www.elsevier.nlrlocateraqua-online
Pharmacokinetics of oxytetracyline in Arctic charr
ž
Sal
Õ
elinus alpinus L. in freshwater at low
/
temperature
Tor Haug
a,), Petter Arnt Hals
b aInstitute of Marine Biotechnology, The Norwegian College of Fishery Science, UniÕersity of Tromsø, BreiÕika, Tromsø N-9037, Norway
b
Nycomed Amersham, P.O. Box 4220, TorshoÕ, Oslo N-0401, Norway
Accepted 6 December 1999
Abstract
Ž . Ž
The pharmacokinetics of oxytetracycline OTC was studied in Arctic charr SalÕelinus alpinus
. Ž .
L. in freshwater at a mean water temperature of 6.38C, after intravascular i.v.; 10 and 20 mgrkg
Ž .
and oral p.o.; 50 and 100 mgrkg administration. Two different oral formulations were tested as carriers for OTC; one in which OTC was dissolved in agar, the other where OTC was encapsulated in liposomeralginate particles. Blood samples were collected via a cannula placed in the dorsal aorta, and the concentration of OTC in plasma was assayed using solid phase extraction
ŽSPE and high-performance liquid chromatography HPLC . Pharmacokinetic analysis of plasma. Ž .
concentration–time data after i.v. administration indicated that a three-compartment model best
Ž .
described OTC disposition in Arctic charr. The volume of distribution VdŽarea. and total body
Ž . y1 y1
clearance ClT ranged from 2.57 to 2.90 lrkg and from 6.27 to 6.54 ml kg h , respectively.
Ž .
The elimination of OTC after i.v. administration was relatively slow; the half-lives t1r2 were 266.3 and 326.9 h in fish receiving 10 and 20 mg OTCrkg, respectively. No dose-dependent pharmacokinetics could be observed. The absorption of OTC was incomplete for both
formula-Ž .
tions tested. The mean bioavailability F of OTC ranged from 3.2% to 7.3%. Dose and drug formulation had, however, no significant effect on the bioavailability. The mean maximum
Ž .
concentration of OTC in plasma Cmax was significantly higher in the group receiving OTC in
Ž . Ž .
agar 3.93mgrml compared to the liposomeralginate group 0.97mgrml . The mean time to
Ž .
reach Cmax Tmax was also significantly longer in fish receiving the liposomeralginate
formula-Ž . Ž .
tion 136.0 h compared to fish receiving the agar formulation 17.8 h . Results from the present
)Corresponding author. Tel.:q47-77-64-60-71; fax:q47-77-64-60-20.
Ž .
E-mail address: [email protected] T. Haug .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
study indicate that liposomeralginate particles show little promise as a drug carrier for OTC in oral formulations to fish since it did not increase the bioavailability of the drug.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Oxytetracycline; Pharmacokinetics; Bioavailability; SalÕelinus alpinus; Liposomes; Aortic
cannula-tion; Therapy of fish diseases
1. Introduction
Ž .
Oxytetracycline OTC is a broad-spectrum antibiotic drug which has been widely used in the treatment of systemic bacterial infections in farmed fish. It is administered orally, incorporated into pelleted feed. The common practice in Norwegian fish farming
is to administer a dose of 100 mg OTCrkg fishrday for 6–10 days.
Ž
The pharmacokinetics of OTC has previously been studied in Atlantic salmon Salmo
. Ž . Ž
salar Pye-MacSwain et al., 1992; Elema et al., 1996 , amago salmon Oncorhynchus
. Ž . Ž . Ž .
rhodurus Uno et al., 1992 , chinook salmon O. tshawytscha Abedini et al., 1998 ,
Ž . Ž
rainbow trout O. mykiss Grondel et al., 1989; Bjorklund and Bylund, 1990,1991;
¨
.
Black et al., 1991; Rogstad et al., 1991; Nouws et al., 1992; Abedini et al., 1998 , carp
ŽCyprinus carpio. ŽHaenen et al., 1985; Grondel et al., 1987; Nouws et al., 1992 ,.
Ž . Ž . Ž .
European eel Anguilla anguilla Nouws et al., 1993 , ayu Plocoglossus altiÕelis
ŽUno, 1996 , African catfish Clarias gariepinus. Ž . ŽGrondel et al., 1989 , striped bass.
ŽMorone saxatilis. ŽXu and Rodgers, 1993 , tench Tinca tinca. Ž . ŽReja et al., 1996 , and.
Ž . Ž .
yellowtail Seriola quinqueradiata Uno et al., 1992 . In addition, the pharmacokinetics
Ž . Ž
of tetracycline has been studied in channel catfish Ictalurus punctatus Plakas et al.,
. Ž . Ž .
1988 and dogfish shark Squalus acanthias Guarino, 1986 . However, to our
knowl-Ž .
edge, there are no studies on the pharmacokinetics in Arctic charr SalÕelinus alpinus , a
fish species now being farmed in northern Norway. In addition, there are few publica-tions concerning the pharmacokinetics of antibacterial agents in fish at low temperatures.
The water temperature shows great seasonal variation, and temperatures down to 08C are
occasionally encountered during winter in the northern part of Norway.
Previous pharmacokinetic studies of OTC have revealed that the bioavailability of this drug is relatively low in fish. Some of the studies, however, have shown that the oral bioavailability of antibacterial agents can be improved by using different
ap-Ž . Ž
proaches in dosage Rogstad et al., 1993 and drug formulation Endo et al., 1987;
.
Martinsen et al., 1993 . Liposomes are microscopic phospholipid vesicles composed of one or more concentric phospholipid bilayers. The use of liposomes as an oral dosage form may protect the encapsulated drug from digestive degradation or interaction with other molecules, and thereby increase absorption of poorly absorbed drugs from the
gastrointestinal tract. Encapsulation of bacterial antigens in liposomeralginate particles
Ž
has been shown to improve the effect of vaccines administered orally to fish Eggset et
.
al., 1995 . Encapsulation in liposomeralginate particles may therefore also improve the
absorption and thereby the bioavailability of antibacterial agents in fish.
safe withdrawal periods, and to minimize the environmental effects of the drugs used in aquaculture. The pharmacokinetics of drugs may be affected by parameters such as fish species, age, water temperature and salinity, route of administration and other experi-mental conditions. Therefore, the extrapolation of pharmacokinetic data obtained in one species to another species, living under different conditions, should be done with caution.
The aim of the present study was twofold; firstly, to describe the pharmacokinetics of
Ž . Ž .
OTC in Arctic charr, living at low temperatures, after intravascular i.v. and oral p.o. administration, and secondly, to determine the relative bioavailability of OTC after p.o.
administration of a liposomeralginate formulation compared to the drug dissolved in
water with agar as a viscosity-increasing agent.
2. Materials and methods
2.1. Chemicals
Ž .
Acetonitrile Rathburn Chemicals, Walkerburn, Scotland and N,
N-dimethylform-Ž .
amide Fluka, Buchs, Switzerland were both of high-performance liquid
chromatogra-Ž .
phy HPLC -grade. All other chemicals were of analytical grade. The water used was
Ž .
distilled and purified with a Milli-Q reagent grade water system Millipore, MA, USA . OTC hydrochloride and tetracycline hydrochloride were purchased from the
Norwe-Ž .
gian Medicinal Depot Oslo, Norway . The formulation of OTC for i.v. administration
Ž .
was made by solving OTC–HCl in 0.9% saline 10 and 20 mgrml . The oral
Ž
formulations were made either by suspending the drug in 0.5% Bacto-Agar Difco
.
Laboratories, Detroit, MI, USA or by encapsulating it into liposomeralginate particles
Žsee below . Dosing solutions of OTC in agar were prepared at 50 and 100 mg. rml.
Methanolic stock solutions of the two tetracyclines for analytical purposes were
pre-pared at a concentration of 1 mgrml and stored in the dark at y208C. Working
standards were prepared daily by dilution from the stock solutions.
2.2. Preparation of OTC liposomeralginate particles
Liposomeralginate particles containing OTC were prepared by mixing 6 g OTC with
Ž Ž . .
6 g soya bean phospholipids Pro-Lipo S ; Lucas Meyer, Hamburg, Germany and adding 20 ml of water dropwise. After 15 min of stirring, 80 ml of water was added, and
the solution was set for sedimentation for 16 h at q48C. The excess of OTC was
removed from the preparation by centrifugation at 27,000=g for 1 h and the
super-natant was discarded. A volume of 140 ml of water was added to the liposome pellet,
the solution was centrifuged at 27,000=g for 1.5 h, and the supernatant was discarded.
Ž . Ž
The pellet 9.2 g was mixed with 9.2 ml 2% Na-alginate Protanal LF 10r40 RB;
.
Protan Biopolymer, Drammen, Norway . The solution was stirred and allowed to stand for 10 min in order to remove air bubbles from the preparation. The suspension was loaded into a 10-ml syringe with a 27-gauge needle attached and sprayed down into
0.5–2.0 mm diameter liposomeralginate particles were formed. After 2 h, the particles
were washed in water in order to remove salt and free OTC, and stored at q48C until
administration. The concentration of OTC in the particles was determined by suspending crushed particles in water and analyzing the material by SPE and HPLC. The
concentra-Ž .
tion of OTC was found to be 79.2"7.9 mg OTCrg particles ns4 . The particles
were suspended in water to a concentration of 100 mg OTCrml before administration.
2.3. Experimental fish
Ž . Ž .
Healthy Arctic charr S. alpinus L. weighing 514"154 g mean"S.D. , obtained
Ž .
from the Aquaculture Research Station Tromsø, Norway , were used. The fish were held in fibre-glass circulation tanks, supplied with aerated freshwater at a temperature
ranging from 4.28C to 8.68C, with a mean value of 6.38C. The fish were fed a
commercial pelleted fish diet.
2.4. Experimental procedure
Dorsal aorta cannulation was performed by a modification of the procedures of
Ž . Ž .
Houston 1971 and Soivio et al. 1972,1975 . The fish were anaesthetized in well-aerated
Ž .
water containing 100 mgrl tricaine methane sulphonate MS-222, Sigma and 200 mgrl
Ž .
sodium hydrogen carbonate NaHCO , Sigma . When the fish showed no respiratory3
activity, they were taken out of the water, weighed, and placed on an operating table.
Ž
The cannula consisted of a 35-cm polyethylene tubing PE 50, intramedic
non-radio-. Ž .
opaque, non-toxic; Clay Adams, NJ, USA . The top ca. 0.5 mm of a 1-ml pipette tip was pulled outside the tubing, ca. 5 cm from the end, which was to be inserted into the aorta.
Ž .
A guitar string d;0.5 mm was placed inside the cannula, and the cannula was
inserted through the palate between the first and second gill arches and pushed ca. 2 cm into the dorsal aorta. Upon successful entry into the aorta, blood filled the cannula as the guitar string was removed. A tube clip was put on the cannula to prevent blood loss. A
17-gauge=2 in. needle was inserted through the snout and the distal end of the cannula
was guided outside through the needle. The pipette tip prevented the cannula from being pulled out of the fish snout and the dorsal aorta. The needle was then removed and the
Ž .
cannula was flushed and filled with heparinized 200 IErml 0.9% saline, and melted at
the end with a flame. The cannula was sutured to the roof of the mouth.
During the experiments, the fish were individually housed in flowthrough chambers
Ž .
2.5. Drug administration and sampling
The fish were fasted for 2 days before administration of the drug. Dosing
formula-tions were administered at 1 mlrkg body weight. The i.v. administration was made
Ž . Ž .
directly into the dorsal aorta via the cannula at doses of 10 ns6 or 20 ns6 mg
OTCrkg body weight. The fish given OTC orally were lightly sedated with MS-222
before dosing. The drug formulation was given by gavage into the stomach, using a tube
Ž . Ž
which was connected to a disposable syringe. The doses were 50 ns7 and 100 agar:
.
ns9, liposomeralginate: ns3 mg OTCrkg body weight. The fish were placed in the
sampling chambers immediately after drug administration.
Ž .
Blood ca. 600ml was sampled via the aortic cannula at 1, 4, 8, 12, 24 and 48 h, and
at regular time intervals between 3 and 20 days after i.v. administration. In the fish given OTC orally, blood samples were taken at 4, 8, 12, 24, 48, 72 and 96 h, and at regular
intervals between 6 and 24 days after administration. In the group given 50 mgrkg
OTC, p.o., additional samples were taken from four fishes after 33 days. The heparin– saline solution was removed from the cannula before each sampling and discarded. The
blood was centrifuged for 2 min at 10,000=g and the obtained plasma was stored at
y208C until analysis.
2.6. Analytical procedures
Ž .
The analysis of OTC in plasma was performed by the method of Bjorklund 1988 ,
¨
with minor modifications. Due to different analytical equipment, the injection volume and the mobile phase of the HPLC method had to be adjusted in order to obtain
Ž
symmetric peaks and satisfactory resolution between OTC and tetracycline TC; used as
. Ž . Ž .
internal standard . The solid phase extraction SPE method used by Bjorklund 1988
¨
gave a relatively low recovery of the tetracyclines on Bond Elut C18 columns. In order to increase the recovery, the washing volume was reduced from 30 to 5 ml, and the elution volume was reduced from 10 to 1 ml. By reducing the elution volume, a time-consuming evaporation step was also eliminated. Thus, the analytical procedure
used in the present study was as follows. Plasma samples were spiked with 0.5mg TC,
Ž .
and 10 ml of cold q48C extraction buffer, containing 0.1 M citric acid and 0.2 M
Ž . Ž .
disodium hydrogenphosphate 62:38 pH 4.0 , was added. The samples were shaken
and sonicated for 5 min and allowed to stand for 15 min atq48C. The plasma samples
Ž
were then transferred to 1 ml Bond Elut C18 SPE columns Varian, Harbor City, CA,
. Ž .
USA , which had been activated by flushing with 5 ml methanol and 10 ml 5% wrv
disodium EDTA. After the samples had run through the columns, they were washed with 5 ml water. OTC and TC were eluted with 1 ml of 0.01 M methanolic oxalic acid,
and 10ml aliquots of the eluate were subjected to reverse-phase HPLC. The analysis of
each plasma sample was performed in duplicates.
Ž .
The HPLC system Waters Assosiates, Millipore, MA, USA consisted of a model 600E system controller, a model 717 autosampler, and a model 484 variable wavelength UV detector set at 355 nm. The response was recorded using a model 745 Data Module
Ž .
integrator. Separation was achieved on a Nova-Pak RP-C18 column 100=3.9 mm i.d.
Ž . Ž .
Ž . Ž . Ž
acetonitrile, 6% vrv N, N-dimethylformamide and 80% vrv 0.01 M oxalic acid pH
.
2.1 and had a flow rate of 1 mlrmin. The HPLC system was operated at 258C.
Quantification of OTC was accomplished using a standard curve constructed on the
basis of extracted OTCrTC ratio. Peak height was used to compare OTC and TC levels
in the plasma samples. The extraction recoveries of OTC and TC were determined by
comparing peak heights from the analysis of plasma spiked with 0.1–5.0 mgrml with
peak heights resulting from direct injection of methanolic standards. The detection limit
ŽLOD was defined as the concentration of OTC in plasma which gave an HPLC–UV.
signal three times greater than the signal-to-noise ratio. The lowest calibration standard,
Ž .
i.e., 0.1mg OTCrml, was used as the limit of quantitation LOQ .
2.7. Pharmacokinetic and statistical analysis
The plasma OTC concentration vs. time data were analysed for each fish and treatment, using non-linear least-squares regression. The pharmacokinetic computer
Ž
program, SAAM II, version 1.1 SAAM Institute, University of Washington, Seattle,
.
WA, USA was used for the calculations. The data obtained in the groups given 10 and
20 mgrkg, i.v., were best described by a three-compartment open model. The model
was selected on the basis of the residual sum of squares and the minimum Akaike’s
Ž .
information criterion AIC . All diffusion processes were assumed to follow first-order
Ž .
Ž .
kinetics. The area under the concentration–time curve AUC from zero to infinity was
calculated using the formula: AUCsAraqBrbqCrg. The mean residence time
ŽMRT was determined as: MRT. sAUMCrAUC, where AUMC is the AUC of a plot
of the product of time and plasma drug concentration vs. time, from zero to infinity
ŽRitschel, 1992 ..
The serum concentration–time curves after oral dosing could not be fitted by the non-linear least squares method using compartment models with first-order absorption.
Ž .
The elimination rate constant ke after p.o. administration was estimated from the
Ž .
terminal part of the elimination curve using AIC , including at least the six last data
Ž .
points. The elimination half-life t1r2 of OTC was calculated using: t1r2sln 2rk . Thee
AUC was determined by the trapezoidal rule, including the terminal portion. The percent
Ž .
oral bioavailability F was determined by comparing the areas under the plasma
Ž . Ž
concentration vs. time curves AUC using the equation: Fs100= AUCp.o.=
. Ž .
Dosei.v. r AUCi.v.=Dosep.o., where Dosei.v.rAUCi.v. is the mean value from both the
Ž . Ž .
10 and 20 mgrkg, i.v., study. The peak OTC concentration Cma x and peak time Tmax
for each fish were read directly from the concentration vs. time curve.
All statistical analyses were performed with Mann–Whitney’s non-parametric test,
Ž
using the computer program, StatvieweSEq, version 1.04 Abacus Concepts,
Berke-.
ley, CA, USA . The level of significance was chosen to be ps0.05. The
pharmacoki-netic data are presented as mean"S.D.
Ž .
3. Results
3.1. Method of analysis
By reducing the washing volume and the elution volume in the SPE method, the
recovery for OTC and TC from plasma was increased from 45.3"12.4% and 40.1"
Ž .
12.0% to 83.0"8.9% and 78.5"8.7% ns10 , respectively. The retention time for
OTC and TC in the HPLC system was 3.07 and 3.59 min, respectively. The LOD was
Ž .
calculated to be 0.05 mg OTCrml. The response of the UV detector peak height
Ž .
correlated well with OTC concentrations rs0.9985 and was found to be linear over
the range from 0.1 to 5.0mg OTCrml; thus, 0.1mg OTCrml was used as LOQ. Three
samples from fish given 50 mgrkg of OTC in agar and one sample from fish given 100
mgrkg of OTC in liposomeralginate contained concentrations above LOD but below
LOQ, and here the concentrations were quantitated by extrapolation of the standard
curve. Plasma samples with concentrations above 5.0mg OTCrml were diluted in water
and reanalysed.
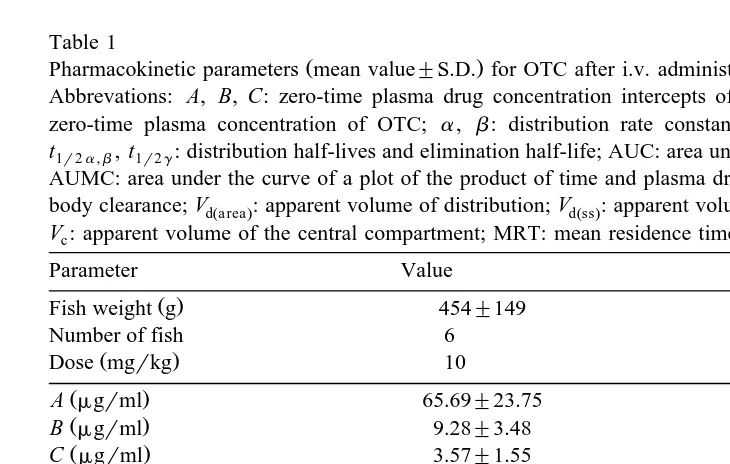
Table 1
Ž .
Pharmacokinetic parameters mean value"S.D. for OTC after i.v. administration to Arctic charr
Abbrevations: A, B, C: zero-time plasma drug concentration intercepts of triphasic disposition curve; C0: zero-time plasma concentration of OTC; a, b: distribution rate constants; g: elimination rate constant;
t1r2a,b, t1r2g: distribution half-lives and elimination half-life; AUC: area under the concentration–time curve; AUMC: area under the curve of a plot of the product of time and plasma drug concentration–time; Cl : totalT body clearance; VdŽa rea.: apparent volume of distribution; VdŽss.: apparent volume of distribution at steady state;
V : apparent volume of the central compartment; MRT: mean residence time.c
3.2. The i.Õ. administration
The plasma concentration–time curves for both doses were best described with the
tri-exponential three-compartment open model: CpsAeyatqBeybtqCeygt where Cp
is the concentration of OTC in plasma; A, B and C are zero-time plasma drug
concentration intercepts of the triphasic concentration–time curve; a and b are
distribution rate constants;g is the elimination rate constant; t is time; and e represents
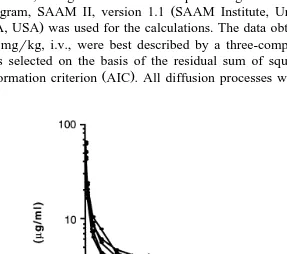
the base of the natural logarithm. The observed individual plasma drug concentrations of OTC after i.v. administration are depicted in Figs. 1 and 2. The pharmacokinetic parameters for OTC after i.v. administration are listed in Table 1.
Ž .
The volume of distribution VdŽa rea. was 2.57"1.03 and 2.90"0.61 lrkg at doses
of 10 and 20 mgrkg, respectively. The volume of distribution of the central
compart-Ž . Ž .
ment Vc ranged from 0.10 to 0.13 lrkg. Total body clearance ClT was 6.54"1.33
ml kgy1
hy1
at the lower dose and 6.27"1.39 ml kgy1
hy1
at the higher dose. The
Ž .
elimination half-life t1r2g of the terminal part of the elimination phase was estimated
to be 266.3"61.7 and 326.9"60.4 h at 10 and 20 mgrkg, respectively. The MRT was
calculated to be 301.2"49.2 and 357.1"97.6 h in the low and high doses,
respec-tively.
Ž .
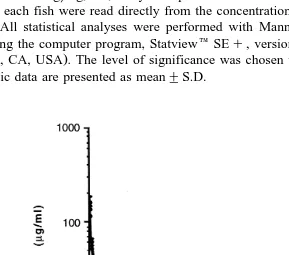
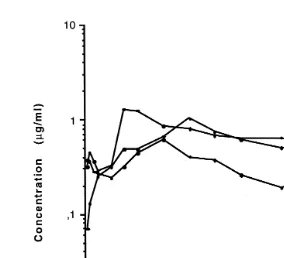
Fig. 3. Semilogarithmic plot of plasma OTC concentration vs. time in Arctic charr ns7 after p.o.
Ž .
3.3. The p.o. administration
The observed individual plasma drug concentrations of OTC after p.o. administration
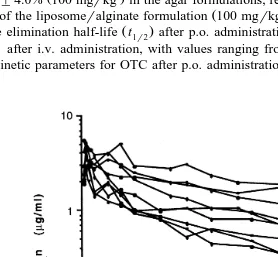
of agar formulations and the liposomeralginate formulation are depicted in Figs. 3–5.
Ž .
The maximum plasma concentration of OTC Cmax , 1.51"0.86mgrml, was achieved
30.3"33.3 h after p.o. administration of 50 mgrkg in agar. At 100 mgrkg, the Cma x
Ž3.93"0.99 mgrml. was achieved 17.8"17.4 h after administration. The
Ž . Ž .
liposomeralginate formulation 100 mgrkg delayed the Cmax 0.97"0.33mgrml to
136.0"60.4 h after administration. A large individual variation in the Cma x and in the
Ž .
time to reach Cmax Tmax was observed. With the agar formulations, the Tmax ranged
from 4 to 96 h, while with the liposomeralginate formulation, the Tmax ranged from 72
to 192 h.
Ž .
The mean bioavailability of OTC was estimated to be 4.2"1.3% 50 mgrkg and
Ž .
7.3"4.0% 100 mgrkg in the agar formulations, respectively. The mean
bioavailabil-Ž .
ity of the liposomeralginate formulation 100 mgrkg was estimated to be 3.2"0.9%.
Ž .
The elimination half-life t1r2 after p.o. administration was apparently longer than the
t1r2 after i.v. administration, with values ranging from 367.0 to 468.5 h. The
pharma-cokinetic parameters for OTC after p.o. administration are given in Table 2.
Ž .
Fig. 4. Semilogarithmic plot of plasma OTC concentration vs. time in Arctic charr ns9 after p.o.
Ž .
Ž .
Fig. 5. Semilogarithmic plot of plasma OTC concentration vs. time in Arctic charr ns3 after p.o.
Ž .
administration of a single dose of 100 mgrkg body weight liposomeralginate formulation . Each curve represents data from one fish.
It was observed that feed intake and swimming activity were reduced during the experimental period in all test groups.
Table 2
Ž .
Pharmacokinetic parameters mean value"S.D. for OTC after p.o. administration to Arctic charr
Abbrevations: Cma x: maximum concentration; Tmax: time to reach maximum concentration; k : eliminatione rate constant; t1r2: elimination half-life; AUC: area under the concentration–time curve; F: bioavalability.
Parameter Value
Ž .
Fish weight g 541"107 562"179 707"107
Number of fish 7 9 3
Formulation agar agar liposomeralginate
Ž .
Dose mgrkg 50 100 100
Ž .
Cma x mgrml 1.51"0.86 3.93"0.99 0.97"0.33
Range 0.82–3.03 2.66–5.36 0.61–1.27
Ž .
Tma x h 30.3"33.3 17.8"17.4 136.0"60.4
Range 8–96 4–72 72–192
y1
Ž .
ke h 0.002"0.001 0.002"0.001 0.002"0.000
Ž .
t1r2 h 367.0"175.8 444.2"133.3 468.5"85.1
Ž .
AUC0 –` mg hrml 341.9"105.2 1188.1"643.4 510.6"160.5 AUCrDose 6.8"2.1 11.9"6.4 5.2"1.5
Ž .
4. Discussion
Plasma concentrations of OTC in the fish given the drug by i.v. injection decreased rapidly during the first day after administration. This was probably due to tissue distribution since the further decline in the later two phases was much slower. The terminal half-life was in the range of 266–327 h, and the total clearance was relatively
Ž y1 y1.
low 6.3–6.5 ml kg h . The elimination half-life observed in this study was
considerably longer than those reported for other fish species living at higher water
Ž .
temperatures. In carp, the elimination half-life was 169.0 h at 88C Nouws et al., 1992
Ž .
and 139.8 h at 208C Grondel et al., 1987 . In rainbow trout, half-lives of 81.5–130.0 h
Ž . Ž
80.3 h in African catfish at 258C Grondel et al., 1989 . In Atlantic salmon in seawater,
Ž .
half-lives of 50.7 and 12.0 h were calculated at 7–88C Elema et al., 1996 and 158C
ŽPye-MacSwain et al., 1992 , respectively. Despite the differences in species and.
experimental conditions, these results confirm that water temperature has a major effect on the elimination of OTC in fish. The slower elimination of drugs in fish at low temperatures compared to high temperatures may partly be due to the lower production
Ž . Ž .
of bile Curtis et al., 1986 and urine Hunn, 1982 at low temperatures.
Comparing the pharmacokinetic parameters calculated at the two dose levels used in the present study, no dose-dependent pharmacokinetics of OTC in Arctic charr after i.v.
Ž .
administration was indicated. Neither the volume of distribution VdŽa rea. and VdŽss. , the
Ž .
total body clearance ClT , the MRT nor the distribution and elimination half-lives
Žt1r2a,b,g.differed significantly between the two dose groups.
Ž .
The apparent volume of distribution VdŽa rea. of 2.57–2.90 lrkg indicates that OTC is
well-distributed throughout the body, and that the major part of the drug in the body is extravascularly at distribution equilibrium. This is considered an advantage for OTC, since many of the fish pathogenic bacteria cause abscesses and lesions in the skin and
Ž .
muscles, which are poorly vascularized in fish Ferguson, 1989 . The VdŽa rea. is relatively
Ž
high, considering the low octanolrwater partition coefficient of OTC Coliazzi and
. Ž
Klink, 1969 and the high plasmaprotein binding in salmonids Bjorklund and Bylund,
¨
.
1991 . However, the affinity of tetracyclines for bone tissue and skin may contribute to
Ž .
the high VdŽa rea.. The volume of the central compartment Vc was approximately three
Ž
times larger than the blood volume of salmonids at low temperatures 35 mlrkg;
.
Nikinmaa et al., 1981 .
The absorption of OTC in Arctic charr was relatively rapid, but incomplete, for both
formulations tested in this study. Although the number of fish in the liposomeralginate
Ž .
group was small ns3 , it was considered sufficient for the assessment of whether this
formulation increased the bioavailability of OTC as compared to the agar formulation. The mean oral bioavailability of OTC varied between 3.2% and 7.3%. Dose and formulation had no significant effect on the bioavailability. Our results are in agreement with those previously reported. The bioavailability was found to be 1.9–6.9% in Atlantic
Ž . Ž .
Ž .
0.4–0.6% in carp Grondel et al., 1987; Nouws et al., 1992 , and 1.2–5.6% in rainbow
Ž . Ž .
trout Bjorklund and Bylund, 1991; Nouws et al., 1992 . Cravedi et al. 1987 and
¨
Ž .
Rogstad et al. 1991 found that less than 10% of orally administered OTC was absorbed
Ž .
in rainbow trout. In a recent publication Abedini et al., 1998 , the bioavailability of OTC was 24.8% and 30.3% in chinook salmon and rainbow trout, respectively. In the latter study, an oral formulation consisting of gelatin capsules containing a methanolic solution of OTC was used, and this may be the explanation for the increased bioavail-ability compared with the other studies.
Low oral bioavailability may be due to several factors. Tetracyclines are known to
Ž .
form stabile complexes with di- and trivalent cations Ghandour et al., 1992 . These complexes do not pass biological membranes easily and this may prevent absorption of
Ž .
tetracyclines Cravedi et al., 1987; Grondel et al., 1987 . The absorption of OTC may also be reduced because of unfavourable pH values in the intestine of fish. For instance,
Ž .
the intestinal pH of rainbow trout was as high as 9.5 Dauble and Curtis, 1990 , and at
Ž .
this pH, only a small fraction of OTC is non-ionized Stephens et al., 1956 and thereby readily available for absorption. High levels of tetracyclines in liver and bile shortly
Ž
after p.o. administration Ingebrigtsen et al., 1985; Plakas et al., 1988; Bjorklund and
¨
.
Bylund, 1990 indicate that OTC undergoes significant first pass effect, i.e., extraction in liver and further elimination into the bile. This process is not directly affected by drug
Ž
formulation, and may be the explanation for the relatively low bioavailability 60% or
. Ž
less even in warm-blooded animals Fabre et al., 1971; Schifferli et al., 1982; Mevius et
.
al., 1986; Dyer, 1989 .
Ž .
At 100 mgrkg, the Cmax was significantly higher ps0.013 in the group receiving
Ž . Ž
OTC in agar 3.93"0.99 mgrml compared to the liposomeralginate group 0.97"
. Ž .
0.33mgrml . Tmaxalso differed significantly ps0.011 between the two formulations,
with mean values of 17.8 and 136.0 h for the agar and liposomeralginate formulation,
respectively. These differences could be ascribed to a better disintegration and
dissolu-tion of the drug from the agar formuladissolu-tion compared to the liposomeralginate
formula-tion. The absorption of OTC in the liposomeralginate formulation would thereby be
delayed. For comparison, the Cmax after administration of the same dose in medicated
Ž
feed was 2.05mgrml in amago salmon and 1.14mgrml in rainbow trout Uno et al.,
.
1992 . In the present study, the Cmax of OTC was 1.51"0.86 mgrml after p.o.
administration of 50 mgrkg in agar, while the Cma x was 0.42mgrml in Atlantic salmon
Ž .
held in seawater, administered the same dose in medicated feed Elema et al., 1996 .
The lower Cmax value obtained in fish held in seawater andror given medicated feed
may be due to complex binding of OTC to di- and trivalent cations, present both in feed and water. Fractional gastric emptying of the drug formulations could explain the large
individual differences observed in Cmax and Tmax.
Our data indicate that the elimination of OTC from Arctic charr was slower after p.o. administration than after i.v. administration. This may be caused by slow gastric
Ž .
evacuation due to the low temperatures Amundsen and Klemetsen, 1988 and entero-hepatic recycling of the drug, meaning that absorption is the rate-limiting factor for elimination. Long elimination half-lives of OTC after p.o. administration have also been
Ž .
observed in rainbow trout Bjorklund and Bylund, 1990; Rogstad et al., 1991 . Further-
¨
Ž .
the half-life was calculated to be as long as 713 h after p.o. administration of 10 mgrkg,
Ž .
encapsulated in liposomeralginate particles unpublished results .
The dorsal aorta cannulation technique made it possible to take blood samples from individual fish over prolonged periods of time, thus allowing the advantage of establish-ing individual pharmacokinetic profiles. However, several publications have reported that the pharmacokinetics of drugs is different in cannulated and non-cannulated fish
ŽKleinow, 1991; Martinsen et al., 1993; Sohlberg et al., 1996 , and this could limit the.
value of using this technique. Reduced appetite and swimming activity, which were
Ž .
observed in this study, are indicators of stress. Soivio et al. 1975 and Mazik et al.
Ž1994 also reported increase in stress parameters in fish due to cannulation and repeated.
Ž
blood sampling. Stress, in itself, may increase the metabolic rate in fish Wendelaar
.
Bonga, 1997 , and in addition, swimming activity has been shown to affect blood flow
Ž . Ž .
to the intestines Stevens, 1968 as well as urine flow Hofmann and Butler, 1979 . These factors may have influenced the pharmacokinetics of OTC in this study, but the significance for the calculated values is uncertain.
In the treatment of bacterial fish diseases, antibacterial agents are commonly adminis-tered in feed. Due to the low oral bioavailability of OTC, a major part of the administered drug would thus enter the environment in an unchanged, active form via faeces, without having had a therapeutic effect. The fact, that the bioavailability of OTC
Ž .
is lower in diseased fish compared to healthy fish Uno, 1996 and that addition of OTC
Ž .
to feed reduces the feed intake in fish Hustvedt et al., 1991 , reduces the efficacy of this
Ž
drug even more. Based on its pharmacokinetics and its toxicological Toften and
. Ž . Ž
Jobling, 1996 , environmental Smith et al., 1994 and immunological effects Rijkers et
.
al., 1980; Wishkovsky et al., 1987; Siwicki et al., 1989 , the future use of OTC in the treatment of diseases in farmed fish may be questionable. The pharmacological research in fish should be directed towards alternative antibacterial agents with higher bioavail-ability, satisfactory distribution and short half-lives, without interfering with normal physiology and immunological defense mechanisms.
5. Conclusion
Ž
This study of the pharmacokinetics of OTC in Arctic charr at low temperatures mean
.
of 6.38C has shown that the drug is widely distributed in the fish, with an apparent
volume of distribution ranging from 2.57 to 2.90 lrkg. The elimination of OTC was
relatively slow, with terminal half-lives ranging between 266.3 and 326.9 h. The
Ž .
bioavailability was low mean values 3.2–7.3% and generally unaffected by dose and formulation, but comparable to data reported previously from similar studies in various
fish species. Encapsulating OTC in liposomeralginate particles did not increase the
bioavailability as compared to a formulation where OTC was dissolved in water added agar as a viscosity-increasing agent.
Acknowledgements
Ž
methods is highly appreciated. The staff at the Aquaculture Research Station Tromsø,
.
Norway is acknowledged for technical assistance and for providing fish to this study.
References
Abedini, S., Namdari, R., Law, F.C.P., 1998. Comparative pharmacokinetics and bioavailability of oxytetracy-cline in rainbow trout and chinook salmon. Aquaculture 162, 23–32.
Amundsen, P.A., Klemetsen, A., 1988. Diet, gastric evacuation rates and food consumption in a stunted population of Arctic charr, SalÕelinus alpinus L., in Takvatn, northern Norway. J. Fish Biol. 33, 697–709.
Bjørklund, H., 1988. Determination of oxytetracycline in fish by high-performance liquid chromatography. J. Chromatogr. 432, 381–387.
Bjorklund, H.V., Bylund, G., 1990. Temperature-related absorption and excretion of oxytetracycline in¨
Ž .
rainbow trout Salmo gairdneri R. . Aquaculture 84, 363–372.
Bjorklund, H.V., Bylund, G., 1991. Comparative pharmacokinetics and bioavailability of oxolinic acid and¨
Ž .
oxytetracycline in rainbow trout Oncorhynchus mykiss . Xenobiotica 21, 1511–1520.
Black, W.D., Ferguson, H.W., Byrne, P., Claxton, M.J., 1991. Pharmacokinetic and tissue distribution study of oxytetracycline in rainbow trout following bolus intravenous administration. J. Vet. Pharmacol. Ther. 14, 351–358.
Coliazzi, J.L., Klink, P.R., 1969. pH partition behavior of tetracyclines. J. Pharmacol. Sci. 58, 1184–1189. Cravedi, J.-P., Chubert, G., Delous, G., 1987. Digestibility of chloramphenicol, oxolinic acid and
oxytetracy-cline in rainbow trout and influence of these antibiotics on lipid digestibility. Aquaculture 60, 133–141.
14 Ž
Curtis, L.R., Kemp, C.J., Svec, A.V., 1986. Biliary excretion of C-taurocholate by rainbow trout Salmo
.
gairdneri is stimulated at warmer acclimation temperature. Comp. Biochem. Physiol. C 84, 87–90.
Dauble, D.D., Curtis, L.R., 1990. Influence of digestive processes on the absorption and fate of quinoline
Ž .
ingested by rainbow trout Oncorhynchus mykiss . Environ. Toxicol. Chem. 9, 505–512.
Dyer, D.C., 1989. Pharmacokinetics of oxytetracycline in the turkey, evaluation of biliary and urinary excretion. Am. J. Vet. Res. 50, 522–524.
Eggset, G., McQueen Leifson, R., Mikkelsen, H., Hjelmeland, K., Homme, J.M., Syvertsen, C., Wathne, E., Bøgwald, J., 1995. Oral vaccination of Atlantic salmon, Salmo salar L., against furunculosis with vaccines encapsulated in liposomeralginate particles. J. Mar. Biotechnol. 3, 196–199.
Elema, M.O., Hoff, K.A., Kristensen, H.G., 1996. Bioavailability of oxytetracycline from medicated feed
Ž .
administered to Atlantic salmon Salmo salar L. in seawater. Aquaculture 143, 7–14.
Endo, T., Onozawa, M., Hamaguchi, M., Kusuda, R., 1987. Enhanced bioavailability of oxolinic acid by ultra-fine size reduction in yellowtail. Nippon Suisan Gekkaishi 53, 1711–1716.
Fabre, J., Milek, E., Kalfopoulos, P., Merier, G., 1971. Tetracycline kinetics in man. Digestive absorption and serum concentration. Schweiz. Med. Wochenschr. 101, 593–598.
Ferguson, H.W., 1989. Systemic Pathology of Fish. Iowa State University Press, IA, 263 pp.
Ghandour, M.A., Azab, H.A., Hassan, A., Ali, A.M., 1992. Potentiometric studies on the complexes of
Ž . Ž .
tetracycline TC and oxytetracycline OTC with some metal ions. Monatsh. Chem. 123, 51–58. Grondel, J.L., Nouws, J.F.M., De Jong, M., Schutte, A.R., Driessens, F., 1987. Pharmacokinetics and tissue
distribution of oxytetracycline in carp, Cyprinus carpio L., following different routes of administration. J. Fish Dis. 10, 153–163.
Grondel, J.L., Nouws, J.F.M., Schutte, A.R., Driessens, F., 1989. Comparative pharmacokinetics of oxytetra-cycline in rainbow trout, Salmo gairdneri and African catfish, Clarias gariepinus. J. Vet. Pharmacol. Ther. 12, 157–162.
Guarino, A.M., 1986. In vivo metabolism and disposition of drugs by aquatic species. Vet. Hum. Toxicol. 28
ŽSuppl. 1 , 31–37..
Haenen, O.L.M., Grondel, J.L., Nouws, J.F.M., 1985. Pharmacokinetics of oxytetracycline, trimethoprim, sulphatroxazole and sulphamidine in the carp. In: Van Miert, A.S.J.P.A.M., Bogaert, M.G., Debackere, M.
ŽEds. , Comparative Veterinary Pharmacology, Toxicology and Therapy. Proceedings of the Third EAVPT.
Hofmann, E.L., Butler, D.G., 1979. The effect of increased metabolic rate on renal function in the rainbow trout, Salmo gairdneri. J. Exp. Biol. 82, 11–23.
Houston, A.H., 1971. A simple improvement in the vascular catheterization procedure for salmonid and other teleost fishes. J. Fish. Res. Board Can. 28, 781–783.
Hunn, J.B., 1982. Urine flow rate in freshwater salmonids, a review. Prog. Fish Cult. 44, 119–124. Hustvedt, S.O., Storebakken, T., Salte, R., 1991. Does oral administration of oxolinic acid or oxytetracycline
affect feed intake of rainbow trout?. Aquaculture 92, 109–113.
Ingebrigtsen, K., Nafstad, I., Maritim, A., 1985. The distribution of3H-tetracycline after a single, oral dose in
Ž .
the rainbow trout Salmo gairdneri as observed by whole body autoradiography. Acta Vet. Scand. 26, 428–430.
Kleinow, K.M., 1991. Experimental techniques for pharmacokinetic data collection in free-swimming fish. In:
Ž .
Mayes, M.A., Barron, M.G. Eds. , Aquatic Toxicology And Risk Assessment Vol. 14 American Society for Testing and Materials, Philadelphia, PA, USA, pp. 131–138.
Martinsen, B., Horsberg, T.E., Sohlberg, S., Burke, M., 1993. Single dose kinetic study of sarafloxacin after
Ž .
intravenous and oral administration of different formulations to Atlantic salmon Salmo salar held in sea water at 8.58C. Aquaculture 118, 37–47.
Mazik, P.M., Plakas, S.M., Stehly, G.R., 1994. Effects of dorsal aorta cannulation on the stress response of
Ž .
channel catfish Ictalurus punctatus . Fish Physiol. Biochem. 12, 439–444.
Mevius, D.J., Vellenga, L., Breukink, H.J., Nouws, J.F., Vree, T.B., Driessens, F., 1986. Pharmacokinetics and renal clearance of oxytetracycline in piglets following intravenous and oral administration. Vet. Q. 8, 274–284.
Nikinmaa, M., Soivio, A., Railo, E., 1981. Blood volume of Salmo gairdneri, influence of ambient temperature. Comp. Biochem. Physiol. A 69, 767–769.
Nouws, J.F.M., Boon, J.H., Driessens, F., Mengelers, M.J.B., Booms, G.H.R., Van der Heijden, M.H.T., 1993. Residue depletion of oxytetracycline in European eel. In: Haagsma, N., Ruiter, A., Czedik-Eysenberg, P.B.
ŽEds. , Euroresidue II: Conference of Residues of Veterinary Drugs in Food, 3–5 May 1993, Veldhoven,.
the Netherlands. pp. 514–517.
Nouws, J.F.M., Grondel, J.L., Boon, J.H., Van Ginneken, V.J.T., 1992. Pharmacokinetics of antimicrobials in
Ž .
some freshwater species. In: Michel, C., Alderman, D.J. Eds. , Chemotherapy in Aquaculture: From Theory to Reality. Symposium, 12–15 March 1991, Paris. Office International des Epizooties, pp. 437–447.
Plakas, S.M., McPhearson, R.M., Guarino, A.M., 1988. Disposition and bioavailability of3H-tetracycline in
Ž .
the channel catfish Ictalurus punctatus . Xenobiotica 18, 83–93.
Pye-MacSwain, J., Rainnie, D., Cawthorn, E., Johnson, G., 1992. Plasma pharmacokinetics and bioavailability of oxytetracycline in Atlantic salmon held in seawater. Bull. Aquacult. Assoc. Can. 92, 70–72.
Reja, A., Moreno, L., Serrano, J.M., Santiago, D., Soler, F., 1996. Concentration–time profiles of
oxytetracy-Ž .
cline in blood, kidney and liver of tench Tinca tinca L. after intramuscular administration. Vet. Hum. Toxicol. 38, 344–347.
Rijkers, G.T., Teunissen, A.G., Van Oosterom, R., Van Muiswinkel, W.B., 1980. The immune system of
Ž .
cyprinid fish. The immunosuppressive effect of the antibiotic oxytetracycline in carp Cyprinus carpio L. . Aquaculture 19, 177–189.
Ritschel, W.A., 1992. Handbook of Basic Pharmacokinetics. Drug Intelligence Publications, Hamilton Press, Hamilton, 588 pp.
Rogstad, A., Ellingsen, O.F., Syvertsen, C., 1993. Pharmacokinetics and bioavailability of flumequine and oxolinic acid after various routes of administration to Atlantic salmon in seawater. Aquaculture 110, 207–220.
Rogstad, A., Hormazabal, V., Ellingsen, O.F., Rasmussen, K.E., 1991. Pharmacokinetic study of oxytetracy-cline in fish: 1. Absorption, distribution and accumulation in rainbow trout in freshwater. Aquaculture 96, 219–226.
Schifferli, D., Galeazzi, R.L., Nicolet, J., Wanner, M., 1982. Pharmacokinetics of oxytetracycline and therapeutic implications in veal calves. J. Vet. Pharmacol. Ther. 5, 247–257.
Smith, P., Hiney, M.P., Samuelsen, O.B., 1994. Bacterial resistance to antimicrobial agents used in fish farming, a critical evaluation of method and meaning. Annu. Rev. Fish Dis. 4, 273–313.
Sohlberg, S., Martinsen, B., Horsberg, T.E., Søli, N.E., 1996. Evaluation of the dorsal aorta cannulation
Ž .
technique for pharmacokinetic studies in Atlantic salmon Salmo salar in sea water. J. Vet. Pharmacol. Ther. 19, 460–465.
Soivio, A., Nyholm, K., Westman, K., 1975. A technique for repeated sampling of the blood of individual resting fish. J. Exp. Biol. 62, 207–217.
Soivio, A., Westman, K., Nyholm, K., 1972. Improved method of dorsal aorta catheterization, haematological
Ž .
effects followed for three weeks in rainbow trout Salmo gairdneri . Finn. Fish. Res. 1, 11–21. Stephens, C.R., Murai, K., Brunings, K.J., Woodward, R.B., 1956. Acidity constants of the tetracycline
antibiotics. J. Am. Chem. Soc. 78, 4155–4158.
Stevens, E.D., 1968. The effect of exercise on the distribution of blood to various organs in rainbow trout. Comp. Biochem. Physiol. 25, 615–625.
Toften, H., Jobling, M., 1996. Development of spinal deformities in Atlantic salmon and Arctic charr fed diets supplemented with oxytetracycline. J. Fish Dis. 49, 668–677.
Ž
Uno, K., 1996. Pharmacokinetic study of oxytetracycline in healthy and vibriosis-infected ayu Plecoglossus
.
altiÕelis . Aquaculture 143, 33–42.
Uno, K., Aoki, T., Ueno, R., 1992. Pharmacokinetic study of oxytetracycline in cultured rainbow trout, amago salmon and yellowtail. Nippon Suisan Gakkaishi 58, 1151–1156.
Wendelaar Bonga, S.E., 1997. The stress response in fish. Physiol. Rev. 77, 591–625.
Wishkovsky, A., Roberson, B.S., Hetrick, F.M., 1987. In vitro suppression of the phagocytic response of fish
Ž .
macrophages by tetracyclines. J. Fish Biol. 31 Suppl. A , 61–65.