A physiological characterization of Mn-tolerant tobacco plants
selected by in vitro culture
Geraldina Santandrea, Tiziana Pandolfini, Andrea Bennici *
Department of Plant Biology,Uni6ersity of Florence,P.le Cascine28,50144Florence,Italy
Received 4 June 1999; received in revised form 13 September 1999; accepted 13 September 1999
Abstract
In previous research, an in vitro stepwise procedure permitted us to obtain Nicotiana tabacumregenerated plant lines able to grow in the presence of Mn at 2 and 5 mM (Mn-tolerant plants). These plants showed several morpho-physiological and cytological differences in comparison to the Mn-sensitive regenerated plants. In particular, the number of xylem cells and the degree of lignification appeared to be influenced differently by these Mn concentrations. In the present work these Mn-tolerant and Mn-sensitiveN.tabacumplants, maintained in the presence of Mn 2 and 5 mM, have been characterized with regards to the uptake of Mn and Fe, the activity of extracellular peroxidases in the stems, and the activity of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in the leaves. The leaf response to an increasing Mn concentration in the medium, corresponded a parallel decrease of Fe content. Plants tolerant of 5 mM Mn showed almost a doubling Mn content over that of the 5 mM Mn-sensitive plants. In the stem, 2 and 5 mM Mn inhibited the extracellular free peroxidases (guaiacol peroxidases) either in the Mn-tolerant plants or in the Mn-sensitive plants. In the Mn-sensitive plants treated with 2 mM Mn the activity of the peroxidases of the ionically and covalently bound wall peroxidases was also depressed. In 5 mM Mn-tolerant plants, an enhanced activity of the covalently bound wall peroxidases was observed. The effect of Mn on the covalently bound wall syringaldazine peroxidases was identical to that observed in the guaiacol peroxidases; the activity was significantly higher in the Mn-tolerant plants grown in the presence of 5 mM Mn. In the leaf, the increase of Mn content inhibited the activity of guaiacol peroxidase, ascorbate peroxidase and superoxide dismutase in the Mn-tolerant as well as in the Mn-sensitive plants. However, the effect was greater in the Mn-sensitive plants. Only glutathione reductase did not show significant variation except for the 2 mM Mn-sensitive plants, where an increased activity was detected. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Mn-tolerance;Nicotiana tabacum; Plant regeneration; Peroxidases; Glutathione reductase; Superoxide dismutase
www.elsevier.com/locate/plantsci
1. Introduction
Manganese is an essential trace element for plants where it is involved in redox reactions as a cofactor for many enzymes. In particular it is the metal component of superoxide dismutase (SOD) [1,2]. The most important function of Mn is its role as a constitutive element in the water-splitting system of PSII [3,4].
An excess of Mn can lead to toxicity conditions in natural and agricultural sites and especially in acid soils [5,6].
Manganese toxicity symptoms are observed in shoots as growth retardation, brown lesions, foliar chlorosis and crinkled leaves [7,8]. The response of plants to excess Mn at physiological level consists principally in a decline in photosynthetic rate and also in a reduction of respiration [9,10]. The Mn critical toxicity content varies widely depending on the plant species, age, temperature, light intensity and soil nutrient balance [4].
Toxic Mn effects are found also in different in in vitro developmental processes such as germina-tion, callus induction and growth, and shoot re-generation from callus [3,8,11,12]. Moreover, high Mn level has direct cytotoxic effects such as exten-sive cytoplasmic injures, mitochondria
modifica-* Corresponding author. Tel.:+39-55-328-8270; fax:+ 39-55-360-137.
E-mail address:[email protected] (A. Bennici)
tion and plasma membrane ruptures in the outer root cap and meristematic cells [13].
The physiological mechanisms of Mn toxicity and tolerance are still not well known. Many reports suggest that Mn excess may cause the induction of oxidative stress [4]. In this case, as a consequence of the uptake of toxic concentrations of the metal, several defense enzymes such as SOD, ascorbate peroxidase (ASPX), glutathione reductase (GR), guaiacol peroxidase (GPX), are induced in the chloroplasts and cytosol as protec-tion against oxidative stress [14 – 17].
Peroxidases (POD) are oxido-reductases which occur practically in all plants. They are present as several isoenzymes which are found in soluble, ionically bound and covalently bound forms [18]. Their form and distribution is, probably, related to different physiological functions [19,20] An in-crease of POD activity has been considered as a metabolic response under various stress conditions including heavy metal stress [21]. In the cell wall-located (bound) POD activity plays a key role in controlling the production and deposition of lignin in vascular tissue [22,23]. In non-lignified primary cell walls, POD may be involved in the cross-link-ing of cinnamic acids such as ferulic acid, the production of which is implicated in the enhance-ment of cell adhesion in parenchyma tissues [24 – 26]
POD may also prevent oxidative damage to plasmamembranes, acting as peroxide radical scavengers [14].
A previous research established an in vitro pro-cedure for the selection of Nicotiana tabacum var. BEL W3 callus lines and regenerated plants toler-ant to high doses of Mn (2 and 5 mM). The performance of the Mn-tolerant plants with regard to several morphological, anatomical and cytologi-cal characteristics, in comparison to the Mn-sus-ceptible regenerated plants, was described [12]. In particular, Mn was also shown to affect the num-ber of xylem elements and the degree of lignifica-tion, which differed from plants grown in the presence of 2 and 5 mM Mn compared to the control. Moreover, in these plants, damaged chloroplasts and a reduction in their number were also observed, especially for those treated with 5 mM Mn.
With an aim of better explaining these previous results and to characterize Mn-tolerant plants from the physiological point of view, the present
study was planned to determine in selected plants growing in the presence of Mn at 2 and 5 mM, (1) the activity of extracellular POD in the stems using two electron donors such as guaiacol and syringaldazine, (2) the activity of SOD, ASPX and GR in the leaves.
2. Materials and methods
2.1. Plant material and culture conditions
The selection procedure ofN.tabacumvar. BEL W3 plants tolerant to 2 and 5 mM Mn has been
previously reported [12]. Briefly, Nicotiana
into leaves and stems for enzyme extraction and for the determination of the Mn and Fe content (Mn/Fe ratio) in the leaves.
The cultures were maintained in a growth room at 2591°C under a 16 h light photoperiod (35 mmol m−2 s−1) provided by Sylvania Day-light fluorescent tubes F36W/154-ST.
2.2. Extraction of extracellular enzymes and enzymic assays
2.2.1. Stem
All stems of N. tabacum, previously weighed,
were vacuum infiltrated with 66 mM
K-phos-phate buffer pH 7.0 (5 ml g−1 FW) containing
100 mM KCl and then centrifuged at 1500 g for 10 min at 4°C, as described by Castillo and Greppin [23]. A fraction designated as ‘extracellu-lar fluid’ was obtained containing extracellu‘extracellu-lar free and ionically bound wall POD. The residual plant material was then homogenized with cold mortar and pestle, using cold absolute acetone (2 ml g−1 FW) plus quartz sand, until no fibrous residue could be seen. The homogenate was then filtered in vacuo and the resulting acetone pow-ders were dissolved in a 30 mM Tris buffer pH
7.6 (2 mg g−1 FW) containing 5 mM
mercap-toethanol, 0.5% (v/v) Tween 20, 0.1% (w/v) insol-uble PVP and 1 mM EDTA and centrifuged at 800 g for 10 min. The pellet was washed twice in 30 ml Tris buffer (pH 7.6) and then suspended in the same buffer containing 2% (v/v) Triton X-100 and centrifuged at 800 g for 10 min. The pellet, after washing in distilled water and Tris buffer, was treated with 1 mM NaCl for 2 h at room temperature and centrifuged at 800 g for 10 min. The supernatant gave the fraction containing the ‘ionically bound wall POD fraction’. The pellet was rinsed several times in distilled water, treated with 0.5% (w/v) cellulase and 2.5% pectinase in 0.1 M Na-acetate buffer, pH 5.0 for 15 h 30 min at room temperature and centrifuged at 800 g for 10 min. The supernatant represented the fraction containing the ‘covalently bound wall POD’. The activity of POD was assayed in the extracellular fluid and in the ionically and covalently bound wall fractions, using guaiacol and syringaldazine as substrates.
GPX (EC. 1.11.1.7) activity was assayed in 60 mM K-phosphate buffer pH 6.1 containing 28 mM guaiacol and 5 mM H2O2 [28]. The increase
in absorbance was recorded at 470 nm.
Syringal-dazine POD activity was assayed in 0.1 M NaK
phosphate buffer pH 6.0 containing 4.2×10−2 mM syringaldazine (dissolved in methanol – diox-ane 1:1 v/v) and 1.6 mM H2O2 [28]. The increase in absorbance was recorded at 530 nm. Guaiacol and syringaldazine POD activities were referred (measured with reference) to the protein content of each sample and expressed as DA470 min−1
mg−1 protein and DA
530 min−1 mg−1 protein, respectively. All enzyme activities were tested us-ing a Perkin – Elmer UV 55IS spectrophotometer. Protein concentration was determined by Bio-Rad Protein Assay (Biorad) with bovine plasma globulin as a standard
2.2.2. Leaf
Crude extracts of leaves were obtained from homogenization with cold acetone (2 ml g−1FW) as described for the stems. The resulting acetone powders obtained after drying the sample were dissolved in 30 mM Tris buffer pH 7.6 (3 ml g−1
FW) containing 5 mM mercaptoethanol, 0.5% (v/
v) Tween 20, 0.1% (w/v) insoluble PVP and 1
mM EDTA, and then kept at 4°C for 90 min. The extracts were filtered with Miracloth
(Cal-biochem) and centrifuged at 15 000 g at −4°C
for 20 min. The supernatant was collected for the enzyme assays. GPX (EC. 1.11.1.7) activity was assayed at 470 nm with 28 mM guaiacol, 5 mM H2O2 and 50 – 100 ml of the extract in 60 mM K-phosphate buffer (pH 6.1) [28]. ASPX (EC. 1.11.1.11) activity was measured at 290 nm by the method of Kato and Shimizu [29]. The assay mixture contained 0.5 mM Na-ascorbate, 1 mM H2O2, 0.1 mM EDTA and 100 ml of the extract in 50 mM K-phosphate buffer (pH 7.0) GR (EC. 1.6.4.2) activity was determined at 340 nm using the method of Foster and Hess [30] in 100 mM
K-phosphate buffer pH 7.0 containing 150 mM
NADPH, 500 mM GSSG, 1.0 mM EDTA and
200 ml of the extract. SOD (EC. 1.15.1.1) activity was assayed at 550 nm by the method of Mc Cord and Fridovich [31]. The assay mixture con-tained 50 mM K-phosphate buffer pH 7.8, 0.1
mM EDTA, 50 mM xanthine, 10 mM
ferricy-tochrome c, enough xanthine oxidase to cause
2.3. Mn and Fe analyses
Mn and Fe concentrations (mg g FW) were
determined in leaves of Mn-tolerant and Mn-sensi-tive plants by atomic absorption spectrophotome-try (Perkin – Elmer 370) after the samples had been rinsed in distilled water, dried at 80°C for 24 h and wet-ashed in a nitric and perchloric acid mixture (5:2 v/v) on an electric thermostatic plate at 300 – 350°C.
2.4. Statistical analyses
All measurements were made in triplicate. All values reported have been expressed as means of triplicates9SE. The significance of differences be-tween control and treated mean values have been evaluated by Student’s t-test and reported in the tables as *(P50.05) and **(P50.01).
3. Results
3.1. Mn and Fe uptake
Leaf Mn content increased with rising metal concentrations in the medium for both
Mn-toler-ant and Mn-sensitive plMn-toler-ants. At 2 mM Mn medium concentration the tolerant and Mn-sensitive plants showed approximately the same leaf metal concentrations, whereas at 5 mM Mn the tolerant plants showed a higher concentration of Mn (almost double) than the Mn-sensitive plants treated with the same metal concentration (Table 1). Furthermore to the increase in leaf Mn concentration, a corresponding parallel decrease of leaf Fe concentration occurred, also shown in Table 1 (r= −0.84, PB0.01).
3.2. Enzyme acti6ity
3.2.1. Stem
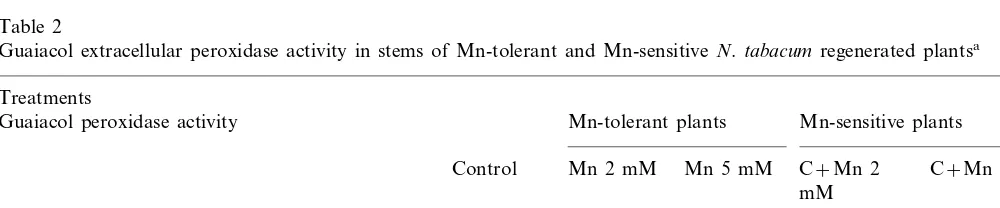
The GPXs of the extracellular fluid showed a reduced activity compared to the control either in the Mn-tolerant or Mn-sensitive plants treated with high concentrations of Mn (Table 2). In the Mn-sensitive plants treated with 2 mM Mn the activity of the POD of the ionically and covalently bound wall fractions was also depressed. By con-trast, 5 mM Mn tolerant plants showed an en-hanced activity of the covalently bound wall POD (Table 2).
The activity of syringaldazine POD in the extra-cellular fluid and in the ionically bound wall
frac-Table 1
Mn and Fe concentrations in leaves of Mn-tolerant and Mn-sensitiveN.tabacumregenerated plantsa
Treatments
Mn-sensitive plant Mn-tolerant plant
Metal content
Mn 2 mM
Control Mn 5 mM C+Mn 2 mM C+Mn 5 mM
422.3949.5 7255.09104.5 11521.69304.7 8021.59630.5 6247.391040.3 Mn
171.399.2 Fe 483.7951.1 277.7937.7 156.0915.7 248.0937.0
aValues are means of three replicates9SE. Concentrations are reported inmg g−1DW.
Table 2
Guaiacol extracellular peroxidase activity in stems of Mn-tolerant and Mn-sensitiveN.tabacumregenerated plantsa
Treatments
Mn-sensitive plants Mn-tolerant plants
Guaiacol peroxidase activity
C+Mn 5 mM Mn 2 mM
Control Mn 5 mM C+Mn 2 mM
582**932 570*9130 1518970
Extracellular fluid (D470min−1 mg−1protein) 825*9167 569*987
7.092.2
6.190.9 4.090.5
Ionically wall bound (D470min−1mg−1 protein) 7.691.5 2.0*90.3
3.691.2 2.7*90.1
6.4*90.6 3.990.6
Covalently wall bound (D470min−1mg−1 FW) 3.890.3
Table 3
Syringaldazine extracellular peroxidase activity in stems of Mn-tolerant and Mn-sensitiveN.tabacumregenerated plantsa
Treatments
Siringaldazine peroxidase activity Mn-tolerant plants Mn-sensitive plants
Mn 2 mM Mn 5 mM
Control C+Mn 2 C+Mn 5 mM mM
Extracellular fluid (D530min−1 mg−1protein) 505199380 41349400 49799745 64839880 41629713
Ionically wall bound (D530min−1mg−1 protein) 4.690.5 4.490.1 3.390.1 2.3*90.5 3.590.2
6.790.9 9.3*90.9 5.190.9
6.490 4 4.990.9
Covalently wall bound (D530min−1mg−1 FW)
aValues are means of three replicates9SE. *P]0.05; **P]0.01.
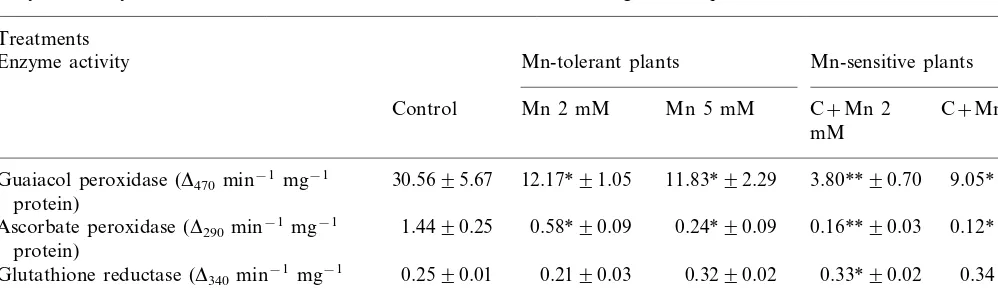
Table 4
Enzyme activity in leaves of Mn-tolerant and Mn-sensitive N.tabacumregenerated plantsa
Treatments
Enzyme activity Mn-tolerant plants Mn-sensitive plants
Mn 2 mM
Control Mn 5 mM C+Mn 2 C+Mn 5 mM mM
30.5695.67 12.17*91.05 11.83*92.29 3.80**90.70
Guaiacol peroxidase (D470min−1 mg−1 9.05*92.75
protein)
Ascorbate peroxidase (D290min−1mg−1 1.4490.25 0.58*90.09 0.24*90.09 0.16**90.03 0.12*90.01
protein)
0.2590.01 0.2190.03 0.3290.02
Glutathione reductase (D340min−1mg−1 0.33*90.02 0.3490.04
protein)
36.87*914.79 40.87*910.66
Superoxide dismutase (U mg−1protein) 91.9393.07 33.23*92.12 56.50*98.68
aValues are means of three replicates9SE. *P]0.05; **P]0.01.
tion did not vary in the different treatments, ex-cept for the Mn-sensitive plants treated with 2 mM Mn (Table 3). In this case the ionically bound wall activity decreased in comparison to the control. The effect of Mn on the covalently bound wall syringaldazine POD was identical to that observed in the guaiacol: the activity was significantly higher in the Mn-tolerant plants grown in the presence of 5 mM Mn (Table 3).
3.2.2. Leaf
The behavior of the enzymes involved in the antioxidant defense in the presence of elevated tissue Mn concentrations was practically similar for the Mn-tolerant and Mn-sensitive plants. The increase of Mn content inhibited the activity of GPX, ASPX and SOD in the Mn-tolerant as well as in the Mn-sensitive plants (Table 4). GPX activity was reduced to about 40% of the control in Mn-tolerant plants and to 12% in the Mn-sensi-tive ones. ASPX activity was 11% of the control in the Mn-sensitive plants treated with 2 mM Mn,
whereas it was 40% of the control in the Mn-toler-ant ones. The activity of SOD was depressed to 40% of the control in both sensitive and Mn-tolerant plants with the 2 mM Mn treatment. At the highest Mn treatment (5 mM) the inhibitory effect remained constant in the Mn-tolerant plants and Mn-sensitive plants. However, the effect was greater in the Mn-sensitive plants. The activity of GR did not show significant variation, except for the 2 mM Mn-sensitive plants, where an enhanced activity was detected.
4. Discussion
con-stituted by a compartmentalization process which can segregate excess metal from the key metabolic reactions [33,34]. In fact, accumulation has been observed especially in the leaf tissues [35,36]. These findings are consistent with our observa-tions, which, although preliminary, seem to indi-cate that a potential Mn compartmentalization occurs in the leaf mesophyll intercellular spaces and in the outer cell wall of the leaf epidermis; no accumulation of Mn has been seen in the vacuoles (Bennici, pers. comm.). An important factor of Mn-toxicity is represented by its interactions with other mineral elements and in particular with Fe: increasing Fe content in the soil decreases Mn uptake, and Mn seems to interfere with Fe metabolism [9,37,38].
An in vitro stepwise procedure employed to select calli able to survive at increasing Mn con-centrations was a useful method for obtaining N. tabacum plants tolerant of 2 and 5 mM Mn [12]. These plants appeared more resistant to Mn than control plants, when both were grown under the same conditions. An interesting difference found between 2 and 5 mM Mn tolerant plants was a greater xylem cell number and reduced lignifica-tion at 2 mM with respect to the control; in contrast, tolerant plants growing at 5 mM Mn possessed fewer but more strongly differentiated xylem cells than control plants or plants main-tained in 2 mM Mn. In fact, a quantitative analy-sis showed in stems of 2 mM Mn-tolerant plants, the number of completely lignified xylem elements to be significantly lower (22%) than the control (31%). Instead, 5 mM Mn-tolerant plants exhib-ited a higher number of differentiated xylem cells (44%) in comparison to the control.
These observations are in agreement with the significant increased activity of covalently wall bound POD found in plants tolerant of the highest Mn dose (5 mM), using both guaiacol and sy-ringaldazine as substrates. In fact, excess Mn, like other metal stress, may increase dry matter pro-duction [12,39,40] and seems to cause a higher activity of the extracellular POD covalently bound to the wall, which are thought to be involved in lignification processes [21,41,42].
In particular, syringaldazine has been indicated as a specific substrate of PODs involved in lignifi-cation, because only cell walls that are undergoing this process are able to oxidize syringaldazine [41,43]. It has been suggested that the increased
wall lignification induced by POD activity, which probably reduces cell growth, might represent a mechanical adaptation to biotic and abiotic stress conditions [21,28,44].
Because Mn might promote oxidative stress, it is important to characterize the response of antioxi-dant enzymes GPX, ASPX and SOD. High levels of Mn reduced the activity of these enzymes di-rectly involved in the detoxification of free radi-cals, thus reducing the protection capacity against oxidative stress. In plants growing in the presence of Mn 2 and 5 mM, the tolerance to the metal seems to be related, or is the result of a lower susceptibility of some of these enzymes to Mn inhibition.
POD have a heme group and some isoforms of SOD, typical of chloroplasts, contain Fe as a
metal component of the prosthetic group
(FeSOD). In Mn-tolerant and Mn-sensitive
plants, the uptake of a high amount of Mn is associated with a strong decrease in Fe concentra-tion in the leaves. This may affect the synthesis of POD and SOD. However, in Mn-tolerant plants the activity of GPX and SOD is less depressed than in Mn-sensitive plants, despite the similar degree of foliar Fe reduction.
The very high Mn accumulation found in the leaves in comparison to the control, correlated to the increase of the content of the metal in medium culture is in agreement with the observations of Ohki [45] and Macfie and Taylor [10]. This result may explain the alterations of chloroplasts de-scribed in our previous work, especially in plants treated with 5 mM Mn. In these plants spongy mesophyll cells showed a reduced number of chloroplasts with disrupted grana, and rich in plastoglobuli and proteic bodies [12]. At normal concentration Mn plays a role in protecting chlorophyll from photooxidation [10]. Therefore, it is possible that the presence of high concentra-tion of Mn in the leaves causes oxidaconcentra-tion of either chlorophyll or chloroplast membranes [46], in ad-dition to other effects at photosynthetic levels [47,48].
Further research is underway to better under-stand the role of compartmentalization and/or the possible presence of metal-chelating compounds in relation to the tolerance mechanism to manganese.
Acknowledgements
This research was supported by a Grant from an Italian National Research Project of the Minis-tero dell’Universita` e della Ricerca Scientifica (MURST).
References
[1] J.N. Burnell, The biochemistry of manganese in plants, in: R.D. Graham, R.J. Hannam, N.C. Uren (Eds.), Manganese in Soils and Plants, Kluwer, The Nether-lands, 1988, pp. 125 – 137.
[2] M.J. Mukhopadhay, A. Sharma, Manganese in cell metabolism of higher plants, Bot. Rev. 57 (2) (1991) 117 – 149.
[3] K.B. Clairmont, W.G. Hagar, E.A. Davis, Manganese toxicity to chloryphyll synthesis in tobacco callus, Plant Physiol. 80 (1986) 291 – 293.
[4] A. Gonzales, K.L. Steffen, J.P. Lynch, Light and excess manganese, Plant Physiol. 118 (1998) 493 – 504.
[5] C.D. Foy, Physiological effects of hydrogen, aluminium and manganese toxicities in acid soil, in: F. Adams (Ed.), Soil acidity and liming, Agronomy monograph No. 12, second ed., Am. Soc. Agron. Madison, WI, 1984, pp. 57 – 97.
[6] M. Kitao, T.T. Lei, T. Koike, Effects of manganese toxicity on photosynthesis of white birch (Betula platy
-phylla var.japonica) seedlings, Physiol. Plant 101 (1997) 249 – 256.
[7] B.T. Kang, R.L. Fox, A methodology for evaluating the manganese tolerance of cawpea (Vigna unguiculata) and some preliminary results of field trials, Field Crops Res. 3 (1980) 199 – 210.
[8] J.F. Petolino, G.B. Collins, Manganese toxicity in to-bacco (Nicotiana tabacumL.) callus and seedlings, J. Pl. Physiol. 118 (1985) 139 – 144.
[9] C.D. Foy, R.L. Chaney, M.C. White, The physiology of metal toxicity in plants, Ann. Rev. Plant Physiol. 29 (1978) 511 – 566.
[10] S.M. Macfie, G.J. Taylor, The effects of excess man-ganese on photosynthetic rate and concentration of chlorophyll in Triticum aesti6um grown in solution
cul-ture, Physiol. Plant 85 (1992) 467 – 475.
[11] G. Santandrea, S. Schiff, A. Bennici, Manganese toxicity to different growth processes in vitro inNicotiana, Plant Cell Tiss. Org. Cult. 50 (1997) 125 – 129.
[12] G. Santandrea, S. Schiff, A. Bennici, Effects of man-ganese on Nicotianaspecies cultivated in vitro and char-acterization of regenerated Mn-tolerant tobacco plants, Plant Sci. 132 (1998) 71 – 82.
[13] G. Santandrea, C. Tani, A. Bennici, Cytological and ultrastructural response ofNicotiana tabacumL. roots to manganese stress, Plant Biosyst. 132 (1998) 197 – 206. [14] F. Van Assche, H. Clijsters, Effects of metals on enzyme
activity in plants, Plant Cell Environ. 13 (1990) 195 – 206. [15] C.H.R. De Vos, H. Schat, Free radicals and heavy metal tolerance, in: J. Rozeman, J.A.C. Verkleij (Eds.), Ecolog-ical Responses to Environmental Stresses, Kluwer, Dor-drecht, 1991, pp. 22 – 30.
[16] R.G. Alscher, J.L. Hess, Antioxidants in Higher Plants, CRC Press, Boca Raton, FL, 1993.
[17] C. Foyer, P. Mullineaux, Causes of Photooxidative Stress and Amelioration of Defense System in Plants, CRC Press, Boca Raton, FL, 1994.
[18] L. Vamos-vigyazo, Polyphenol oxidase and peroxidase in fruits and vegetables, CRC Crit. Rev. Food Sci. Nutr. 15 (1981) 49 – 127.
[19] T. Gaspar, C. Penel, T.A. Thorpe, H. Greppin, Peroxi-dases 1970 – 1980: A survey of their biochemical and physiological roles in higher plants, University of Geneve, Geneve, 1982.
[20] P. Schloss, C. Walter, M. Mader, Basic peroxidases in isolated vacuoles of Nicotiana tabacum L, Planta 170 (1987) 225 – 229.
[21] F.J. Castillo, Extracellular peroxidases as markers of stress?, in: H. Greppin, C. Penel, T. Gasper (Eds.), Molecular and Physiological Aspects of Plant Peroxi-dases, Centre de Botanique, University de Gene`ve, 1986, pp. 419 – 426.
[22] K. Freudenberg, Biosynthesis and constitution of lignin, Nature 183 (1959) 1152 – 1155.
[23] F.J. Castillo, H. Greppin, Balance between anionic and cationic extracellular peroxidase activities in Sedum al
-bum leaves after ozone exposure. Analysis by higher performance liquid chromatography, Physiol. Plant 68 (1986) 201 – 208.
[24] M.L. Parker, K.W. Waldron, Texture of Chinese water chestnut: Involvement of cell wall phenolics, J. Sci. Food Agric. 68 (1995) 337 – 346.
[25] K.W. Waldron, A.J. Parr, A. Ng, J. Ralph, Cell wall esterified phenolic dimers: identification and quantifica-tion by reverse phase high performance liquid chro-matography and diode array detection, Phytochem. Anal. 7 (1996) 305 – 312.
[26] K.W. Waldron, A.C. Smith, A.J. Parr, A. Ng, M.L. Parker, New approaches to understanding and con-trolling cell separation in relation to fruit and vegetable texture, Trends Food Sci. Technol. 8 (1997) 213 – 221. [27] T. Murashige, F. Skoog, A revised medium for rapid
growth and bioassays with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 473 – 497.
[28] T. Pandolfini, R. Gabbrielli, C. Comparini, Nickel toxic-ity and peroxidase activtoxic-ity in seedlings ofTriticum aes
-ti6umL, Plant Cell Environ. 15 (1992) 719 – 725.
[29] M. Kato, S. Shimizu, Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing to-bacco leaves; phenolic-dependent peroxidative degrada-tion, Can. J. Bot. 65 (1987) 729 – 735.
[31] J.M. Mc Cord, I. Fridovich, Superoxide dismutase: on enzymic function for erythrocuprein (hemocuprein), J. Biol. Chem. 244 (1969) 6049 – 6055.
[32] C.N. Giannopolitis, S.K. Ries, Superoxide dismutases, Plant Physiol. 59 (1977) 309 – 314.
[33] A.R. Memon, M. Chino, Y. Takeoka, K. Hora, M. Yatazawa, Distribution of manganese in leaf tissues of a manganese accumulator: Acanthopanax sciadophylloides
as revealed by electron probe X-ray microanalyzer, J. Pl. Nutr. 2 (1980) 457 – 476.
[34] A.R. Memon, M. Chino, H. Hidaka, Manganese toxicity in field grown tea plants as micro-distribution of man-ganese in the leaf tissue as revealed by electron probe micrography, Soil Sc. Pl. Nutr. 27 (1981a) 317 – 328. [35] R.D. Gilbert, Mineral Nutrition and the Balance of Life,
University of Oklahoma Press, Norman, OK, 1957, pp. 111 – 124.
[36] M. Masui, A. Nakaya, A. Ishida, T. Ogura, The man-ganese excess of muskmelons: 6. Manman-ganese distribution in the parts, Engei Gakkai Zasshi 49 (1980) 79 – 84. [37] C.D. Foy, Manganese and plants, in: Manganese, Natl.
Acad. Sci. Natl. Res. Counc, Washington, WA, 1973, pp. 51 – 76.
[38] M. Masui, A. Nakaya, A. Ishida, Studies on the man-ganese excess muskmelon: V. The manman-ganese, calcium and iron concentrations in nutrient solutions, Engei Gak-kai Zasshi 45 (1976) 267 – 274.
[39] M. Maro`ti, J. Bogna`r, Effect of toxic metals inhibiting the growth of plant callus tissue (IV), Acta Agronom. Hung. 40 (1991) 39 – 47.
[40] P. Gori, S. Schiff, G. Santandrea, A. Bennici, Response of in vitro cultures of Nicotiana tabacum L. to copper stress and selection of plants from Cu-tolerant callus, Plant Cell Tiss. Org. Cult. 53 (1998) 161 – 169.
[41] A. Imberty, R. Goldberg, A.M. Catesson, Isolation and characterization of Populus isoperoxidases involved in the last step of lignin formation, Planta 164 (1985) 221 – 226.
[42] S.C. Fry, Cross-linking of matrix polymers in the grow-ing cell walls of angiosperms, Plant Physiol. 37 (1986) 165 – 186.
[43] N.G. Lewis, E. Yamamoto, Lignin: occurrence, biogene-sis and biodegradation, Ann. Rev. Plant Physiol. Plant Mol. Biol. 41 (1990) 455 – 496.
[44] T. Gaspar, C. Penel, F.J. Castillo, H. Greppin, A two step control of basic and acidic peroxidases and its significance for growth and development, Physiol. Plant 64 (1985) 418 – 423.
[45] K. Ohki, Manganese deficiency and toxicity effects on growth development and nutrient composition in wheat, Agron. J. 76 (1984) 213 – 218.
[46] T. Horiguchi, Mechanism of manganese toxicity and tolerance of plants VII. Effect of light intensity on man-ganese-induced chlorosis, J. Plant Nutr. 11 (1988) 235 – 245.
[47] H. Clijster, F. Van Assche, Inhibition of photosynthesis by heavy metals, Photosynth. Res. 7 (1985) 31 – 40. [48] R.O. Nable, R.L. Houtz, G.M. Cheniae, Early inhibition
of photosynthesis during development of manganese tox-icity in tobacco, Pl. Physiol. 86 (1988) 1136 – 1142.