Lack of effect of serum amyloid A (SAA) on the ability of
high-density lipoproteins to inhibit endothelial cell adhesion
molecule expression
Dale Ashby
a,b,c, Jenny Gamble
c, Mathew Vadas
c, Noel Fidge
d, Sarah Siggins
d,
Kerry – Anne Rye
a,b,c, Philip J. Barter
a,b,c,*
aDepartment of Medicine,Royal Adelaide Hospital,Uni6ersity of Adelaide,North Terrace,Adelaide,SA5000,Australia bCardio6ascular In6estigation Unit,Royal Adelaide Hospital.Adelaide,SA,Australia
cHanson Centre for Cancer Research,Department of Human Immunology,IMVS.Adelaide,SA,Australia dBaker Medical Research Institute,Prahran,Vic,Australia
Received 27 August 1999; received in revised form 25 January 2000; accepted 18 February 2000
Abstract
Studies have been conducted to determine whether the ability of high density lipoproteins (HDL) to inhibit the cytokine-in-duced expression of vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells is altered by the presence in HDL of the acute phase reactant, serum amyloid-A (SAA). Preparations of HDL3were isolated on two separate occasions from the plasma
of each of 19 patients: the first was collected before and the second 3 days after undergoing coronary artery bypass graft surgery. Whereas the preoperative HDL3sample contained no SAA, in the postoperative sample SAA accounted for an average of 42%
of the HDL3protein. The preoperative HDL3and postoperative, SAA-enriched HDL3were identical in terms of their ability to
inhibit the tumour necrosis factor-a (TNF-a)-induced expression of VCAM-1 in human umbilical vein endothelial cells (HUVECs). To assess the effect of having an even greater SAA enrichment of HDL3, samples of HDL3 were incubated with
purified SAA, which displaced almost all of the apoAI and about 40% of the apoAII from the HDL3. This in vitro SAA-enriched
HDL3 inhibited the TNF-a-induced expression of VCAM-1 in HUVECs in a concentration dependent manner, which was
identical to that of the unmodified HDL3. The presence of SAA did not alter the cell-surface binding of HDL3to endothelial cells.
It has been concluded that the presence of SAA in HDL has no effect on the ability of these lipoproteins either to inhibit the expression of VCAM-1 in endothelial cells or to bind to proteins on the endothelial cell surface. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Adhesion molecules; Endothelial cells; High density lipoprotiens; Serum amyloid A
www.elsevier.com/locate/atherosclerosis
1. Introduction
There is a growing consensus that atherosclerosis is a chronic inflammatory condition in which an early event is the recruitment of blood monocytes in a process mediated by the expression of adhesion proteins on the surface of endothelial cells [1]. Our recent observation [2] that human high density lipoproteins (HDL) inhibit the cytokine-induced expression of the endothelial cell adhesion proteins (VCAM-1, ICAM-1 and E-selectin)
has raised the possibility that anti-inflammatory prop-erties of HDL may contribute to the well documented anti-atherogenic effects of these lipoproteins.
We have been systematically investigating how the composition of HDL impacts on their anti-inflamma-tory potential. In studies with both native HDL [3] and reconstituted HDL (rHDL) [4], we have shown that inhibition of the cytokine-induced expression of VCAM-1 in endothelial cells is unaffected by replacing all of the HDL apolipoprotein (apo) AI with apoAII. We have also shown that changing the HDL particle size and the cholesteryl ester and triglyceride content of the particles has no effect on inhibitory activity [4]. We now investigate whether the ability of HDL to inhibit * Corresponding author. Tel.: +61-88-2225608; fax: +
61-88-2225938.
E-mail address:[email protected] (P.J. Barter).
the cytokine-induced expression of VCAM-1 in en-dothelial cells is altered when the apoAI in HDL is replaced by the acute phase reactant, serum amyloid-A (SAA).
SAA is synthesised by the liver during times of inflammation. Once released into the circulation, SAA rapidly associates with HDL [5 – 7] in which it may account for up to 87% of HDL protein [8,9]. There have been suggestions that SAA may be pro-athero-genic. For example, HDL enriched with SAA have a reduced reactivity with lecithin:cholesterol acyltrans-ferase (LCAT) which may compromise their efficiency as promoters of reverse cholesterol transport [10]. SAA-enriched HDL have also been reported to have a decreased ability to protect LDL against oxidation [11]. Furthermore, SAA which is unassociated with HDL has been shown to induce the migration, adhesion and tissue infiltration of monocytes, polymorphonuclear leukocytes and T lymphocytes, and to increase the expression of cellular adhesion molecules [12]. It is not known whether the expression of endothelial cell adhe-sion molecules is also promoted by the SAA, which is associated with HDL. Nor is it known whether the presence of SAA in HDL interferes with the docu-mented ability of HDL to inhibit the cytokine-induced expression of adhesion molecules in endothelial cells. This latter issue is addressed in the studies described in this report.
2. Methods
2.1. Isolation of high density lipoproteins
Blood was collected from subjects by standard venipuncture in tubes containing disodium EDTA (final concentration 1 mg/ml) after a 14 h fast and was placed immediately on ice. In the subjects undergoing coro-nary artery bypass graft (CABG) surgery, blood was collected on the day prior to surgery and again from the same subjects on the third post-operative day. Blood was collected from healthy control subjects on two occasions, 4 days apart. Plasma was separated by centrifugation at 4°C. HDL3 were isolated in the
den-sity interval 1.13 – 1.21 g/ml by sequential ultracentrifu-gation at 4°C for 17 h at 100 000 rpm in a Beckman TLA 100.4 rotor using a Beckman TL-100 Ultracen-trifuge; density adjustments were achieved by the addi-tion of solid KBr [13]. Purificaaddi-tion was achieved by two further centrifugations, one at the lower and the other at the higher density. Supernatants and infranatants in each spin were recovered by tube slicing. The resulting preparations of HDL3were dialysed against either 3×
1 L of endotoxin-free phosphate buffered saline (pH 7.4) (PBS) or Tris-buffered saline (TBS) (pH 7.4).
Approval for collection of plasma samples from pa-tients and normal controls was obtained from the Hu-man Ethics Committee of the Royal Adelaide Hospital.
2.2. Characterisation of HDL
HDL particle size distribution was assessed by elec-trophoresis on 3 – 40% nondenaturing polyacrylamide gradient gels (Gradipore, Sydney, Australia) as de-scribed previously [14]. Concentrations of total protein, apoAI, apoAII, triglyceride, total and unesterified cholesterol and phospholipids were assayed as de-scribed elsewhere [15]. The SAA content of HDL3 was
estimated by two methods. (i) HDL3 samples were
subjected to SDS polyacrylamide gel electrophoresis. The gels were then stained and scanned [8] and the percentages of SAA, apoAI and apoAII were calculated from the integrated curves on the assumption that dye uptake is similar for each peptide and proportional to apolipoprotein mass. (ii) HDL3 were assayed directly
for total protein, apoAI and apoAII, with SAA being estimated by difference. All samples were assayed for SAA by both methods, with the two values never varying by more than 8%. The values presented repre-sent the means of the two methods.
2.3. Isolation of SAA
SAA was purified from the plasma collected from patients 3 days after they had undergone surgery for coronary artery bypass grafting (CABG). HDL3 was
isolated as above and delipidated [16]. The apoAI, apoAII and SAA were separated by a modification of the method of Weisweiler [17]. The modification in-volved using a salt gradient of 0.5 M NaCl from 0 – 50% over 3 h using a Superose 6 Pharmacia column contain-ing Q-sepharose. A total of 100 mg protein (apoAI, apoAII and SAA) was loaded onto the column. The apoAI, apoAII and SAA each appeared as a single band after electrophoresis on a 20% SDS-polyacryla-minde gradient gel stained with Coomassie R 350. The purified SAA was dialysed against 3×5 L of 20 mM NH4HCO3, lyophilised and stored at −20°C until
used. Prior to use, the SAA was reconstituted in 0.01 M Tris – HCl, 3.0 M guanidine HCl, 0.01% (w/v) EDTA-Na2 (pH 8.2) for 1 – 2 h and then exhaustively dialysed
against TBS (5×1 l).
2.4. Preparation of SAA-enriched HDL3 in6itro
HDL3 was isolated from healthy subjects and
incu-bated in vitro with purified SAA at a mass ratio of SAA:HDL3 total protein of 2:1. As a control another
aliquot of the HDL3 was incubated in the absence of
the supernatant following ultracentrifugation for 17 h at 1.25 g/ml at 100 000 rpm in a Beckman TLA 100.4 rotor in a Beckman TL-100 Ultracentrifuge. The re-covered HDL3 samples were dialysed against 3×1 l
of endotoxin- free PBS before use.
2.5. Preparation of discoidal reconstituted HDL
Discoidal reconstituted HDL (rHDL) containing g -palmityl-b-linoleoyl-phosphatidylcholine (PLPC) and either apoAI or SAA were prepared by the cholate dialysis method described by [18].
2.6. Expression of VCAM-1 in human umbilical 6ein endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated as described previously [2]. They were cultured on gelatin coated culture flasks in medium M199 with Earles Salts (Trace Biosciences, Australia) supplemented with 20% foetal calf serum (FCS) (Commonwealth Serum Laboratories, Melbourne, Australia), 20 mmol/l HEPES, 2 mmol/l glutamine, 1 mmol/l sodium pyruvate, non essential amino acids, penicillin, streptomycin, 20 mg/ml endothelial growth supplement (Collaborative Research, Australia) and 20 mg/ml heparin (Sigma). Confluent preparations of passage three, four or five HUVECs were pre-incu-bated for 1 h (or for 17 h in some experiments) in the presence of the various preparations HDL3. TNF-a (100 U/ml) (R&D Systems, USA) was then added to the culture medium and 5 h later the cell surface expression of VCAM-1 was measured by flow cytometry analysis.
2.7. Flow cytometry analysis
The expression of VCAM-1 on the surface of HU-VECs was measured as described previously [2]. In brief, the HUVECs were first washed with FACS wash (RPMI 1640, containing 10 mmol/l HEPES, 0.02% azide and 2.5% foetal calf serum). They were then incubated with mouse monoclonal antibody to VCAM-1 (51-10C9) for 30 min at 4°C, then washed again with FACS wash. Binding of the primary anti-body was detected by incubating with FITC conju-gated secondary antibody (Immunotech FITC conjugated F(ab)2 fragment goat (mouse IgG)) for 30 min at 4°C. After washing with PBS, the cells were harvested by trypsinization. FACS wash was added to neutralise the trypsin. The cells were centrifuged and the cell pellet resuspended in FACS fixative (PBS containing 2% glucose, 0.02% azide and 2.5% formal-dehyde). The expression of VCAM-1 was measured as fluorescence intensity using a Coulter Epics Profile II
flow cytometer (Coulter, USA.). A total of 10 000 cells was counted for each sample. Controls included absence of primary antibody and substitution of an isotype-matched, nonrelevant antibody.
2.8. Labeling of HDL
Unmodified HDL3 and the HDL3 that were
en-riched with SAA during incubation in vitro were la-belled with Na125I using the iodo-bead iodination
method according to the manufacturers’ instructions. Free 125
I was removed by gel filtration through Sep-hadex 6-25M (PD10 column, Pharmacia Biotech); samples were then further dialysed against 0.05M Tris – HCl, pH 7.4. Specific activities of metabolised HDL ranged from 70 – 110 cpm/ng protein.
2.9. Binding studies
The binding studies were conducted with bovine aortic endothelial cells (BAECs). The BAECs were prepared from primary culture and characterised as previously described [19]. Cells were grown in 75cm2
flasks in RPMI 1640 modified medium (ICN) supple-mented with 10% foetal calf serum. 4 mM L-glu-tamine and 1% penicillin/streptomycin antibiotic solution (ICN). Cells were incubated at 37°C in a 5% CO2, 95% air, water-jacketed incubator.
Cells were seeded into 12 well micro plates at (1 – 2)×105 cells per well and when 90% confluent, the
3ml of medium (RPMI with 10% FBS) was replaced with 1ml serum free RPMI and incubated for 2 – 3 h to remove adherent lipoproteins. After washing the cells once with serum free RPMI, binding studies were commenced by the addition of 1 ml fresh RPMI (serum free) containing varying amounts of 125
I-la-belled HDL preparations or unlaI-la-belled HDL (see Sec-tion 3) and incubated for 3 h at 37°C. To determine bound HDL, the medium was removed, cells were washed four times with 0.1% BSA in PBS and twice with PBS. Surface bound lipoproteins were removed by adding 1 ml 0.05% (w/v) trypsin/EDTA (ICN) to each well and the cells were quickly detached by gen-tle scraping and pelleted by centrifugation at 1000×g for 5 min in a Heraeus Christ Minifuge GL. Surface bound lipoproteins were collected in the supernatant (trypsin releasable) and internalised lipoproteins were released from the cell pellet (trypsin resistant HDL) using 1 ml 0.1 M NaOH to dissolve cell material.
2.10. Statistical analyses
Table 1
Composition and particle size of the different preparations of native HDL3
Percentage by mass
Phospholipid Unesterified cholesterol Cholesteryl Triglyceride
Total protein Particle diameter
3were isolated from each of 19 patients on the day before they underwent coronary artery bypass graft (CABG) surgery. bAnother sample was taken from each subject 3 days postoperatively for isolation of a second preparation of HDL
3. cSamples of HDL
3 were also collected on two separate occasions (4 days apart) from normal control subjects. All values represent the
means9SEM.
* pB0.05 for the difference between the two samples.
3. Results
3.1. Inhibition of HUVEC VCAM-1 expression by HDL: effect of the in6i6o enrichment of HDL3 with SAA
Preparations of HDL3were isolated on two separate
occasions from the plasma of each of 19 subjects: the first sample was collected 1 day before and the second 3 days after undergoing coronary artery bypass graft surgery. The total protein content of the HDL3 was
unaffected by the surgery (Table 1). The composition of the protein, however, was considerably changed (Table 2). Whereas the preoperative HDL3 sample contained
no SAA, in the postoperative sample SAA had replaced a proportion of both the apoAI and apoAII and ac-counted for an average of 42% of the HDL3 protein
(Table 2). The postoperative HDL3 were also larger,
were enriched in unesterified cholesterol, triglyceride and phospholipids and were depleted in cholesteryl esters when compared with the preoperative samples (Table 1). As a control, samples of HDL3 were also
isolated on two separate occasions 4 days apart from each of six healthy volunteers who did not undergo surgery. In contrast to the surgical patients, the compo-sition and size of the HDL3 isolated from the normal
subjects did not change over the 4 days (Tables 1 and 2).
The preoperative HDL3 and postoperative
SAA-en-riched HDL3were compared in terms of their ability to
inhibit the TNF-a-induced expression of VCAM-1 in HUVECs. Both samples inhibited VCAM-1 expression in a concentration dependent manner (Fig. 1). Further-more, when added at equal cholesterol concentrations, the extent of inhibition of VCAM-1 expression was identical for the two samples. The samples of HDL3
obtained from control subjects also inhibited VCAM-1
expression in a concentration dependent manner, with no significant difference in the degree of inhibition between the first and second samples of HDL3(Fig. 2).
3.2. Inhibition of HUVEC VCAM-1 expression by HDL: effect of enriching HDL3 with SAA in 6itro
To assess the effect of having an even greater propor-tion of the HDL3protein replaced by SAA, samples of
HDL3were incubated in vitro with purified SAA. This
resulted in SAA replacing almost all of the apoAI and about 40% of the apoAII; SAA accounted for an average of 81.5% of the protein in these in vitro modified HDL3(Table 3). The in vitro incorporation of
SAA into HDL3was associated with an increase in the
particle size from 8.8 to 10.2 nm (Table 3), comparable
Table 2
Protein composition of the different preparations of native HDL3
Percentage by mass
3 were isolated from each of 19 patients on the
day before they underwent coronary artery bypass graft (CABG) surgery.
bAnother sample was taken from each subject 3 days
postopera-tively for isolation of a second preparation of HDL3. cSamples of HDL
3were also collected on two separate occasions
(4 days apart) from normal control subjects. All values ignore any contribution made by other minor HDL apolipoproteins. Values represent means9SEM.
Fig. 1. The Effect of preoperative HDL3and post-op SAA-HDL3on
VCAM-1 expression in HUVECs. HUVECs were preincubated for 1 h with preoperative HDL3 and postoperative SAA-enriched HDL3
before being activated with TNF-a(100 U/ml) and incubated for a further 5 h. The expression of VCAM-1 was quantitated by flow cytometry. Values are presented as a percentage of that in a sample without HDL. The preoperative HDL3(") and postoperative
SAA-enriched HDL3(2) were added to the HUVECs according to total
cholesterol concentration. The results are expressed as the mean and standard error of the mean,n=19.
Table 3
Protein composition of in vitro modified native HDL3a
Percentage by mass
ApoAI ApoAII SAA
Unmodified 72.991.6 27.191.6 0 HDL3
81.591.7 17.291.3
1.490.4 SAA-enriched
HDL3
aSamples of HDL
3 were isolated from each of three normal
subjects and incubated in vitro with lipid-free SAA which displaced both apoAI and apoAII from the particles. Values represent the means9SEM.
manner (Fig. 3). As in the studies using HDL3enriched
with SAA in vivo, the in vitro SAA-enriched HDL3
were identical to the unmodified HDL3 in terms of the
magnitude of the inhibition.
In order to determine whether there would be an effect of SAA if there was a longer preicubation of the HUVECs with the HDL3, the duration of the
preincu-bation HUVECs was increased from 1 to 17 h. The longer preincubation was associated with a greater de-gree of VCAM-1 inhibition for a given cholesterol concentration of each preparation (Fig. 4). However, as with the shorter preincubations, there was no difference between the two preparations in terms of the degree of the inhibition.
to the increase in HDL3 size associated with SAA
enrichment in vivo.
The unmodified HDL3and the in vitro SAA-enriched
HDL3 both inhibited the TNF-a-induced expression of
VCAM-1 in HUVECs in a concentration dependent
Fig. 3. The effect of enriching HDL3 with SAA during in vitro
incubation on cytokine-induced VCAM-1 expression in HUVECs. SAA-enriched HDL3was prepared in vitro by displacing apoAI with
lipid-free SAA as descibed in Methods. HUVECs were preincubated for 1 h with either the unmodified HDL3or the SAA-enriched HDL3
before being activated with TNF-a(100 U/ml) and incubated for a further 5 h. The expression of VCAM-1 was quantitated by flow cytometry. Values are presented as a percentage of that in a sample without HDL. The unmodified HDL3( ) and in vitro SAA-HDL3
() were added to the HUVECs according to total cholesterol concentration. The results are presented as the mean and standard error of the mean from three experiments, where each was performed in duplicate.
Fig. 2. The effect of samples of HDL3 collected 4 days apart from
normal subjects on VCAM-1 expression in HUVECs. HUVECs were preincubated for 1 h with pre HDL3and post HDL3 before being
activated with TNF-a(100 U/ml) and incubated for a further 5 h. The expression of VCAM-1 was quantitated by flow cytometry. Values are presented as a percentage of that in a sample without HDL. The first sample of HDL3(") and the second sample of HDL3
Fig. 4. The effect of longer preincubation with unmodified and SAA-enrched HDL3 on the inhibition of VCAM-1 expression in
HUVECs. SAA-enriched HDL3was produced in vitro as described in
the legend to Fig. 3. HUVECs were preincubated for 17 h with either unmodified HDL3 or with the SAA-enriched HDL3 before being
activated with TNF-a(100 U/ml) and incubated for a further 5 h. The expression of VCAM-1 was quantitated by flow cytometry. Values are presented as a percentage of that in a sample without HDL. The unmodified HDL3( ) and SAA-enriched HDL3() were
added to the HUVECs according to total cholesterol concentration. The results are presented as the mean values obtained in a single experiment, which was performed in duplicate.
3.3. Inhibition of HUVEC VCAM-1 expression by HDL: effects of discoidal rHDL containing apoAI or SAA
As a final investigation of whether SAA influenced the inhibitory activity of HDL, studies were conducted with discoidal rHDL which contained PLPC as the sole lipid component and SAA or apoAI as the sole protein component. The AI-rHDL were 26% protein and 74% phospholipid and, as reported previously [13], com-prised a homogeneous population of particles with of diameter 9.4 nm as determined by non-denaturing gra-dient gel electrophoresis (results not shown). The SAA-rHDL were 28% protein and 72% phospholipid but, unlike the AI-rHDL, were heterogeneous in size, con-sisting of three distinct subpopulations of particles with diameters of 19.7, 9.3 and 7.6 nm (results not shown). The preparations of both AI-rHDL and SAA-rHDL inhibited the TNF-a-induced expression of VCAM-1 in HUVECs in a concentration-dependant fashion. When equated for phospholipid composition, if anything the inhibitory activity of the SAA-rHDL was superior to that of the AI-rHDL (Fig. 5). However, we have demonstrated previously that the inhibition is a func-tion of the HDL particle molarity. Our inability to determine the number of SAA molecules per particle of SAA-rHDL made it impossible to equate them with the AI-rHDL in terms of particle molarity. Thus, all that can be concluded is that both preparations were effec-tive inhibitors but that it cannot be determined whether either is superior to the other.
We also investigated the effects of lipid-free SAA on VCAM-1 expression. As we have observed previously with lipid-free apoAI, the SAA had no effect: it neither stimulated nor inhibited the TNF-a-induced expression of VCAM-1 in HUVECs (result not shown).
3.4. Binding of unmodified and SAA-enriched HDL3 to endothelial cells
Having found that SAA did not influence the ability of HDL3 to inhibit the cytokine-induced expression of
VCAM-1 in HUVECs, further studies were conducted to determine whether other aspects of the interaction of HDL with endothelial cells may be influenced by the presence of SAA in the HDL. Specifically, the effect of SAA on the binding of native HDL3 to BAECs was
assessed.
In competition displacement experiments, both unla-belled HDL3 and SAA-enriched HDL3 (prepared by
incubating native HDL3 in vitro with purified SAA as
described above) competed almost identically for bind-ing and internalisation of125I-labelled HDL
3, with 43 –
50% of the label being displaced by the addition of 100
mg of the competitor (Fig. 6A). Similarly, in cross-com-petition experiments, unlabelled HDL3 and
SAA-en-riched HDL3 competed almost equally for binding of
125I-labelled SAA-enriched HDL
3 to cultured BAECS.
SAA-enriched HDL3 were less effective in reducing
internalisation of 125I-SAA-enriched HDL
3, suggesting
that factors other than specific binding sites may con-tribute to uptake of HDL3.
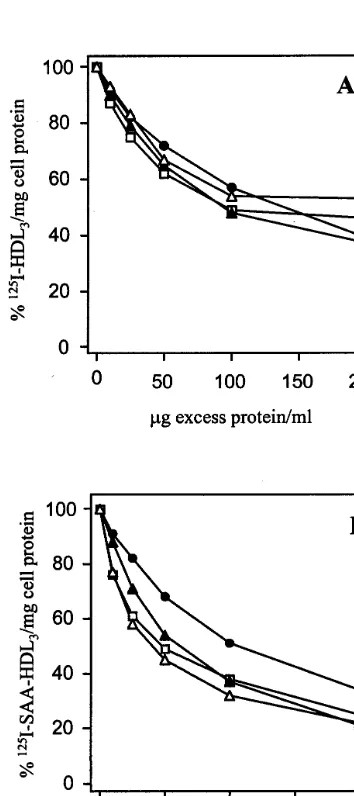
Concentration dependence studies were also carried out to investigate further possible differences in cell interaction between the two lipoprotein preparations. Specific binding and internalisation is shown in Fig. 7A and B. For both lipoprotein preparations, saturation
Fig. 7. Concentration dependent binding and internalisation of125
I-HDL3or125I-SAA-HDL3to BAEC. Data shown is specific binding
which was determined by subtracting non-specific binding from total binding as described in the Methods section. Panel A shows specific binding ( ) and internalisation () of125I-HDL
3determined in the
presence of unlabeled HDL3. Panel B shows specific binding ( ) and
internalisation () of 125I-SAA-HDL
3, with non-specific binding
having been determined in the presence of unlabeled HDL3;
corre-sponding parameters {binding (), internalisation ()} were de-termioned in incubations performed in the presence of excess SAA-HDL3.
Fig. 6. Competitive displacement of 125I-HDL
3 (A) or 125
I-SAA-HDL3(B) by unlabeled lipoprotein. BAEC cells were incubated with
3 ng (protein) of125I-labeled HDL
3and increasing concentrations of
either unlabeled HDL3or SAA-HDL3. Surface bound or internalised
labeled lipoprotein was determined as trypsin releasable or trypsin resistant radioactivity, respectively, as described in the Methods section. Panel A shows the surface bound 125I-HDL
3 displace by
either excess HDL3 () or SAA-HDL3 () and the amount of 125I-HDL
3 internalised after incubating with increasing
concentra-tions of HDL3 ( ) or SAA-HDL3 (). Panel B shows the
corre-sponding displacement of 125I-SAA-HDL
3 bound (,) or
internalised ( ,) in the presence of HDL3 (,) or SAA-HDL3
(, ), respectively.
was approached at 100 mg protein. Binding parameters were calculated from Scatchard plots. Kds for specific binding of SAA-enriched HDL3 were 1.18×10−
9
and 0.3×10−9
M for native HDL3. Capacity of binding
(Bmax) was correspondingly higher for SAA-enriched
HDL3, being 648 ng/mg protein compared with 198
ng/mg protein for native HDL3. Although the cells
appeared to show a higher capacity of binding for SAA-enriched HDL3 than for native HDL3, the
num-bers of binding sites for SAA-enriched HDL3could not
4. Discussion
SAA is an acute phase reactant that is synthesised in the liver and is released into the plasma in a variety of inflammatory conditions [20]. It is also released into plasma in response to major trauma or, as in the present study, major surgery. Within plasma most of the SAA is transported as a component of HDL [5 – 7] for which it has a high affinity. SAA displaces apoA1 and, to a lesser extent, also apoAII from HDL in a process that leaves the HDL increased in both particle size and density [8,9]. Thus, a typical SAA-enriched HDL circulating in plasma during times of inflamma-tion has the size of HDL2 but the density of HDL3
[8,9]. As in the present study, this association of SAA with HDL is also associated with a depletion of HDL cholesteryl esters and an enrichment in HDL triglyce-ride and phospholipids [5 – 9].
SAA has been reported to be potentially pro-athero-genic in both its lipid-free form and when associated with HDL3. For example, in its lipid-free form,
recom-binant human SAA is chemoattractant and induces the migration, adhesion and tissue infiltration of mono-cytes, polymorphonuclear leukocytes and T lymphocytes [11,21]. Recombinant human SAA has also been shown to increase the expression of the cellular adhesion molecules Mac-1 and leukocyte cell adhesion molecule-1 [12]. When associated with HDL, SAA enhances HDL binding to macrophages [22]. SAA-enriched HDL also bind to human monocytic THP-1 cells more avidly than normal HDL [23]. The presence of SAA in HDL inhibits the LCAT-mediated esterification of HDL cholesterol [10] and decreases the ability of HDL to protect LDL against oxidation [11]. Given this list of potentially pro-inflammatory and pro-atherogenic effects of SAA, it was surprising in the present studies to find that the incorporation of SAA into HDL did not compromise the ability of the HDL to inhibit the cytokine-induced endothelial cell adhesion molecule expression. It was also unexpected to find that the presence of SAA in HDL had no significant effect on the binding of the lipoproteins to endothelial cells. In the present study, SAA was incorporated into HDL by three different methods. When it was incorpo-rated in vivo following surgery it accounted for about 40% of the HDL3 protein. When incorporated in vitro
by displacing all of the apoAI and much of the apoAII it accounted for about 80% of the HDL protein, while in the SAA-rHDL it was the only protein constituent of the particle. The results with each of these preparations were consistent with a conclusion that SAA had no effect on the ability of HDL either to inhibit the expression of VCAM-1 in endothelial cells or to influ-ence binding of the lipoproteins to cell surface proteins on BAECs. Whether or not the ability of HDL to inhibit VCAM-1 expression is dependent on the bind-ing of the HDL to cell surface receptors is not known.
The absence of an effect of SAA on the HDL-medi-ated inhibition of endothelial cell VCAM-1 should be viewed in the light of our recent finding that replacing the apoAI in HDL with apoAII does not compromise the inhibitory activity of the particles [3,4]. This sug-gests that the apolipoprotein composition of HDL does not regulate the process. The fact that discoidal com-plexes of SAA as the only protein and PLPC as the only lipid were able to mimic native HDL in their ability to inhibit endothelial cell VCAM-1 expression raises the possibility that it may be the phospholipid component of the HDL that is responsible for the VCAM-1 inhibition. Perhaps apoAI, apoAII and SAA are involved only to the extent that they provide a transport vehicle that targets the phospholipid to the site where it can act. Whether or not the process is dependant on the binding of HDL to a cell surface protein cannot be determined from these studies.
We have reported previously that the HDL3 isolated
from different human subjects vary widely in their ability to inhibit the expression of VCAM-1 in cytokine activated HUVECs [3]. We have also shown that the inhibitory activity of HDL3is superior to that of HDL2
[3]. These differences cannot be explained in terms of differing HDL particle size, nor in differences in the composition of apolipoproteins, cholesteryl esters or triglyceride [4]. Such findings lend support to the possi-bility raised by the present study that the phospholipids in HDL may be the key constituents responsible for this anti-inflammatory property of HDL. Such a propo-sition is the subject of studies currently underway in this laboratory.
Acknowledgements
This work was supported by funding from the Na-tional Health and Medical Council of Australia, the National Heart Foundation of Australia and the Royal Adelaide Hospital Research Fund. D.T.A. was sup-ported by a fellowship from the Helpman Bequest. We thank Jenny Drew for technical assistance in the growth of endothelial cells. TNF-a was a generous gift of Genetech, South San Francisco USA. We also thank staff of the delivery wards of the Women’s and Chil-dren’s Hospital and Burnside War Memorial Hospital, Adelaide for the collection of umbilical cords.
References
[1] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801 – 9.
[3] Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arte-rioscler Thromb Vasc Biol 1998;18:1450 – 5.
[4] Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. The ability of reconstituted high density lipoproteins to inhibit cy-tokine-induced expression of vascular endothelial cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res 1999;40:345 – 53.
[5] Benditt EP, Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci USA 1977;74:4025 – 8.
[6] Malmendier CL, Christophe J, Ameryckx JP. Separation and partial characterization of new apolipoproteins from human high density lipoproteins. Clin Chim Acta 1979;99:167 – 76.
[7] Eriksen N, Benditt EP. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci USA 1980;77:6860 – 4. [8] Clifton PM, Mackinnon AM, Barter PJ. Effects of serum
amy-loid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J Lipid Res 1985;26:1389 – 98.
[9] Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid-A containing human high density lipoprotein 3: density, size and apolipo-protein composition. J Biol Chem 1986;261:9644 – 51.
[10] Steinmetz A, Hocke G, Saile R, Puchois P, Fruchart JC. Influ-ence of serum amyloid-A on cholesterol esterification in human plasma. Biochim Biophys Acta 1989;1006:173 – 8.
[11] Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase re-sponse: loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995;96:2758 – 67. [12] Badolato R, Wang JM, Murphy WJ, et al. Serum amyloid A is
a chemoattractant: induction of migration, adhesion, and tissue
infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med 1994;180:203 – 9.
[13] Hatch FT, Lees RS. Practical methods for plasma lipoprotein analysis. Adv Lipid Res 1968;6:1 – 68.
[14] Rye KA. Interaction of the high density lipoprotein conversion factor with recombinant discoidal complexes of egg phos-phatidylcholine, free cholesterol and apolipoprotein A-I. J Lipid Res 1989;30:335 – 46.
[15] Hopkins GJ, Barter PJ. Apolipoprotein A-I inhibits transforma-tions of high density lipoprotein subpopulatransforma-tions during incuba-tion of human plasma. Atherosclerosis 1989;75:73 – 82. [16] Osborne JC. Delipidation of plasma lipoproteins. Meth Enzymol
1986;128A:213 – 22.
[17] Weisweiler P. Isolation and quantitation of apolipoproteins A-I and a-II from human high density lipoproteins by fast-protein liquid chromatography. Clin Chim Acta 1987;169:249 – 54. [18] Matz CE, Jonas A. Micellar complexes of human apolipoprotein
AI with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J Biol Chem 1982;257:4535 – 50. [19] Myers, Larkins. Bradykinin-induced changes in
phosphoinosi-tides, inositol phosphate production and intracellular free cal-cium in cultured bovine aortic endothelial cells. Cell Signal 1989;1:335 – 41.
[20] Malle E, Stienmetz A, Raynes JG. Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis 1993;102:131 – 46.
[21] Xu L, Badolato R, Murphy WJ, et al. A novel biologic function of serum amyloid A. Induction of T lymphocyte migration and adhesion. J Immunol 1995;155:1184 – 90.
[22] Kisilevsky R, Subrahmanyan L. Serum amyloid A changes high density lipoprotein’s cellular affinity. A clue to serum amyloid A’s principal function. Lab Invest 1992;66:778 – 85.
[23] Banka CL, Yuan T, de Beer MC, Kindy M, Curtis LK, de Beer FC. Serum amyloid-A (SAA): influence on HDL mediated cellu-lar cholesterol efflux. J Lipid Res 1995;36:1058 – 65.