decapoda). Physiol. Behav. 31, 733–734.
Shuttleworth, K.L., Unz, R.F., 1991. Influence of metals and metal speciation on the growth of filamentous bacteria. Water Res. 25, 1177–1186.
Spicer, J.I., Weber, R.E., 1991. Respiratory impairment in crustaceans and molluscs due to exposure to heavy metals. Comp. Biochem. Physiol. 100C (3), 339–342.
StatSoft, Inc., 1995. STATISTICA for Windows (Computer program manual). Tulsa, OK: StatSoft, Inc., 2300 East 14th Street, Tulsa OK, 74104-4442, (918) 749–1119.
Sutterlin, A.M., 1974. Pollutants and chemical senses of aquatic animals – perspective and review. Chem. Senses Flavor 1, 167–178.
Taylor, G.J., Stadt, K.J., Dale, M.R.T., 1992. Modeling the interactive effects of aluminium, cadmium, manganese, nickel and zinc stress using the Weibull frequency-distribution. Environm. Exper. Botany 32, 281–293.
Thurberg, F.P., Sawson, M.A., Collier, R.S., 1973. Effects of copper and cadmium on osmoregulation and oxygen consumption in two species of estuarine crabs. Mar. Biol. 23, 171–175.
´
Valentini, H., D’Incao, F., Rodrigues, L.F., Rebelo Neto, J.E., Rahn, E., 1991. Analise da pesca do
˜ ˜ ˆ
camarao-rosa (Penaeus brasiliensis e Penaeus paulensis) nas Regioes Sudeste e Sul do Brasil. Atlantica 13 (1), 143–157.
Wang, J.Y., Zhang, M.P., Xu, J.G., Wang, Y., 1995. Reciprocal effect of Cu, Cd, Zn on a kind of marine alga. Water Res. 29, 209–214.
White, S.L., Rainbow, P.S., 1982. Regulation and accumulation of copper, zinc and cadmium by the shrimp
Palaemon elegans. Mar. Ecol. Prog. Ser. 8, 95–101.