Effects of combination therapy with estrogen plus simvastatin on
lipoprotein metabolism in postmenopausal women with type IIa
hypercholesterolemia
Akihiko Wakatsuki *, Yuji Okatani, Nobuo Ikenoue
Department of Obstetrics and Gynecology,Kochi Medical School,Oko cho,Nankoku,Kochi783-8505,Japan
Received 12 March 1999; received in revised form 26 July 1999; accepted 18 August 1999
Abstract
We investigated the effects of estrogen and simvastatin, administered both alone and in combination, on the plasma lipid levels and lipoprotein-related enzymes in 45 postmenopausal women with type IIa hypercholesterolemia. They received 0.625 mg conjugated equine estrogen (n=15), 5 mg simvastatin (n=15), or the combination (n=15) daily for 3 months. We measured the concentrations of cholesterol and triglyceride in the plasma, and in the very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL)1 (1.019BdB1.045 g/ml) and LDL2 (1.045BdB1.063 g/ml), and high-density lipoprotein (HDL)2 (1.063BdB1.125 g/ml) and HDL3 (1.125BdB1.210 g/ml) subfractions, and apolipoproteins, and the activities of lipoprotein-metabolizing enzyme before and after treatment. All three treatments significantly lowered the plasma levels of total cholesterol, LDL1 cholesterol, and apolipoprotein B, C-II, and E. In combination therapy, significantly reduced levels of VLDL, IDL, and LDL2 cholesterol were also obtained. Combination therapy lowered total and LDL1 cholesterol significantly more than did estrogen alone. Estrogen and combination therapy significantly increased the levels of cholesterol in the HDL2 subfraction, triglyceride in the HDL2 and HDL3 subfractions, and apolipoprotein A-I and A-II. Estrogen treatment, but not combination therapy, also significantly raised the levels of total and IDL triglyceride. Estrogen and combined therapies significantly lowered the activities of hepatic triglyceride lipase and lecithin cholesterol acyltransferase. Findings indicate that combination therapy with estrogen plus simvastatin favorably affected lipid metabolism by reducing the concentrations of VLDL and IDL particles as well as large and small LDL particles, increasing the concentration of HDL particles, and preventing estrogen-induced increases in plasma triglyceride levels. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Estrogen; Simvastatin; Combination; Very low-density lipoprotein; Low-density lipoprotein; High-density lipoprotein; Postmenopausal women
www.elsevier.com/locate/atherosclerosis
1. Introduction
Low-density lipoprotein (LDL), a major cholesterol (Ch)-carrying lipoprotein in the plasma, is implicated in the formation of atherosclerotic lesions. In women, the plasma levels of LDL cholesterol (LDL-Ch) increase after menopause [1], resulting in age-related increase in the susceptibility to coronary heart disease [2]. We previously demonstrated that in both postmenopausal and oophorectomized women, the decrease in the plasma concentration of estrogen leads to an enhanced activity of lipoprotein lipase (LPL), which may increase
the plasma LDL concentration [3]. Arca et al. suggested that hypercholesterolemia in postmenopausal women is due to an impairment of the LDL receptor [4].
High-density lipoprotein (HDL) is regarded as a protective factor against coronary heart disease, be-cause HDL particles remove cholesterol from the foam cells and transport it to the hepatocytes. According to Jensen et al., the plasma level of HDL-cholesterol (HDL-Ch) decreased after menopause [5]. However, our previous study demonstrated that the plasma level of HDL-Ch did not change significantly after either natural or surgically induced menopause [6].
We previously also found that estrogen replacement therapy favorably affects lipid metabolism by reducing the plasma concentration of LDL particles and increas-* Corresponding author. Tel.:+81-888-80-2382; fax:+
81-888-80-2384.
ing that of HDL particles [6]. Other studies also demonstrated, however, that estrogen stimulates the hepatic production of very low-density lipoprotein (VLDL) which, like LDL, is considered an atherogenic lipoprotein particle [7]. In addition, estrogen induces hypertriglyceridemia, which is associated with an in-creased incidence of coronary heart disease [8]. Plasma triglyceride (TG) levels are closely related to the size of the LDL particles [9]. Thus, the smaller, denser LDL particles are associated with an increased risk of coro-nary heart disease [10]. We previously reported that estrogen-induced hypertriglyceridemia may lead to a reduction in the size of LDL particles and an increased prevalence of LDL subclass pattern B [11], which con-sists of particles having diameters less than 25.5 nm and which is strongly associated with an increased risk for atherosclerosis [10]. Campos et al. reported [12] that estrogen therapy decreases the proportion of large, but not of small, LDL particles.
The National Cholesterol Education Program [13] and the Japanese Atherosclerosis Society [14] have stated the indications and goals of dietary or drug therapy for hypercholesterolemia based on the patients’ plasma levels of total-Ch and LDL-Ch. The estrogen-induced reduction in plasma LDL-Ch is less than 20% [6]. We previously demonstrated that in post-menopausal women with moderate to severe hyperc-holesterolemia, it may be difficult to achieve target levels of total-Ch or LDL-Ch in the plasma by estrogen therapy alone [15], especially since the estrogen-induced reduction in LDL-Ch is less than 20%.
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, effectively reduces the plasma concentrations of VLDL and intermediate-density lipo-protein (IDL), as well as of LDL [16]. Simvastatin has also been shown to reduce plasma total-TG levels in patients with hypertriglyceridemia [17]. Simvastatin slightly increases the plasma levels of HDL-Ch [18]. Combination with estrogen plus simvastatin may provide additional favorable effects on lipid metabolism.
The present study investigated the effects of estrogen and simvastatin, alone and in combination, on the plasma lipid levels and the activities of enzymes in-volved in lipoprotein metabolism in postmenopausal women with type IIa hypercholesterolemia.
2. Materials and methods
2.1. Subjects
Between April 1, 1995 and March 31, 1996, we evaluated 45 postmenopausal Japanese women with type IIa hypercholesterolemia with plasma total-Ch levels of 220 mg/dl or greater and plasma total-TG
levels of less than 150 mg/dl, as defined by Japanese Atherosclerosis Society [14]. Their mean age was 55 years (range, 46 – 64 years); their mean body mass index was 23.192.0 kg/m2(range, 19.7 – 25.8 kg/m2). Subjects with familial hypercholesterolemia (FH), as defined by the Ministry of Health Welfare [14], were excluded from the study. None of the women had had a men-strual period for at least 1 year. None had a history of coronary heart disease or of risk factors for coronary heart disease, such as hypertension, diabetes mellitus, or low HDL cholesterolemia. None of the subjects smoked, used caffeine or alcohol, had a history of thyroid disease, liver disease, or was currently taking any medication known to influence lipoprotein metabolism. Written informed consent was obtained from each subject before admission to the study. The study design was approved by the Ethics Committee of Kochi Medical School.
2.2. Treatment protocol
Each subject had received dietary counseling by nu-tritionists at our institution before drug therapy. In none of the patients did the plasma total-Ch levels decrease to less than 220 mg/dl after 6 months of this dietary regimen. The 45 patients were then randomly assigned in open, parallel-group fashion to the three treatment groups. Subjects in the estrogen group (n= 15) received 0.625 mg oral conjugated equine estrogen daily, those in the simvastatin group (n=15) received 5 mg simvastatin daily, and those in the combination group (n=15) received both agents daily for 3 months. After they had signed informed consent forms, the subjects were randomized by means of a random num-ber generator. Endometrial biopsies and blood samples were obtained from each subject before and after treatment.
2.3. Blood collection and lipoprotein isolation
Blood samples were drawn between 08:00 and 10:00 am following a 12-h fast and centrifuged immediately at 1500×g for 20 min at 4°C to obtain the plasma. Subsequently, heparin (10 U/kg body weight) was ad-ministered intravenously, followed by 5.0 ml saline infusion to flush the line. Ten minutes after heparin administration, blood was collected and postheparin plasma was obtained by centrifugation at 1500×g, for 20 min at 4°C to determine LPL and hepatic triglyce-ride lipase (H-TGL) activities.
Concentrations of total-Ch and total-TG in the plasma, as well as the Ch and TG levels in the lipoproteins, were measured enzymatically [20]. Plasma concentra-tions of apolipoproteins (apo) A-I, A-II, B, C-II, C-III, and E were determined by a turbidimetric im-munoassay [21]. Assays of plasma lipids were per-formed within 24 h of storing the samples at 4°C. Assays of plasma apolipoproteins, lipoprotein lipids, and enzyme activities were performed within 7 days of storing the samples at −80°C.
2.4. Enzyme assays
To determine LPL activity, 0.49 ml assay mixture (50 mmol/l glyceryl trioleate, 15% gum arabic emulsion, 0.2 mol/l Tris – HCl at pH 8.2, 5% bovine serum albumin, 0.1% NaCl, 140ml serum) was incubated for 80 min at
37°C. Postheparin plasma and 100 mmol/l sodium lau-ryl sulfate mixture was incubated for 60 min at 26°C. The reaction was started by adding 0.01 ml postheparin plasma-sodium lauryl sulfate mixture to the assay mix-ture and incubation at 28°C for 60 min. Subsequently, 2.5 ml of an extraction mixture containing 40:10:1 isopropyl alcohol,n-heptane and 1 N H2SO4was added to the incubation mixture and shaken vigorously. After standing for 10 min, the mixture was separated into two phases, and each phase was centrifuged. An aliquot (1 ml) of the upper phase was dried with nitrogen and dissolved with 500 ml 5% Triton X. Fatty acid levels in
the eluate were determined enzymatically [3].
To determine H-TGL activity, the reaction was started by adding 0.01 ml of a mixture of postheparin plasma and 0.2 mol/l Tris – HCl at pH 8.8 to 0.49 ml assay mixture (50 mmol/l glyceryl trioleate, 15% gum arabic emulsion, 0.2 mol/l Tris – HCl at pH 8.8, 5% bovine serum albumin, 0.75 mol/l NaCl). After incuba-tion for 60 min at 28°C, fatty acid levels were measured as already described [3].
Plasma lecithin cholesterol acyltransferase (LCAT) activity was measured using a commercially available enzymatic kit (Nippon Shoji, Osaka).
Cholesterol ester transfer protein (CETP) activity was quantified as the capacity of the samples to pro-mote the transfer of radiolabeled cholesteryl esters from a tracer amount of labeled HDL3 to apo B-containing lipoproteins. Serum samples (25ml) were incubated with
radiolabeled HDL3, 2.5 nmol cholesterol and 75 nmol iodoacetate in a final volume of 50ml at 37°C for 3 h.
A negative control for each sample was carried out by incubating the mixture at 0°C. Of the incubation mix-ture, 45ml were then added to 2 ml potassium bromide
solution (d=1.07 g/ml) and centrifuged at 260,000×g for 4 h at 4°C. Both the supernatant which contained VLDL, IDL, and LDL fractions, and the subnatant, which contained the HDL fraction, were recovered, and radioactivities were measured in both fractions. The
results were expressed as nanomoles of radiolabeled cholesterol esters transferred from HDL3 to apo B-con-taining lipoproteins per minute [22].
2.5. Statistical analysis
All data are expressed as the mean9standard devia-tion (S.D.). Treatment-induced changes were analyzed by a pairedt-test. Differences in the lipid levels among the three groups before and after treatment were ana-lyzed by one-way analysis of variance (ANOVA). If the ANOVA indicated a significant difference, a multiple comparison procedure was performed by Fisher’s pro-tected least-significant difference. A level of PB0.05 was accepted as statistically significant.
3. Results
3.1. Patient characteristics
No significant differences among the groups were found in mean age (estrogen, 54.994.7 years; simvas-tatin, 53.597.1 years; combination, 55.894.7 years) and body mass index (estrogen, 22.892.1 kg/m2
; sim-vastatin, 23.291.6 kg/m2
; combination, 23.392.3 kg/ m2
). The mean body mass index did not change significantly during the study period. Histologic analy-sis of endometrial biopsy specimens from all patients did not detect hyperplasia in any of the patients before or after treatment. Baseline levels of lipids, apolipo-proteins, and the enzyme activities were not signifi-cantly different among the three groups.
3.2. Changes in cholesterol le6els
3.3. Changes in triglyceride le6els
In addition to cholesterol levels, we evaluated the effects of each treatment on triglyceride levels in the
various lipoprotein subfractions. Estrogen treatment resulted in significant increases in the plasma levels of total-TG, an increase of 22.5%, IDL-TG (46.6%), HDL2-TG (69.1%), and HDL3-TG (47.7%). No
signifi-Table 1
Changes in cholesterol levels in the lipoproteins in hypercholesterolemic postmenopausal women treated with estrogen, simvastatin, or a combination of estrogen plus simvastatina
Combination (n=15) Cholesterol Estrogen (n=15) Simvastatin (n=15)
266.5928.9
Change (%) −11.2910.6 −22.3910.0†† −27.597.5†††
36.9912.9 39.3912.3
Before 31.3910.5
VLDL (mg/dl)
33.3911.0 23.798.8* 25.398.4**
After
−20.0930.0 −31.5924.2†
−5.6929.0
−40.6925.2†† −17.6936.9 3.6960.9
−33.5912.1†† −41.9911.5†††
Change (%) −21.0913.2
25.596.7 23.798.6 24.896.2
Before LDL2 (mg/dl)
19.496.3*
After 22.696.6 20.999.0
−6.6978.4 −15.4940.0
−4.6937.2
After 18.792.2 17.592.4 18.793.4
3.2925.3
−7.4916.8 2.7911.6
Change (%)
aData are expressed as mean9S.D. *PB0.05, **PB0.01, ***PB0.001 versus before treatment as determined by pairedt-test. †PB0.05, ††PB0.01, †††PB0.001 versus estrogen treatment as determined by multiple comparison analysis.
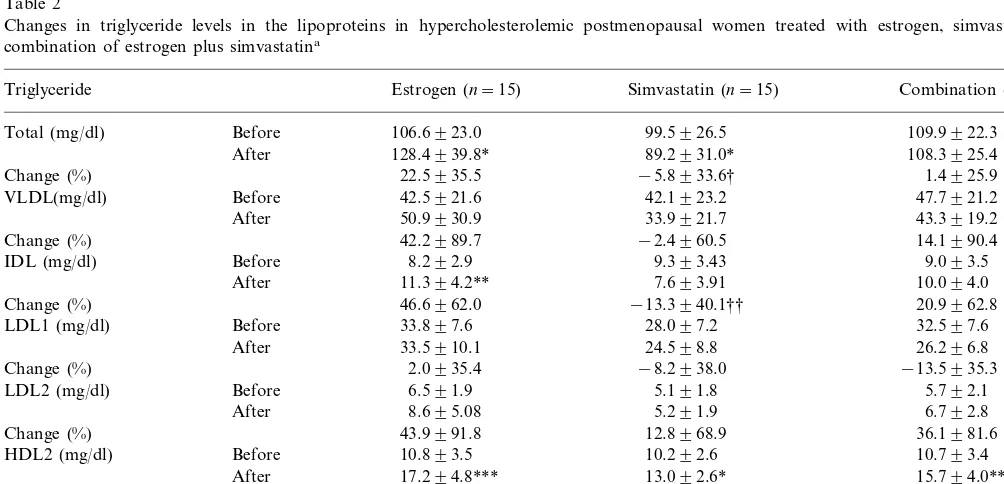
Table 2
Changes in triglyceride levels in the lipoproteins in hypercholesterolemic postmenopausal women treated with estrogen, simvastatin, or a combination of estrogen plus simvastatina
Triglyceride Estrogen (n=15) Simvastatin (n=15) Combination (n=15)
109.9922.3 99.5926.5
Total (mg/dl) Before 106.6923.0
108.3925.4
After 128.4939.8* 89.2931.0*
−5.8933.6† 1.4925.9
Change (%) 22.5935.5
47.7921.2
VLDL(mg/dl) Before 42.5921.6 42.1923.2
43.3919.2 33.9921.7
50.9930.9 After
42.2989.7 −2.4960.5 14.1990.4
Change (%)
Before 9.393.43 9.093.5
IDL (mg/dl) 8.292.9
7.693.91 10.094.0
After 11.394.2**
46.6962.0 −13.3940.1†† 20.9962.8
Change (%)
32.597.6
Before 33.897.6 28.097.2
LDL1 (mg/dl)
8.695.08 5.291.9 6.792.8
After
Before 4.791.15 4.391.3
HDL3 (mg/dl)
5.191.7 6.993.0*
After 6.592.4**
8.2937.5
Change (%) 47.7951.6‡ 61.0966.1‡‡
Table 3
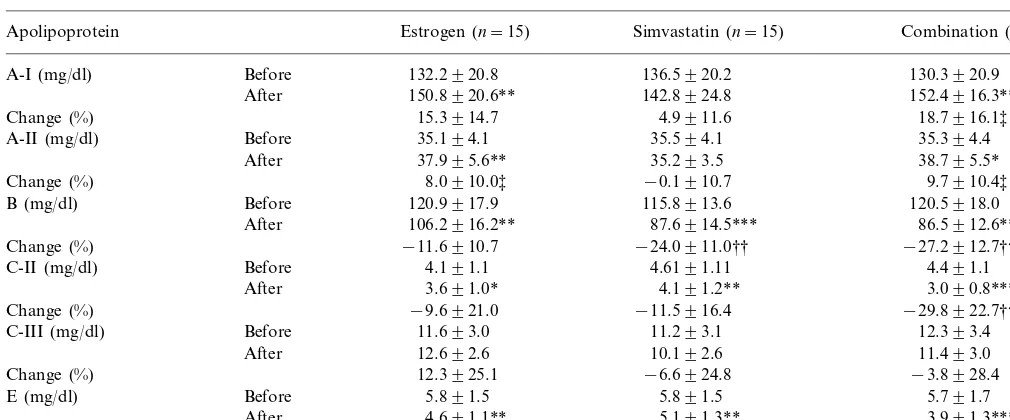
Changes in apolipoprotein levels in hypercholesterolemic postmenopausal women treated with estrogen, simvastatin, or a combination of estrogen plus simvastatina
Estrogen (n=15)
Apolipoprotein Simvastatin (n=15) Combination (n=15)
A-I (mg/dl) Before 132.2920.8 136.5920.2 130.3920.9
150.8920.6** 142.8924.8
After 152.4916.3**
15.3914.7 4.9911.6
Change (%) 18.7916.1‡
35.194.1 35.594.1
Before 35.394.4
A-II (mg/dl)
37.995.6**
After 35.293.5 38.795.5*
8.0910.0‡ −0.1910.7 9.7910.4‡
Change (%)
120.9917.9 115.8913.6
B (mg/dl) Before 120.5918.0
106.2916.2** 87.6914.5***
After 86.5912.6***
−11.6910.7 −24.0911.0††
Change (%) −27.2912.7†††
4.191.1 4.6191.11
Before 4.491.1
C-II (mg/dl)
3.691.0* 4.191.2** 3.090.8***
After
−9.6921.0 −11.5916.4 −29.8922.7††‡
Change (%)
11.693.0 11.293.1
C-III (mg/dl) Before 12.393.4
12.692.6 10.192.6
After 11.493.0
Change (%) 12.3925.1 −6.6924.8 −3.8928.4
Before
E (mg/dl) 5.891.5 5.891.5 5.791.7
4.691.1** 5.191.3**
After 3.991.3***
Change (%) −18.5915.6 −10.6916.2 −31.6915.4††‡‡
aData are expressed as mean9S.D. *PB0.05, **PB0.01, ***PB0.001 versus before treatment as determined by pairedt-test. ††PB0.01, †††PB0.001 versus estrogen treatment and ‡PB0.05, ‡‡PB0.01 versus simvastatin treatment as determined by multiple comparison analysis.
cant changes occurred in LDL-TG levels (Table 2). Simvastatin treatment, in contrast, significantly reduced the plasma level of total-TG by 5.8%, but did not significantly affect the plasma levels of IDL-TG, LDL-TG, and HDL3-TG. Simvastatin did, however, signifi-cantly increase the concentration of HDL2-TG by 32.3%. Finally, combination therapy significantly in-creased the levels of HDL2-TG by 52.6% and of HDL3-TG by 61.0%. When comparing the three treat-ment approaches, the post-treattreat-ment levels of total-TG and IDL-TG were significantly lower in the simvastatin group than in the estrogen group.
3.4. Apolipoprotein changes
We also assessed the effects of estrogen, simvastatin, and combination therapy on the patients’ apolipo-protein levels. These analyses found that estrogen treat-ment significantly increased the plasma levels of apo A-I, an increase of 15.3%, and A-II (8.0%), and signifi-cantly reduced the plasma levels of apo B, a decrease of 11.6%, apo C-II (9.6%) and apo E (18.5%) (Table 3). Simvastatin treatment also resulted in significant reduc-tions in the plasma levels of apo B decrease of 24.0%, apo C-II (11.5%), and apo E (10.6%), but did not significantly alter the levels of apo A-I and A-II. With combination therapy, the plasma levels of apo A-I were elevated by 18.7% and apo A-II by 9.7%, whereas the plasma levels of apo B were significantly reduced by 27.2%, apo C-II by 29.8%, and apo E by 31.6%. Comparison of the three groups found that the percent
decreases in the levels of apo B, apo C-II and apo E were significantly greater in the combination group than in the estrogen group.
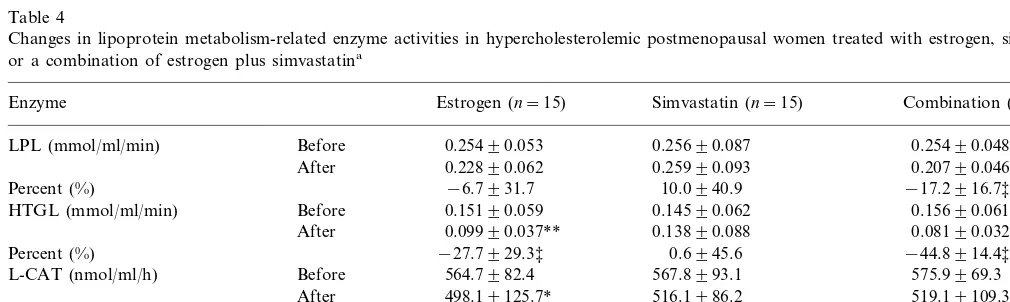
3.5. Enzymatic changes
Finally, we evaluated the effects of estrogen and simvastatin, individually or in combination, on the activities of enzymes involved in lipoprotein metabo-lism. Estrogen treatment significantly inhibited the ac-tivities of H-TGL by 27.7%, and LCAT by 11.6%. The activities of LPL and CETP did not change significantly (Table 4). Simvastatin caused no significant changes in the activities of any of these enzymes tested. Combina-tion therapy, in contrast, significantly suppressed the activities of LPL by 17.2%, H-TGL by 44.8%, and LCAT by 9.8%, but had no effect on CETP activity.
4. Discussion
particles in the liver, which like LDL, are considered atherogenic lipoprotein particles [7]. In addition, estro-gen increases plasma TG levels that are also considered a risk factor of coronary heart disease [8].
Simvastatin has previously been shown to reduce the plasma level of LDL particles as well as those of VLDL and IDL particles [16]. Simvastatin also decreases plasma total-TG levels in patients with hypertriglyce-ridemia [17]. The adverse effects of estrogen on lipid metabolism could be minimized by the combination of simvastatin. In the present study, although estrogen did not alter the plasma levels of VLDL-Ch, VLDL-TG and IDL-Ch, simvastatin or combination therapy with estrogen plus simvastatin reduced those levels. Thus, combination therapy has a favorable effect by reducing plasma levels of VLDL and IDL particles. VLDL is readily metabolized to IDL particles by LPL. This reaction requires apo C-II bound to the surface of the lipoproteins as an essential co-factor. Previous studies found that VLDL particles containing apo E have a high affinity for the LDL receptor and are easily taken up via this receptor [25]. In the present study, all treatments reduced plasma levels of apo C-II and E. Although we did not measure apolipoprotein concen-trations in each lipoprotein particle, these observations suggest that the catabolism of VLDL may be inhibited and the amount of VLDL particles may be directly catabolized by the hepatic LDL receptor in each ther-apy. However, combination therapy markedly reduced the plasma level of apo E, indicating that combination with estrogen plus simvastatin may enhance the stimu-lation of hepatic LDL receptors and reduced hepatic apo B production.
Plasma levels of LDL-Ch are closely associated with the incidence of coronary heart disease. For example, the Lipid Research Clinics Coronary Prevention Trial concluded that an 11% decrease in LDL-Ch levels
reduced the incidence of coronary heart disease by 19% [26]. In the present study, patients on combination therapy exhibited a 38.7% reduction in the LDL-Ch levels and may therefore be at significantly low risk of coronary heart disease.
LDL particles vary in size, density, and lipid compo-sitions. Gaw et al. [27] have identified three LDL subclasses: LDL I and LDL II, which are equivalent to the LDL1 subclass in the present study, represent large, light LDL particles; LDL III, which is equivalent to the LDL2 subclass in the present study, represents small, heavy LDL particles. Not all LDL subfractions are equally atherogenic. Thus, smaller, denser LDL parti-cles are associated with an increased risk of coronary heart disease [10]. This LDL subfraction has a lower affinity for the LDL receptor and likely accumulates in the blood [28]. Moreover, small LDL particles are more susceptible to oxidative modification [29], the initial step in development of atherosclerosis. In the present study, we have demonstrated that estrogen reduced the levels of LDL1-Ch, but did not affect the levels of LDL2-Ch. This finding is consistent with our previous report that estrogen decreases the plasma levels of LDL1-Ch and LDL1-apo B, but not of LDL2-Ch and LDL2-apo B [15]. Each LDL particle contains one molecule of apo B; therefore, the decrease in LDL1-apo B after estrogen treatment likely indicates that estrogen reduces the plasma concentration of large LDL parti-cles. A decrease in LDL-Ch also may be accompanied by a decrease in the plasma concentration of LDL particles. Consistent with our previous findings and the study by Campos et al. [12], estrogen therapy in the present study decreased the plasma concentration of the large, but not of the small, LDL particles. These obser-vations suggest that estrogen removes large LDL parti-cles via hepatic LDL receptors, whereas small LDL particles may not be affected because of their lower affinity for these receptors [27].
Table 4
Changes in lipoprotein metabolism-related enzyme activities in hypercholesterolemic postmenopausal women treated with estrogen, simvastatin, or a combination of estrogen plus simvastatina
Enzyme Estrogen (n=15) Simvastatin (n=15) Combination (n=15)
Before
LPL (mmol/ml/min) 0.25490.053 0.25690.087 0.25490.048
After 0.22890.062 0.25990.093 0.20790.046**
Percent (%) −6.7931.7 10.0940.9 −17.2916.7‡
0.15690.061 0.14590.062
HTGL (mmol/ml/min) Before 0.15190.059
After 0.09990.037** 0.13890.088 0.08190.032***
Percent (%) −27.7929.3‡ 0.6945.6 −44.8914.4‡‡
Before 564.7982.4
L-CAT (nmol/ml/h) 567.8993.1 575.9969.3
After 498.19125.7* 516.1986.2 519.19109.3*
Percent (%) −11.6920.4 −6.5924.0 −9.8914.9
Before 2.9391.14 2.7391.03 2.7291.15
CETP (nmol/ml/min)
2.3990.44 2.6090.58
After 2.4690.69
−2.0936.4
Percent (%) −3.3934.3 1.9944.8
Simvastatin suppresses the endogenous synthesis of hepatic cholesterol, stimulates LDL receptors in the liver, and reduces the plasma levels of LDL-Ch [30]. Similarly, Cuchel et al. [31] have demonstrated that lovastatin reduces plasma LDL-Ch by decreasing LDL production. The suggested mechanisms for this effect include a reduced hepatic secretion of apo B, and an increased uptake of VLDL remnants. In the present study, simvastatin treatment reduced the concentration of LDL1-Ch, indicating that this agent decreases plasma concentrations of the large LDL particles. This simvastatin-induced reduction in large LDL particles may result from the enhanced removal of LDL particles via LDL receptors or from reduced production of LDL. As with estrogen therapy, simvastatin did not significantly alter the levels of LDL2-Ch, suggesting that simvastatin’s effect may be specific for large LDL particles. According to Tilly-Kiesi and Tikkanen [32], HMG-CoA reductase inhibitors such as lovastatin or simvastatin reduce both light and heavy LDL particles in non-FH subjects. The discrepancy between the present findings and the previous study is difficult to reconcile, but may be related to differences in the density of LDL subfractions. LDL subfractions in our study were denser than those in the previous study. Smaller, denser LDL particles have a low affinity for LDL receptor and easily accumulate in the circulation [28].
Combination therapy with estrogen plus simvastatin markedly reduced the concentrations of LDL1-Ch and apo B, which is the major apolipoprotein in LDL. This reduction was significantly greater than that achieved with estrogen, indicating that the estrogen-induced re-duction in large LDL particles can be enhanced by the simultaneous administration of simvastatin. Combina-tion therapy also resulted in reduced LDL2-Ch concen-trations, which may represent a reduction in the plasma concentration of small LDL particles. Potential mecha-nisms for this favorable effect include an enhanced stimulation of hepatic LDL receptors and a reduction in LDL production.
HDL readily removes excess free cholesterol from the tissues via transfer from the cell membrane. Therefore, the plasma level of HDL-Ch is inversely related to the risk of coronary heart disease. In the present study, estrogen increased the concentrations of HDL2-Ch and -TG, and HDL3-TG as well as the plasma levels of apo A-I and A-II, which are the major apolipoproteins in HDL. These findings suggest that estrogen therapy increases the plasma level of HDL particles. HDL is secreted by the liver, and intestine, and during the degradation of TG-rich lipoproteins. The plasma HDL concentration is regulated by activities of enzymes such as LPL, H-TGL, and CETP. LPL facilitates the degra-dation of TG-rich lipoproteins and the production of HDL particles. CETP plays a key role in cholesterol
metabolism by promoting the transfer of cholesteryl ester from HDL to apo B-containing lipoproteins. H-TGL hydrolyzes the TG in IDL to produce LDL, and converts HDL2 to HDL3. Sex steroid hormones regu-late the plasma level of HDL-Ch via the changes in the activity of H-TGL. Estrogen inhibits the activity of H-TGL and increases the plasma level of HDL-Ch, while androgenic progestins have the converse effect [33]. In the present study, estrogen therapy inhibited the activity of H-TGL, but not of LPL or CETP. The estrogen-induced inhibition of H-TGL activity may re-sult in elevated plasma HDL levels [6] and may con-tribute to a reduced incidence of coronary heart disease. Simvastatin, in contrast, did not affect HDL lipid levels, whereas the combination therapy had an effect similar to that of estrogen in increasing the plasma concentration of HDL particles. These findings suggest that simvastatin may not affect HDL metabolism, and that the estrogen’s favorable effect on increasing the level of HDL particles may be preserved by administering it in combination with simvastatin. LCAT esterifies free cholesterol in HDL that is trans-ferred from the tissue. We previously reported that the estrogen-induced decrease in the LDL-Ch concentra-tion may result in the secondary inhibiconcentra-tion of plasma LCAT activity [6]. In the present study, however, LCAT activity was reduced by the estrogen and combi-nation therapies, but not by simvastatin treatment. This finding suggests that the observed reduction of LCAT activity may be due to a direct effect of estrogen.
Hypertriglyceridemia also is a risk factor for coro-nary heart disease [8]. In the present study, estrogen therapy increased the plasma level of total-TG, consis-tent with our previous finding [11]. The estrogen-in-duced increase in plasma total-TG has been shown to result in smaller LDL particles and a greater prevalence of LDL subclass pattern B [11], which is associated with a higher incidence of coronary heart disease [10]. Simvastatin, in contrast, decreases plasma total-TG levels in patients with hypertriglyceridemia [17]. Consis-tent with those findings, simvastatin lowered the total-and IDL-TG concentrations in the present study. In combination with estrogen, simvastatin reduced the estrogen-induced increase in plasma total-TG, and therefore may minimize the adverse effects of estrogen on LDL particle size.
present study demonstrated that the combination of simvastatin and estrogen effectively reduces the concen-trations of large and small LDL particles in plasma, lowers the concentration of VLDL and IDL particles, and increases the concentration of HDL particles. Fur-thermore, combination therapy with simvastatin can prevent estrogen-induced hypertriglyceridemia. Accord-ing to the Heart and Estrogen/Progestin Replacement Study [35], hormone replacement therapy did not re-duce the overall rate of coronary heart disease events in postmenopausal women with established coronary dis-ease. Additional investigations are needed to determine whether estrogen plus simvastatin combination therapy may reduce the incidence of coronary heart disease in postmenopausal women as compared with estrogen alone.
References
[1] Kannel WB. Metabolic risk factors for coronary artery disease in women: prospective from the Framingham Study. Am Heart J 1987;114:413 – 9.
[2] Castelli WP. Epidemiology of coronary heart diseases: the Fram-ingham study. Am J Med 1984;76:4 – 12.
[3] Wakatsuki A, Sagara Y. Lipoprotein metabolism in post-menopausal and oophorectomized women. Obstet Gynecol 1995;85:523 – 8.
[4] Arca M, Vega GL, Grundy SM. Hypercholesterolemia in post-menopausal women. Metabolic defects and response to low-dose lovastatin. J Am Med Assoc 1994;271:453 – 9.
[5] Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas 1990;12:321 – 31. [6] Wakatsuki A, Sagara Y. Effects of continuous
medroxyproges-terone acetate on lipoprotein metabolism in postmenopausal women receiving estrogen. Maturitas 1996;25:35 – 44.
[7] Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. New Engl J Med 1991;325:1196 – 204.
[8] Carlson LA, Bottiger LE, Ahfeldt PE. Risk factors for myocar-dial infarction in the Stockholm prospective study. A 14-year follow-up focussing on the role of plasma triglycerides and cholesterol. Acta Med Scand 1979;206:351 – 60.
[9] McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb 1992;12:1284 – 90.
[10] Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclasses patterns and risk of myocardial infarction. J Am Med Assoc 1988;260:1917 – 21.
[11] Wakatsuki A, Ikenoue N, Sagara Y. Effect of estrogen on the size of low-density lipoprotein particles in postmenopausal women. Obstet Gynecol 1997;90:22 – 5.
[12] Campos H, Sacks FM, Walsh BW, Schiff I, O’Hanesian MA, Krauss RM. Differential effects of estrogen on low-density lipo-protein subclasses in healthy postmenopausal women. Metabolism 1993;42:1153 – 8.
[13] Anonymous. Summary of the Second Record of the National Cholesterol Education Program (NCEP) Expert Panel on Detec-tion, EvaluaDetec-tion, and Treatment of High Blood Cholesterol in Adults. (Adult Treatment Panel II). J Am Med Assoc 1993;269:3015 – 3023.
[14] Anonymous. Investigating committee of guideline for diagnosis and treatment of hyperlipidemias. J Jpn Atheroscler Soc 1997;25:1 – 34.
[15] Wakatsuki A, Ikenoue N, Izumiya C, Okatani Y, Sagara Y. Effect of estrogen and simvastatin on low-density lipoprotein subclasses in hypercholesterolemic postmenopausal women. Ob-stet Gynecol 1998;92:367 – 72.
[16] Anonymous. Comparison of the efficacy, safety and tolerability of simvastatin and pravastatin for hypercholesterolemia. The Simvastatin Pravastatin Study Group. Am J Cardiol 1993;71:1408 – 1414.
[17] Itoh T, Matsumoto M, Hougaku H, et al. Effects of low-dose simvastatin therapy on serum lipid levels in patients with moder-ate hypercholesterolemia: a 12-month study. The Simvastatin Study Group. Clin Ther 1997;19:487 – 97.
[18] Homma Y, Ozawa H, Kobayashi T, Yamaguchi H, Sakane H, Nakamura H. Effects of simvastatin on plasma lipoprotein subfractions, cholesterol esterification rate, and cholesteryl ester transfer protein in type II hyperlipoproteinemia. Atherosclerosis 1995;114:223 – 34.
[19] Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in hu-man plasma. J Clin Invest 1955;34:1346 – 53.
[20] Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470 – 5.
[21] Ikeda T, Shibuya Y, Senda U, et al. Automated immunoturbidi-metric analysis of six plasma apolipoproteins: correlation with radical immunodiffusion assays. J Clin Lab Anal 1991;5:90 – 5. [22] Lagrost L, Gandjini H, Athias A, Guyard-Dangremont V,
Lalle-mant C, Gambert P. Influence of plasma cholesteryl ester trans-fer activity on the LDL and HDL distribution profiles in normolipidemic subjects. Arterioscler Thromb 1993;13:815 – 25. [23] Assmann G, Schulte H. The Prospective Cardiovascular
Mun-ster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am Heart J 1988;116:1713 – 24. [24] Ma PT, Yamamoto T, Goldstein JL, Brown MS. Increased
mRNA for low density lipoprotein receptor in livers of rabbits treated with 17 alpha ethinyl-estradiol. Proc Natl Acad Sci USA 1986;83:792 – 6.
[25] Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240:622 – 30. [26] Anonymous. The Lipid Research Clinics Coronary Primary
Pre-vention Trial Results. II. The relationship of reduction in inci-dence of coronary heart disease to cholesterol lowering. J Am Med Assoc 1984;251:365 – 374.
[27] Gaw A, Packard JJ, Murray EF, et al. Effects of simvastatin on apo B metabolism and LDL subfraction distribution. Arte-rioscler Thromb 1993;13:170 – 89.
[28] Nigon F, Lesnik P, Rouis M, Chapman MJ. Discrete subspecies of human low density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J Lipid Res 1991;32:1741 – 53.
[29] Tribble DL, Holl LG, Wood PD, Krauss RM. Variations in oxidative susceptibility among six low density lipoprotein sub-fractions of differing density and particle size. Atherosclerosis 1992;93:189 – 99.
[30] Grundy SM, Bilheimer DW. Inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase by mevinolin in familial hypercholes-terolemia heterozygotes: effects on cholesterol balance. Proc Natl Acad Sci USA 1984;81:2538 – 42.
[32] Tilly-Kiesi M, Tikkanen MJ. Low density lipoprotein density and composition in hypercholesterolaemic men treated with HMG CoA reductase inhibitors and gemfibrozil. J Intern Med 1991;229:427 – 34.
[33] Colvin PL Jr, Auerbach BJ, Case LD, Hazzard WR, Ap-plebaum-Bowden D. A dose – response relationship between sex hormone-induced change in hepatic triglyceride lipase and high-density lipoprotein cholesterol in postmenopausal women. Metabolism 1991;40:1052 – 6.
[34] Ettinger B, Friedman GD, Bush T, Quesenberry CP Jr. Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol 1996;87:6 – 12.
[35] Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. J Am Med Assoc 1998;280:605 – 13.