www.elsevier.com / locate / bres
Research report
Early loss of synaptic protein PSD-95 from rod terminals of rhodopsin
P347L transgenic porcine retina
a a,b a a a
Scott M. Blackmon , You-Wei Peng
, Ying Hao , Suk Jin Moon , Leonardo B. Oliveira ,
a d a,b,c ,
*
Misako Tatebayashi , Robert M. Petters , Fulton Wong
a
Department of Ophthalmology, Duke University School of Medicine, Durham, NC 27710, USA
b
Department of Neurobiology, Duke University School of Medicine, Durham, NC 27710, USA
c
Department of Pathology, Duke University School of Medicine, Durham, NC 27710, USA
d
Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA
Received 13 July 2000; accepted 29 August 2000
Abstract
Retinitis pigmentosa (RP), a type of retinal degeneration involving first rod and then slow cone photoreceptor degeneration, can be caused by any of a number of mutations in different genes. In the cases of mutations affecting rod-specific genes such as rhodopsin, it is unclear how the mutations may cause degeneration of cones. We have used the porcine retina, which is rod-dominated and has an abundance of cones, to study the mutation-induced changes in both rod and cone photoreceptors. Like patients with the same mutation, rhodopsin P347L transgenic swine manifest rod–cone degeneration. In addition, the rod bipolar cells fail to form synaptic connections with rods; instead, they form ectopic synapses with cones. The mechanisms that prevent the formation of the rod–rod bipolar cell synaptic connection are not known. We used specific antibodies and immunocytochemistry to show that the synaptic protein, PSD-95, is present in both normal and transgenic porcine retinas. During neonatal development, however, PSD-95 is lost from rod terminals in the transgenic swine. This loss is virtually complete (90%) by postnatal day 5, at a time when greater than 80% of rod cell bodies still remain. Furthermore, the remaining rods retain their outer segments and their gross morphology appears relatively normal. In contrast, PSD-95 expression continues in cone terminals, even in 10-month-old transgenic swine, where the rods have all disappeared and the cones show signs of severe degeneration. These results suggest that loss of PSD-95 may not be a general consequence of the deteriorating cell. Rather, the very early and selective loss of PSD-95 from the rod terminals may be causally related to the absence of rod–rod bipolar cell synapses in the rhodopsin P347L transgenic retina. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Genetic models
Keywords: PSD-95; Retinal degeneration; Retinitis pigmentosa
1. Introduction mutations affecting rod-specific genes such as rhodopsin, however, it is unclear how the mutations may cause Retinitis pigmentosa (RP) is an inherited disorder in degeneration of cones, which do not express rhodopsin. To which mutations in various retina-specific genes may lead address this issue, we have created the rhodopsin P347L to rod and then slow cone photoreceptor degeneration transgenic pig model [26], which has provided the oppor-[2,8,30]. In RP patients, the ultimate loss of cones impairs tunity to study the mutation-induced changes in both rod visual function that is critical to their lives. Therefore, a and cone photoreceptors.
major challenge for RP research is elucidating the mecha- Like patients with the same mutation, these transgenic nisms underlying cone degeneration. In the cases of swine manifest rod–cone degeneration [26]. In these transgenic swine, in addition to photoreceptor death, the rod bipolar cells fail to form synaptic connections with the *Corresponding author. Tel.: 11-919-684-8579; fax: 1
1-919-684-rods; instead, they form ectopic synapses with and receive 8829.
E-mail address: [email protected] (F. Wong). input from the surviving cones [24,25]. This finding has
created a new conceptual framework to address the conse- serves as a rod-specific marker, and anti-G protein-gc quences of the rhodopsin P347L mutation, such as discern- subunit polyclonal antibody (CytoSignal), referred to ing the mechanisms that prevent the formation of the hereafter as anti-cone transducin. G protein-gc is the g rod–rod bipolar cell synaptic connection. subunit of cone transducin and thus serves as a
cone-Synapses are highly specialized structures, the formation specific marker [23]. of which requires the interactions of dozens of unique
synaptic proteins. As a preliminary screen, we performed 2.3. Immunocytochemistry PCR to identify, in normal and transgenic porcine retinas,
some of the expressed genes that encode for proteins For immunoperoxidase cytochemistry, the retinal sec-known to be associated with synapses [31]. Among the tions were incubated with mouse monoclonal anti-PSD-95 genes identified (unpublished results of Blackmon and antibody 1:200 overnight at 48C, followed by three washes Wong), PSD-95 is present in both normal and transgenic in PBS for 15 min each. Triton X-100 (0.3%) was added to porcine retinas. This finding is consistent with recently all incubation and wash buffers. The sections were then published results showing the presence of PSD-95 in the incubated with a biotin-conjugated anti-mouse secondary mammalian retina, including the rat, monkey, and tree antibody (1:200, Vector Laboratories) in PBS for 2 h at shrew [17]. room temperature and then washed three times in PBS for In the studies reported here, we have used specific 15 min each, followed by incubation with an avidin– antibodies and immunocytochemical methods to determine biotin–peroxidase complex (1:100, Vector Laboratories) in the localization of PSD-95 in rod and cone terminals in the PBS for 1 h. After three more washes of 15 min each in porcine retina. We discovered that the rod terminals of the PBS, the staining was developed with a substrate solution transgenic swine lose PSD-95 during early postnatal life, of 20 ml of PBS, 0.1 ml of 3% hydrogen peroxide and 10 before substantial rod degeneration has taken place. Since mg of diaminobenzidine (Sigma). After washing with PBS in the central nervous system (CNS), PSD-95 is known to to terminate the staining reactions, the sections were then play an important role in synapse formation and / or coverslipped with 50% glycerol in PBS.
maintenance, our results suggest that early loss of PSD-95 For fluorescence microscopy, the retinal sections were from the rod terminals may be causally related to the incubated with mixed primary antibodies (rabbit polyclonal absence of rod–rod bipolar cell synapses in the rhodopsin anti-cone transducin antibody 1:1000 and mouse mono-P347L transgenic retina. clonal anti-PSD-95 antibody 1:100) overnight at 48C. After two washes in PBS for 15 min, sections were incubated with mixed secondary antibodies (rhodamine-conjugated
2. Materials and methods goat anti-mouse immunoglobulin secondary antibody 1:50 and fluorescein isothiocyanate-conjugated goat anti-rabbit 2.1. Tissue preparation immunoglobulin secondary antibody 1:50) for 1 h at room
temperature.
Transgenic swine from the line Pig-TgN1Pet [26] and For single-labeling immunocytochemistry using anti-non-transgenic littermates were used in this study. Porcine rhodopsin antibodies, the procedure was the same except eyes of different ages were removed from the animals that one primary antibody (mouse monoclonal anti-rhodop-killed under deep anesthesia. After removal of the anterior sin antibody 1:500) and one secondary antibody (fluores-segments, the posterior eyecups were fixed in 4% parafor- cein isothiocyanate-conjugated goat anti-mouse immuno-maldehyde in 100 mM sodium phosphate buffer (PBS, pH globulin secondary antibody 1:50) were used.
7.3) at 48C overnight. The eyecups were then frozen in For microscopic analysis, the sections were examined isopentane cooled in liquid nitrogen, and embedded in and photographed using a 633 objective with a Zeiss O.C.T. embedding medium (Triangle Biomedical Sciences, LSM-410 confocal microscope with rhodamine isothio-Durham, NC). Retinal sections, 10-mm thick, were pre- cyanate- and fluorescein isothiocyanate-fluorescence exci-pared from a cryostat and mounted on gelatin-coated tations.
slides. One normal and one transgenic pig of each of the
following ages were used in this study: newborn (,24-h- 2.4. Quantifications of immunoreactive rod terminals old), 5-day, 2-week, and 10-month. and nuclei
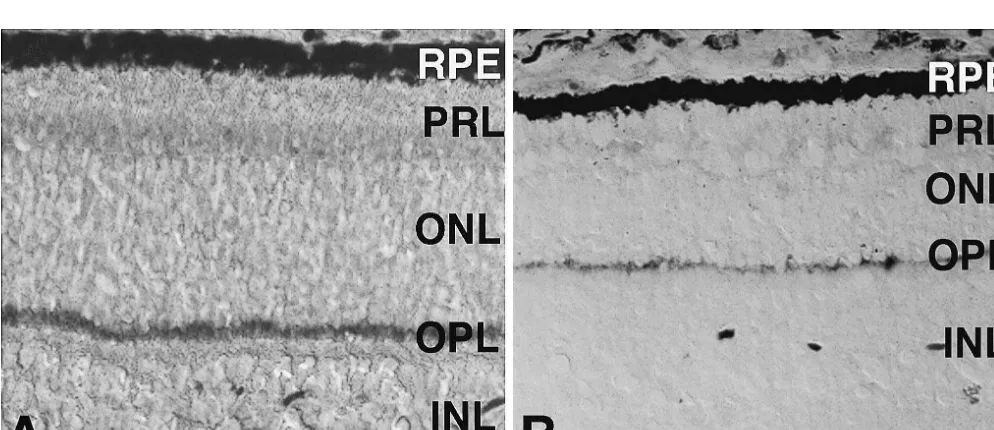
determine the numbers of rod terminals present per high terminals (also known as spherules and pedicles, respec-magnification field. An example of this technique is shown tively), PSD-95 immunoreactivity appears as a very strong in Fig. 2c, which identifies seven rod synaptic terminals band in the outer plexiform layer (OPL) of a normal retina present within the cluster marked by the upper arrow (Fig. 1a). The cellular localization of PSD-95 was further denoted by an asterisk. determined in experiments using antibodies specific for the Twelve micrographs of the sections that were single synaptic vesicle protein synaptophysin, which is known to labeled for rhodopsin were taken with the 633 objective localize in the rod and cone terminals in the OPL [3]. (six from the mid-peripheral retina and six from the central Using double-labelling immunocytochemistry with anti-retina). The numbers of rod cell bodies (labeled with bodies specific to PSD-95 and synaptophysin, respectively, rhodopsin) were then counted per 200 mm of retinal we showed virtually complete colocalization of these two section. Quantitative studies of the numbers of rod cells in proteins in the OPL of normal porcine retinas at all the transgenic porcine retinas were achieved by counting the ages tested (data not shown.)
rhodopsin immunoreactive nuclei (Fig. 5). Staining by PSD-95 immunoreactivity is also present in the OPL of anti-rhodopsin antibodies allowed excellent visualization the 2-week-old transgenic porcine retina (Fig. 1b). Con-of the rod cell bodies in the transgenic swine due to sistent with the expected reduction in the number of delocalization of rhodopsin [20]. The rod cell bodies in the photoreceptors due to degeneration [20,26,29], the band of normal retina were only weakly labeled by anti-rhodopsin PSD-95 immunoreactivity is thinner than that of a normal staining. Therefore, the intensity of the confocal image was retina of the same age.
artificially increased so that the rod nuclei could be quantified in a similar fashion to allow comparisons
between the normal and transgenic retinas. 3.2. PSD-95 is selectively lost from the rod terminals in Differences between the numbers of PSD-95-expressing transgenic swine
rod terminals (or rhodopsin-positive rod cell bodies) in
normal vs. transgenic swine are reported in this study as Rod and cone photoreceptor terminals were identified by mean6standard error. Statistical analyses were performed double-labeling immunofluorescence confocal microscopy using the independent t-test (SigmaPlot). using antibodies specific for PSD-95 and cone transducin (a cone-specific marker), respectively. In the 2-week-old normal porcine retina, as expected from the results
ob-3. Results tained from immunoperoxidase cytochemistry,
immuno-fluorescence signal due to presence of PSD-95 appears as a 3.1. PSD-95 is present in the OPL of normal and thick band in the OPL (Fig. 2a). Furthermore,
double-transgenic swine labeling of PSD-95 and cone transducin reveals two separate but adjacent ‘layers’ in this thick band of the Consistent with its expected localization in rod and cone OPL: a layer of rod terminals in the outer margin
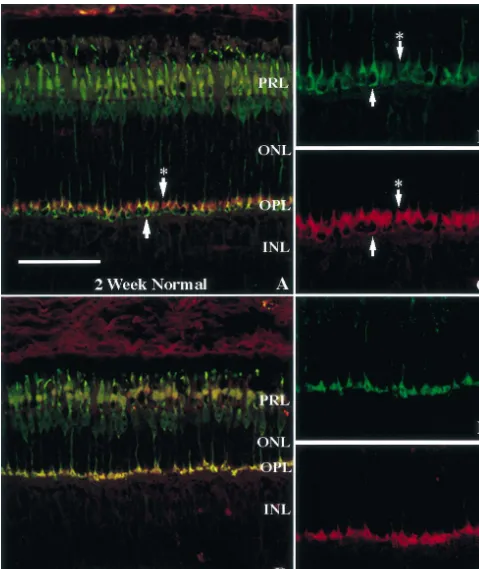
Fig. 2. PSD-95 is selectively lost from the rod terminals of young transgenic swine. Cross sections of 2-week-old normal (A,B,C) and P347L (D,E,F) retinas stained with anti-PSD-95 (red) and anti-cone transducin (green) antibodies using confocal microscopy. Arrowhead marks a cone pedicle. Arrowhead with asterisk marks a cluster of rod terminals. Refer to Fig. 1 for other symbols. Scale550mm.
Fig. 3. Cones continue to express PSD-95 in late stages of retinal degeneration. Cross sections of 10-month-old normal (A) and P347L (B) retinas stained with anti-PSD-95 and anti-cone transducin antibodies using confocal microscopy. IPL, inner plexiform layer; refer to Fig. 1 for other symbols. Scale550 mm.
Fig. 4. The loss of PSD-95 from the rod terminals of transgenic swine is observable early in the neonatal period. Cross sections of newborn normal (A), newborn P347L (B), and 5-day-old P347L (C) retinas stained with anti-PSD-95 and anti-cone transducin antibodies using confocal microscopy. Refer to Fig. 1 for symbols. Scale550mm.
2a. (For comparison, a cone terminal is marked by the comparing 2-week-old (Fig. 2) and 10-month-old (Fig. 3) lower arrow in Fig. 2a.) Individual red-stained rod retinas, then, PSD-95 is selectively and completely lost spherules can be resolved at a higher magnification, as from rod terminals early in postnatal life but continues to explained in Section 2.4 of Materials and methods and be expressed in cone terminals, even in late stages of illustrated in Fig. 2c (upper arrow marked by an asterisk). retinal degeneration.
Identification of rod and cone terminals is further
demonstrated by comparing Fig. 2b and Fig. 2c, which 3.3. Loss of PSD-95 immunoreactivity occurs before correspond to showing the two immunofluorescence labels substantial rod degeneration
separately and at a higher magnification of the same
2-week-old normal retina as shown in Fig. 2a. The upper Studies performed in neonatal swine show that the loss arrows in Fig. 2b and Fig. 2c point to a cluster of rod of PSD-95 is observable even in newborn animals. In the spherules immunoreactive for PSD-95 (Fig. 2c) but not for normal retina, both rod and cone terminals express PSD-95 cone transducin (Fig. 2b). The lower arrows in Fig. 2b and (Fig. 4a). Double-labeling immunofluorescence signals Fig. 2c point to a cone terminal that is immunoreactive for show a pattern typical of normal retinas (Figs. 2a and 3a). both cone transducin and PSD-95. The signal due to The rod terminals are stained red only whereas the cone presence of PSD-95 is stronger in the outer portions of the terminals appear yellowish-green. In contrast, in the trans-cone terminal. genic retina, while there are still a small number of rod In the 2-week-old transgenic porcine retina, similar terminals expressing PSD-95, most of them are no longer double-labeling immunofluorescence studies using PSD-95 PSD-95 immunoreactive (Fig. 4b). By postnatal day 5, and cone transducin as markers identified very few red- even fewer PSD-95 immunoreactive rod terminals are staining rod terminals; instead, only a single row of observed (Fig. 4c). In these neonatal transgenic retinas, yellowish-green photoreceptor terminals remains (Fig. 2d). almost all the terminals appear yellowish-green, suggesting Since these terminals appear yellowish-green due to the that they belong to cones.
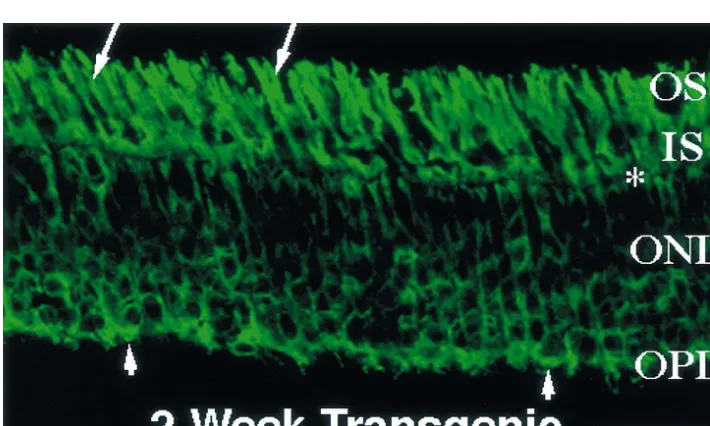
presence of both the red and green signals derived from Results shown in Figs. 2 and 4 indicate an early, PSD-95 and cone transducin, respectively, they must selective loss of PSD-95 from rod terminals. At the ages belong to cones. The presence of only cone terminals in when loss of PSD-95 occurs, it is expected that most of the the transgenic retina is further demonstrated by comparing rods still remain and, at least morphologically, appear Fig. 2e and f, which correspond to showing the two healthy [20,26,29]. Immunofluorescence labeling with immunofluorescence labels separately and at a higher antibodies specific for rhodopsin illustrates the condition of magnification of the same 2-week-old transgenic retina as the 2-week-old transgenic retina (Fig. 5). In normal shown in Fig. 2d. As shown in Fig. 2e and f, for every porcine retinas, intense rhodopsin immunoreactivity is seen PSD-95-staining terminal, there is corresponding immuno- in the rod outer segments with relatively weak staining reactivity for cone transducin. Therefore, PSD-95 immuno- elsewhere in the rod cell bodies. These data are very reactivity is lost from virtually all the rod terminals but similar to those shown in Ref. [20]. In transgenic retinas, continues to be expressed in cone terminals. in contrast, rhodopsin is delocalized, appearing in outer As illustrated by the immunofluorescence signal in Fig. and inner segments, nuclei, and synaptic terminals. This 2a and c (normal retina), PSD-95 appears to concentrate in expected pattern of rhodopsin immunoreactivity [20] is the outer portions of the cone pedicles, leaving a non- shown in Fig. 5. A large number of rod cell bodies are stained interior. In comparison, the non-stained interior is present, and these rods still retain their outer segments, much smaller in the cone pedicles of a transgenic retina of which are generally shortened. Numerous rod synaptic the same age (Fig. 2d and f); thus, the cone pedicles of the terminals can also be observed, although they are no longer transgenic retina appear more compacted than those in PSD-95 immunoreactive.
Table 1
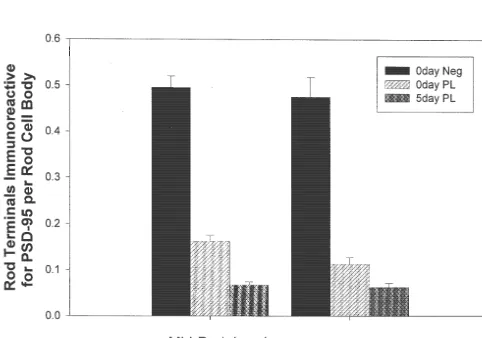
The loss of PSD-95 immunoreactivity in transgenic retinas is much greater than the concurrent loss of rod cell bodies in the same retinas
PSD-95 Rod cell bodies
Rod terminals stained as % Rod cell bodies remaining as % of newborn normal (n57) of newborn normal (n56)
Region of retina Mid peripheral Central Mid peripheral Central
Newborn normal 100 100 100 100
Newborn transgenic 30.5 22.5 92.9 92.4
5-day-old transgenic 11.2 10.8 82.4 81.2
4. Discussion loss of PSD-95 from the rod terminals appears to be selective in that it occurs while the overall morphology of The present study, then, confirms for the first time the the rods is relatively intact and many rod outer segments presence of the synaptic protein PSD-95 in the OPL of the are still present. In addition, electrophysiological record-swine retina. The presence of PSD-95 immunoreactivity in ings from single rod photoreceptors of transgenic swine both the rod spherules and cone pedicles is consistent with demonstrated that rods expressing mutant rhodopsin pos-findings in the retinas of other mammals, including the rat, sess a functioning phototransduction pathway [18]. Taken monkey, and tree shrew [17]. In the normal porcine retina, in aggregate, all the available evidence seems to suggest we show that PSD-95 is present in both the rod and cone that the loss of PSD-95 in rod terminals may be closely synaptic terminals, from birth to 10 months of age. related to, and hence is a more immediate consequence of, Accordingly, this synaptic protein may have a continuous the expression of mutant rhodopsin.
role in photoreceptor function, perhaps in maintaining the Since rhodopsin normally is restricted to the outer integrity of the synapses. Furthermore, in the transgenic segments of the rods where it functions as the light-retina, we have discovered that PSD-95 immunoreactivity detecting molecule of the phototransduction pathway, it is is lost from most of the rod synaptic terminals within a few unclear how a mutation in the rhodopsin gene may lead to days after birth. The loss of PSD-95 from the rod terminals alterations in gene expression of PSD-95, which is a does not seem to represent a general consequence of the synaptic protein. In the transgenic retina, although rhodop-deteriorating cell since PSD-95 expression persisted even sin delocalizes and is present in the rod synaptic terminals, in severely degenerated cones at a late stage of the disease there is no evidence of direct interactions between PSD-95 (in 10-month-old transgenic pigs). Rather, the very early and rhodopsin. Therefore, the link between the defective rhodopsin molecules and the regulation of expression of PSD-95 is likely an indirect one.
On the other hand, it is clear that the rod synapses in the transgenic retina are abnormal from birth [20]. Very few rods with synaptic ribbons and triads can be identified and the rod terminal surface membranes are greatly expanded, giving rise to protrusions that resemble filopodia [20]. The mechanisms underlying the defects in the rod terminals of the transgenic retina are not known. Although the photo-transduction pathway, which is ‘‘a function of the rod outer segment’’, seems to be intact [18], visual information is not relayed from the rods to the second order neurons [1,26] because of the absence of rod–rod bipolar cell synapses [24,25]. If the role of PSD-95 in rods indeed is in synaptic function, our results would suggest that its early loss may be causally related to the absence of rod–rod bipolar cell synapses in the rhodopsin P347L transgenic Fig. 6. A significant difference exists in the number of rod terminals retina. Whether the absence of PSD-95 prevented the expressing PSD-95 per rod cell body in the newborn normal retina vs. the rod–rod bipolar cell synapses from forming, or whether newborn transgenic retina and in the newborn transgenic retina vs. the failure of the formation of these synapses caused the loss 5-day-old transgenic retina. Results are shown as mean6standard error.
of PSD-95 from the rod terminal, however, remains to be (P,0.03; independent t-test). 0d Neg, newborn normal retina; 0d PL,
determined. newborn transgenic retina; 5d PL, 5-day-old transgenic retina; n57
[3] J.H. Brandstatter, S. Lohrke, C.W. Morgans, H. Wassle, Distributions the function of PSD-95 and other members of the family of
of two homologous synaptic vesicle proteins, synaptoporin and synapse-associated proteins [reviewed in Refs. [10,22,28]].
synaptophysin, in the mammalian retina, J. Comp. Neurol. 370 In the CNS, PSD-95 plays a major role in synaptic (1996) 1–10.
function, acting as an anchor for the clustering of mole- [4] J.E. Brenman, D.S. Chao, S.H. Gee, A.W. McGee, S.E. Craven, D.R. cules involved in synaptic transmission, such as NMDA Santillano, Z. Wu, F. Huang, H. Xia, M.F. Peters, S.C. Froehner, D.S. Bredt, Interaction of nitric oxide synthase with the postsynaptic receptors [11,16,21], potassium ion channels [6,11,19], and
density protein PSD-95 and alpha1-syntrophin mediated by PDZ neuronal nitric oxide synthase [4]. These are molecules in
domains, Cell 84 (1996) 757–767.
the postsynaptic densities where their selective concen- [5] G.-Q. Chang, Y. Hao, F. Wong, Apoptosis: final common pathway of tration is essential for reliable and rapid synaptic transmis- photoreceptor death in rd, rds, and rhodopsin mutant mice, Neuron sion and in synaptic plasticity [12,27]. In the retina, it may 11 (1993) 595–605.
[6] N.A. Cohen, J.E. Brenman, S.H. Snyder, D.S. Bredt, Binding of the be hypothesized that PSD-95 functions in an analogous
inward rectifier K1 channel Kir 2.3 to PSD-95 is regulated by fashion, interacting with potential candidates such as
protein kinase A phosphorylation, Neuron 17 (1996) 759–767. potassium channels, neuronal nitric oxide synthase, and [7] W.M. Cowan, J.W. Fawcett, D.D. O’Leary, B.B. Stanfield, Regres-NMDA receptors, although data published so far have not sive events in neurogenesis, Science 225 (1984) 1258–1265. conclusively supported such interactions in the retina [8] A. Gal, E. Apfelstedt-Sylla, A.R. Janecke, E. Zrenner, Rhodopsin
mutations in inherited retinal dystrophies and dysfunctions, Prog. [9,14,15,17,32].
Retin. Eye Res. 16 (1997) 51–79. Like observations made in the rat cerebellum [13,19],
[9] E. Hartveit, J.H. Brandstatter, M. Sassoe-Pognetto, D.J. Laurie, P.H. PSD-95 in the OPL of the retina has a presynaptic Seeburg, H. Wassle, Localization and developmental expression of distribution. The function of PSD-95 in the presynaptic the NMDA receptor subunit NR2A in the mammalian retina, J. membrane has not been established. The molecules in the Comp. Neurol. 348 (1994) 570–582.
[10] M.B. Kennedy, The postsynaptic density at glutamatergic synapses, presynaptic membrane that PSD-95 may interact with
Trends Neurosci. 20 (1997) 264–268. remain to be identified. The transgenic porcine retina may
[11] E. Kim, K.O. Cho, A. Rothschild, M. Sheng, Heteromultimerization serve as a convenient model for investigating these broad and NMDA receptor-clustering activity of Chapsyn-110, a member questions. Future research on defining the roles played by of the PSD-95 family of proteins, Neuron 17 (1996) 103–113. PSD-95 and other important synaptic proteins in the [12] J. Kirsch, H. Betz, Glycine-receptor activation is required for
receptor clustering in spinal neurons, Nature 392 (1998) 717–720. formation, function, and maintenance of synapses at the
[13] U. Kistner, B.M. Wenzel, R.W. Veh, C. Cases-Langhoff, A.M. rod terminals should further our general understanding of
Garner, U. Appeltauer, B. Voss, E.D. Gundelfinger, C.C. Garner, synapses and the mechanisms that govern their function.
SAP 90, a rat presynaptic protein related to the product of the Specific to RP research, elucidating the mechanisms Drosophila tumor suppressor gene dlg-A, J. Biol. Chem. 268 (1993) underlying the synaptic defects of the rods in the trans- 4580–4583.
[14] D.J. Klumpp, E.J. Song, L.H. Pinto, Identification and localization genic retina may be critical for understanding the
patho-1
of K channels in the mouse retina, Vis. Neurosci. 12 (1995) genesis of the disease and its potential treatment.
RP-1177–1190. inducing mutations, including those affecting the
rhodop-[15] D.J. Klumpp, E.J. Song, S. Ito, M.H. Sheng, L.Y. Jan, L.H. Pinto, sin gene, cause rod photoreceptor apoptosis [5,33]. Faulty The Shaker-like potassium channels of the mouse rod bipolar cell synaptic interactions with second-order neurons might and their contributions to the membrane current, J. Neurosci. 15
(1995) 5004–5013. compromise the viability of the rod photoreceptors, a
[16] H.C. Kornau, L.T. Schenker, M.B. Kennedy, P.H. Seeburg, Domain phenomenon known to occur in other parts of the CNS [7].
interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95, Science 269 (1995) 1737–1740.
[17] P. Koulen, E.L. Fletcher, S.E. Craven, D.S. Bredt, H. Wassle, Immunocytochemical localization of postsynaptic density protein
Acknowledgements psd95 in the mammalian retina, J. Neurosci. 18 (1998) 10136– 10149.
[18] T.W. Kraft, E. Banin, W.M. Sessions, A.V. Cideciyan, R.M. Petters, This work was supported in part by NIH grants
S.G. Jacobson, F. Wong, Photoreceptor function in the rhodopsin RO1EY11498 and P30EY05722; Foundation Fighting
P347L transgenic pig, Invest. Ophthalmol. Vis. Sci. 39 (1998) Blindness of Hunt Valley, MD; Research to Prevent S1123.
Blindness, Inc. of New York, NY. [19] G. Laube, J. Roper, J.C. Pitt, S. Sewing, U. Kistner, C.C. Garner, O. Pongs, R.W. Veh, Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux, Mol. Brain Res. 42 (1996) 51–61.
References [20] Z.Y. Li, F. Wong, J.H. Chang, D.E. Possin, Y. Hao, R.M. Petters,
A.H. Milam, Rhodopsin transgenic pigs as a model for human [1] E. Banin, A.V. Cideciyan, T.S. Aleman, R.M. Petters, F. Wong, A.H. retinitis pigmentosa, Invest. Ophthalmol. Vis. Sci. 39 (1998) 808–
Milam, S.G. Jacobson, Retinal rod photoreceptor-specific gene 819.
mutation perturbs cone pathway development, Neuron 23 (1999) [21] M. Niethammer, E. Kim, M. Sheng, Interaction between the C
549–557. terminus of NMDA receptor subunits and multiple members of the
[22] R.J. O’Brien, L.-T. Lau, R.L. Huganir, Molecular mechanisms of the NMDA receptor in hippocampal neurons, Neuron 19 (1997) glutamate receptor clustering at excitatory synapses, Curr. Opin. 801–812.
Neurobiol. 8 (1998) 364–369. [28] M. Sheng, PDZs and receptor / channel clustering: rounding up the [23] O.C. Ong, H.K. Yamane, K.B. Phan, H.K. Fong, D. Bok, R.H. Lee, latest suspects, Neuron 17 (1996) 575–578.
B.K. Fung, Molecular cloning and characterization of the G protein [29] M.O.M. Tso, W.W. Li, C. Zhang, T.T. Lam, Y. Hao, R.M. Petters, F. gamma subunit of cone photoreceptors, J. Biol. Chem. 270 (1995) Wong, A pathologic study of degeneration of the rod and cone 8495–8500. populations of the rhodopsin Pro347Leu transgenic pigs, Trans. Am. [24] Y.W. Peng, T.H. Mahmoud, L.B. Oliveira, M. Tatebayashi, Y. Hao, Ophthalmol. Soc. 95 (1997) 467–479.
B.W. McCuen, R.M. Petters, F. Wong, Rhodopsin mutation induces [30] S. van Soest, A. Westerveld, P.T. DeJong, E.M. Bleeker-Wagemak-ectopic cone-rod bipolar cell synaptic connections in transgenic ers, A.A. Bergen, Retinitis pigmentosa: defined from a molecular swine, Mol. Biol. Cell. 10 (Suppl.) (1999) 76a. point of view, Surv. Ophthalmol. 43 (1999) 321–334.
[25] Y.W. Peng, T.H. Mahmoud, L.B. Oliveira, M. Tatebayashi, Y. Hao, [31] K. von Kriegstein, F. Schmitz, E. Link, T.C. Sudhof, Distribution of B.W. McCuen, R.M. Petters, F. Wong, Synaptic plasticity in mam- synaptic vesicle proteins in the mammalian retina identifies obligat-malian retinas induced by rod-specific mutations, Invest. Ophthal- ory and facultative components of ribbon synapses, Eur. J. Neurosci. mol. Vis. Sci. 41 (2000) S622. 11 (1999) 1335–1348.
[26] R.M. Petters, C.A. Alexander, K.D. Wells, E.B. Collins, J.R. [32] A. Wenzel, J.M. Fritschy, H. Mohler, D. Benke, NMDA receptor Sommer, M.R. Blanton, G. Rojas, Y. Hao, W.L. Flowers, E. Banin, heterogeneity during postnatal development of the rat brain: dif-A.V. Cideciyan, S.G. Jacobson, F. Wong, Genetically engineered ferential expression of the NR2A, NR2B, and NR2C subunit large animal model for studying cone photoreceptor survival and proteins, J. Neurochem. 68 (1997) 469–478.
degeneration in retinitis pigmentosa, Nat. Biotechnol. 15 (1997) [33] F. Wong, Investigating retinitis pigmentosa: a laboratory scientist’s
965–970. perspective, Prog. Retin. Eye Res. 16 (1997) 353–373.