251 (2000) 161–183
www.elsevier.nl / locate / jembe
Breakdown of phytoplankton pigments in Baltic sediments:
effects of anoxia and loss of deposit-feeding macrofauna
a ,* b ,1 c
Thomas S. Bianchi , Birgitta Johansson , Ragnar Elmgren
a
Institute of Earth and Ecosystem Sciences, Department of Ecology and Evolution Biology,
Tulane University, New Orleans, LA 70118-5698, USA b
Department of Zoology, Stockholm University, SE-106 91 Stockholm, Sweden c
Department of Systems Ecology, Stockholm University, SE-106 91 Stockholm, Sweden Received 16 September 1998; received in revised form 15 December 1999; accepted 28 March 2000
Abstract
We examined the decay of chlorophyll a and the carotenoid fucoxanthin in oxic and anoxic sediment microcosms, with and without the deposit-feeding benthic amphipod Monoporeia affinis, over 57 days at 58C. Deep frozen phytoplankton from the Baltic Sea proper was added to all but a few microcosms. The range of chlorophyll a and fucoxanthin decay rate constants observed in
21
microcosms with phytoplankton addition was 0.04–0.07 day . The fastest pigment decay and build-up of chlorophyll breakdown products after phytoplankton addition were found in oxic treatments with amphipods. No effects of amphipods on pigment breakdown were found in anoxic treatments, or in treatments without phytoplankton addition. Greater losses of chlorophyll a in oxic (96%) than in anoxic (80%) treatments after 57 days indicates that preservation of sedimentary organic matter will be enhanced during periods of anoxia. Due to slow recruitment and recolonization in Baltic sediments, a single anoxic event may cause long-term (years) absence of significant macrobenthos. Anoxic events will thus not only reduce decay of plant pigments, and presumably other organic matter, while they last, but will also have longer-term effects, through elimination of macrofauna, which when present enhance organic matter decomposition. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Plant pigments; Decomposition; Baltic Sea; Carbon cycling; Macrobenthos; Bioturbation;
Monoporeia; Oxygen concentration; Anoxia; Hypoxia
*Corresponding author. Tel.:11-504-862-8000; fax:11-504-862-8706.
E-mail addresses: [email protected] (T.S. Bianchi), [email protected] (R. Elmgren).
1
¨
Present address: Restenas, Pl. 32101, SE-459 93 Ljungskile, Sweden. E-mail: [email protected]. 0022-0981 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
1. Introduction
In recent decades, periods of hypoxia and anoxia have markedly modified the distribution and abundance of macro- and meiobenthos below the primary halocline of the Baltic Sea proper (Elmgren, 1975: meiofauna; Andersin et al., 1978; Rumohr et al., 1996: macrofauna). Increasing oxygen deficiency has been mainly caused by nutrient enrichment of the Baltic (Larsson et al., 1985; Rosenberg et al., 1990). This has resulted in increased phytoplankton production (Elmgren, 1989; Stigebrandt, 1991), and a consequent increase in sedimentation of organic matter to sediments (Jonsson and Carman, 1994). Anoxic conditions in bottom waters and sediments in deeper parts of the Baltic proper have resulted in extensive areas of laminated surface sediments (Jonsson et al., 1990). Little is known about the long-term effect of loss of macrofauna on decomposition of sedimenting organic matter, both in the Baltic (Cederwall and Elmgren, 1990; Rosenberg et al., 1990) and in general (Diaz and Rosenberg, 1995).
The direct effect of bioturbation by macrofauna on decomposition of organic matter is well documented in coastal ecosystems (Rhoads, 1974; Berner, 1980; Aller, 1982; Rice, 1986; Lopez and Levinton, 1987; Andersen and Kristensen, 1992; Webb and Montagna, 1993; Alongi, 1995). Bioturbation generally stimulates diagenesis of organic matter by increasing oxygen availability and through mechanical fragmentation by feeding activities (Berner, 1980; Lopez and Levinton, 1987; Bianchi et al., 1989). During prolonged anoxic events, most macrofauna are eliminated and anaerobic metabolism becomes the dominant pathway for breakdown of organic matter. Many studies have shown that anaerobic bacteria are generally less efficient than aerobic bacteria in decomposing organic matter (Fenchel and Finlay, 1995). Kemp (1990) and Lee (1992) proposed that by killing bacterial grazers, lack of oxygen and sulfide toxicity can depress the activity of the sedimentary microbial loop and result in the sequestering of bacteria and bacterial products within anoxic sediments. Thus, periods of anoxia may enhance the preservation of organic matter due to the destruction of macrofauna, a less efficient pathway of anaerobic metabolism, and the disruption of the microbial loop (via changes in bacterial grazers).
lysis, and of metazoan grazing activities (Sanger and Gorham, 1970; Shuman and Lorenzen, 1975; Welschmeyer and Lorenzen, 1985; Hawkins et al., 1986; Bianchi et al., 1988, 1991). The phaeopigment, phaeophorbide, has been shown to be well correlated with the grazing activities of metazoans (Shuman and Lorenzen, 1975; Bianchi et al., 1991). In controlled laboratory feeding experiments, the highest concentrations of total phaeophorbides usually reflect the most intense metazoan grazing activity — which in turn depends upon resource ‘quality’ or availability (Millie et al., 1993).
In this study, we used laboratory microcosms containing sediments collected from the Baltic proper to examine the effects of anoxia and macrobenthos on the degradation of plant pigments in phytodetritus. The macrobenthic species used was the amphipod
¨
Monoporeia affinis (Lindstrom), a dominant deposit-feeder in most of the Baltic Sea ˚
(Segerstrale, 1950, Rumohr et al., 1996). It lives in soft sediments, mainly below 15–20
˚ ¨ ¨
m depth (Segerstrale, 1950; Jarvekulg, 1973), tolerates salinities from fresh water up to
˚
13–15 (Segerstrale, 1950, 1959), feeds on surface sediment (Lopez and Elmgren, 1989), and is an effective bioturbator. Most individuals are found in the upper five centimeters of sediment (Hill and Elmgren, 1987). Recent studies have shown that pigments and most other compounds are more stable under anoxic than oxic conditions (Sun et al., 1991; Sun and Wakeham, 1994; Harvey et al., 1995). Little attention has, however, been given the role of macrofauna and anoxic events in preservation of organic matter, as traced by pigment biomarkers. Our experiments were designed to test for the double effect anoxic events may have on pigment decay. First directly, during anoxia / hypoxia, by restricting breakdown to anaerobic pathways, and then indirectly, even after the return of oxygen to bottom waters, by eliminating bioturbation and grazing on bacteria by macrobenthos.
2. Methods and materials
2.1. Sources of experimental animals, sediments, and phytoplankton
Sediments and subadult amphipods, Monoporeia affinis, of the age class hatched in early spring of the previous year, were collected with a benthic dredge at 30–40 m near
¨
the Asko Laboratory, northern Baltic proper (588499N, 178389E), in spring 1994. Sediments were sieved through a 0.5-mm net to remove all macrofauna. In mid-April, spring-bloom phytoplankton, dominated by diatoms, was collected with a 45-mm plankton net, and deep-frozen until used.
2.2. Laboratory microcosms
The experiment was conducted in summer, in recirculating water tanks at 58C and in total darkness. Artificial seawater was made from aquarium salt (‘hw-Marinemix1 Bio-elements’, Wiegant Gmbh, Germany), to a salinity of 6.5‰, near that at which amphipods and phytoplankton were collected. One experimental tank (2.530.430.2 m, 200 l) each was used for four oxygen concentrations: 0.4 (called anoxia in the
21
air-21
bubbled, called oxic). The water was recirculated at 440 l h by an Eheim pump with an attached bio-filter. An oxygen meter (AOWL2 / KOB / 421, Processtyrning AB, Sweden), regularly calibrated against the Winkler method, monitored and stored oxygen concentrations on a data logger. Oxygen concentration was regulated by a flow of nitrogen gas, which was automatically switched off by a magnetic valve controlled by the oxygen meter, once the desired oxygen level was reached. The water surface was covered with a sheet of Plexiglas to obtain low, stable oxygen concentrations. Ammonium concentrations were determined every 2 weeks in all treatments, using a modification of the method of Solorzano (1969).
Each tank housed 14 boxes (18318 cm), with holes on the sides covered with 45-mm net and filled with 3 cm of sieved sediment (organic content 6%) (Fig. 1). Twelve boxes received phytoplankton additions corresponding to 0.80 g dry weight per box or about
22
10 g C m (assuming 40% of dry weight is carbon, based on normal values for spring ¨
bloom material in the area, A. Sjosten, personal communication). This material settled quickly on the sediment surface, but was not mixed down into the sediment when added. Six boxes were left without animals, and six received 40 amphipods each, corresponding
22
to 1250 m , which is within the normal range of field densities (Ankar and Elmgren, 1976). Two boxes were left without phytoplankton addition, one without and the other with 40 amphipods. The experiment lasted 57 days. Soda-straw (5 mm I.D.) sediment samples were taken weekly from the anoxic and high oxygen treatments (0.4 and 11.6
21 21
mg O l2 ). Low oxic treatments (1.7 and 3.0 mg O l2 ) were sampled only at the end of the experiment. Samples were stored at 2808C before analysis. There was no noticeable loss of sediment from boxes in the tank during the experiment. On ending the
experiment, amphipods were sieved out, transferred to well-oxygenated water and living and dead individuals counted after 30 min. The carbon input to our experimental treatments was similar to that from a coastal spring phytoplankton bloom in the Baltic
22
proper. Larsson et al. (1986) reported a sedimentation of 4.9 g C m during a spring
22 ¨
bloom event near the Asko Laboratory, while Graf et al. (1982) recorded 11.5 g C m over a 4-week spring bloom period in Kiel Bight, southwestern Baltic Sea.
2.3. Pigment analyses
Plant pigments in sediment and spring-bloom material were extracted using 100% acetone according to Bianchi et al. (1995), followed by reversed-phase high-per-formance liquid chromatography (HPLC) after Wright et al. (1991), as modified by Bianchi et al. (1995, 1996). A Waters model 996 photodiode array detector set at 438 nm for absorbance, and a Shimadzu fluorescence detector with excitation at 406 nm and emission at 660 nm were used. The injector was connected to a reversed-phase C18 Alltech absorbosphere column (5 mm particle size; 25034.6 mm I.D.) via a guard
21
column. After injection (100ml), a gradient program (1 ml min ) began isocratically with mobile phase A (80:20 v / v methanol / 0.5 M ammonium acetate, aq.; pH 7.2 v / v), ramped to 100% mobile phase B in 8 min (90:10 v / v acetonitrile / HPLC grade water), and then changed to 20% B and 80% mobile phase C (100% ethyl acetate) in 24 min. A return to 100% B in 10 min followed, with a final ramping to 100% A in 4 min.
High purity HPLC standards of chlorophylls a and b were obtained from Sigma Co. Hoffman LaRoche kindly provided a fucoxanthin standard. Phaeophytins a and b were produced by acidification (30 ml of 2 N HCl added to 1 ml of pigment solution) followed by a clean-up separation using Alltech prep-cartridges to remove the acid. Chlorophyllide a was prepared by activation of chlorophyllase in a diatom culture (Thalassiosira sp.) by overnight incubation in the dark with 50% aqueous acetone. Phaeophorbide a was then prepared by acidification (as above) of this chlorophyllide solution. Although a number of phaeophorbide a-like components have been reported in the literature (Mantoura and Llewellyn, 1983; Bianchi et al., 1988), phaeophorbide a produced like this appears as a single peak. Replicate (n53) and standard precision for plant pigments ranged from 3 to 6%.
2.4. Statistics
Differences in chlorophyll a breakdown rates could not be analyzed with linear regression, since variances differed too much between treatments. Instead, the time at which 70% of the initial pigment concentration had been lost was calculated by linear interpolation, and differences between treatments were tested using the Kruskal–Wallis test. The breakdown of fucoxanthin and the build-up of breakdown products of chlorophylls were mostly analyzed using simple linear regression, logarithmically transformed when needed to homogenize variances. Resulting slopes were tested for significant differences, using Bonferroni’s correction for multiple comparisons where appropriate.
low oxic treatments) were compared using analysis of variance (ANOVA) followed by the Student–Newman–Keuls (SNK) multiple comparison test.. Homogeneity of vari-ances was tested with Cochran’s C-test. Previous experiments have not shown differences between tanks with the same oxygen concentration, and therefore only one tank was used for each oxygen concentration.
3. Results
3.1. Amphipod survival
No amphipods survived in the two lowest oxygen concentrations in treatments with phytoplankton addition and survival was low also at the highest oxygen concentration (Fig. 2). The number of amphipods alive at the end of the experiment was significantly
21 21
greater at 11.6 mg O l2 than at 3.0 mg O l2 (ANOVA, P,0.001, SNK). Also,
21
significantly more dead amphipods were found at 11.6 mg O l2 (ANOVA, P,0.001, SNK). Ammonium concentration showed no significant difference among oxygen
21
treatments (data not shown), and was always below 0.9 mg NH -N l3 , far below the
21
LD50 values (50–150 mg NH -N l3 ) typically reported for amphipods (Kohn et al., 1994).
3.2. Effects of macrobenthos and anoxia on pigment decay
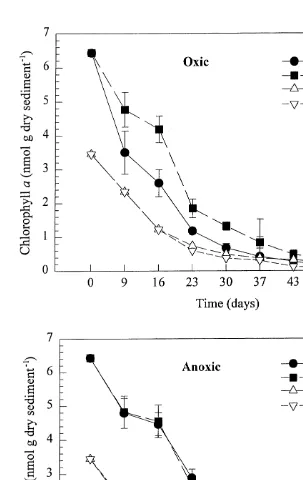
Pigment concentrations for day 0 should be the same in all treatments with additions, and were calculated from analyses of the added spring bloom material and of the bulk sediment without additions (see figures), taking respective weights into account. This means that no value for between-replicate variability is available for day 0.
Fig. 2. Percent living and dead amphipods at the end of the experiment in the four oxygen treatments with phytoplankton addition (%). Animals recovered dead likely died within a week of the end of the 57-day experiment. Error bars denote standard deviation, based on six replicates.
signifi-21
Table 1
Two-factorial ANOVA comparing chlorophyll a and total phaeophoride concentrations (day 57) at oxygen 21
concentrations 0.4, 1.7, 3.0, and 11.6 mg O l2 , with or without amphipods (ns5not significant)
Effect Chlorophyll a Phaeophorbides
df F value P value F value P value
Oxygen 3 221.9 ,0.0001 62.2 ,0.0001
With / without amphipods 1 3.0 ns 34.2 ,0.0001
Interaction 3 1.8 ns 2.9 ,0.046
cantly different), but because it acted for a shorter time, it still gave lower final concentrations.
The concentration of phaeophytin a increased more in the oxic treatment with amphipods than in the oxic treatment without amphipods and in both anoxic treatments (Fig. 5). Excluding the unreplicated day 0 value, the phaeophytin a concentration in the oxic treatment with amphipods seems to have increased in a step fashion between days 23 and 30, with little change before or after. The difference between those two periods is highly significant (two-sample t-test, P50.0001). The other treatments are obviously different, with more of a linear increase from day 9 to day 57 (except that day 57 tends to have lower concentrations than day 50). The slope estimates, oxic without amphipods 0.017160.0009, anoxic with amphipods 0.014760.0009, anoxic without amphipods 0.012660.0014, are not significantly different among treatments.
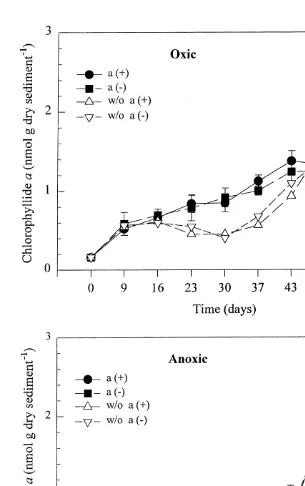
Total phaeophorbide concentrations increased over time, particularly after day 30. Excluding the unreplicated day 0 value, a slope for days 9–57 can be calculated for all treatments with added organic matter, except oxic with amphipods, where values after day 30 were clearly higher than in the other three groups (Fig. 6). The slope is 0.034760.0030 for the oxic treatment without amphipods, which is significantly greater than in both anoxic treatments: with amphipods 0.014060.013, without 0.013960.0017 (no significant difference).
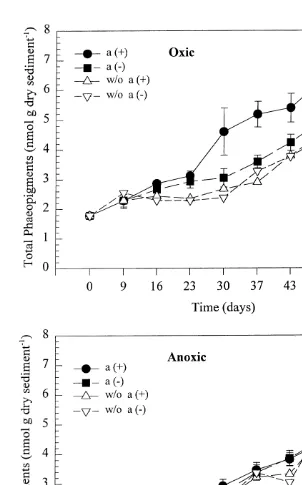
The total phaeopigment concentrations (Fig. 7) showed a development similar to that of phaeophytin a, with both oxic treatments diverging very clearly from the anoxic ones from day 30, and oxic with amphipods clearly higher than without. For the anoxic treatments, a regression for days 9–57 gives identical slopes with amphipods (0.037160.0022) and without (0.037260.0042). Total phaeopigments in oxic and anoxic treatments without added organic matter appeared not to differ between treatments with and without amphipods (Fig. 7). On day 57, total phaeopigment concentrations could be compared for four different oxygen concentrations, with and without amphipods, using ANOVA (Table 1, Fig. 9). For total phaeopigment con-centrations, a significant interaction term was found. The SNK showed significantly
21
lower phaeopigment concentrations at levels of 0.4 and 1.7 mg O l2 for treatments
21
with and without amphipods and at 3 mg O2 l without amphipods. Intermediate concentrations of phaeopigments were found in treatments with amphipods at 3 mg O2
21 21
l , and at 11.6 mg O2 l without amphipods. The highest phaeopigment
con-21
centrations were found at 11.6 mg O l2 with amphipods (Fig. 9).
21
21
21
21
over time (Fig. 8), which can be described by linear regression of logarithmic concentration values. Taking into account that day 0 is unreplicated, the following slopes
2
and degrees of explanation (r ) were estimated:
2
Oxic with amphipods: slope 5 20.0561, r 50.968
2
Oxic without amphipods: slope 5 20.0497, r 50.975
2
Anoxic with amphipods: slope 5 20.0455, r 50.927
2
Anoxic without amphipods: slope 5 20.0446, r 50.973
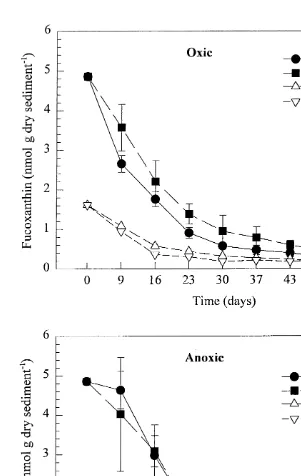
Using Bonferroni’s method, the calculated slopes must differ by at least 0.004 to be significantly different, and all but the two anoxic treatments are thus significantly different. Fucoxanthin concentration thus decreased most rapidly in the oxic treatment with amphipods, slower in the oxic treatment without amphipods and most slowly in the two anoxic treatments (Fig. 8). By day 57, total loss of fucoxanthin was over 90% in all treatments with added organic matter. As for chlorophyll a, more fucoxanthin had been lost in oxic than in anoxic treatments without added spring-bloom material, but the presence on animals seemed to make little difference in any of theses treatments (Fig. 8).
3.3. Pigment decay constants
Apparent first-order decay rate constants were calculated from the relationship:
2kt Gt5G ei
21
where Gt5concentration of pigment at time t (nmol g dry sed ), Gi5initial
con-21
centration of pigment (nmol g dry sed ), t5time (days), and k5decay rate constant
21
(day ). Changes in chlorophyll a and fucoxanthin were fitted using least squares non-linear regression of concentration versus time to yield the rate constant (k, given in Table 2) of this simple first-order equation.
4. Discussion
4.1. Pigment decay constants
The range of chlorophyll a decay rate constants observed in both anoxic and oxic
21
sediments, with and without amphipods (0.04–0.07 day ), was within the range observed for pigments in previous studies (Leavitt and Carpenter, 1990; Bianchi and Findlay, 1991; Sun et al., 1991, 1993a,b; Sun and Wakeham, 1994). Decay rate constants in oxic treatments were faster than in anoxic treatments, which agrees with the general paradigm of slower decay kinetics under anoxic conditions (Fenchel and Finlay, 1995). Fucoxanthin decay rate constants in this study were also similar to chlorophyll a, as were decay constants found in decomposing freshwater epiphytic algae (Bianchi and Findlay, 1991). Decay rate constants under anoxic and oxic conditions found for both
21 21
21
Table 2
21
First-order decay constants (k) (day ) of chlorophylls and carotenoids from phytoplankton detritus in eight a
Decay constants of pigments were calculated for all treatments during the time periods of days 9–57. (6S.D.) Indicates 95% confidence limits.
1), are within range of that found for lipids, proteins, and carbohydrates in phytodetritus decay experiments (Harvey et al., 1995; Canuel and Martens, 1996). This consistency is somewhat surprising, since past studies have shown oxygen-poor, hydrolysis-resistant molecules such as lignins, lipids, and plant pigments to be more sensitive to oxygen effects than more labile substrates such as carbohydrates and amino acids (Hedges and Keil, 1995).
Pigments ‘bound’ to structural compounds such as lignins or surface waxes in higher plants have slower decay rate constants than similar pigments from non-vascular sources (Webster and Benfield, 1986; Bianchi and Findlay, 1991). Thus, despite significant differences in the molecular weight constituents of organic matter in higher plants versus algae, many of these compounds (i.e. lipids, plant pigments) appear to decay at comparable rates under similar redox conditions. These results indicate that much of the decomposition of these compounds is occurring in the ‘free’ state, similar to chlorophyll
a under anoxic conditions (Sun et al., 1991).
4.2. Effects of macrobenthos on pigment decay
protected from oxygen, unlike phytoplankton additions, which were added on top of the sediment. That the added phytoplankton had been frozen may also have made its pigment easier to break down.
Based on changes in chlorophyll a concentrations with time, it appears that Baltic macrofauna (amphipods) can significantly increase the decomposition rate of phyto-plankton pigments in sediments. The highest decay rate for chlorophyll a was found in the oxic treatment with amphipods. Similarly, the highest phaeophorbides and lowest chlorophyll a concentrations were found at the end of the experiment in this treatment (Fig. 9). These results agree with previous studies, which showed that macrofauna enhance the decomposition rate of bulk organic matter (Rhoads, 1974; Aller, 1982; Rice, 1986). More specifically, it has been shown that metazoans are primarily responsible for the conversion of chlorophylls to phaeophorbides (Shuman and Lorenzen, 1975; Welschmeyer and Lorenzen, 1985; Bianchi et al., 1988). Recent work also has shown that pyrolised phaeopigments such as pyrophaeophorbide a and pyrophaeophytin a accounted for more than 70% of the breakdown pigments formed during grazing on phytoplankton by copepods (Head and Harris, 1992, 1994, 1996). Pyrophaeophorbide a has the same structure as phaeophorbide a except that the methylated carboxyl group has been eliminated from the isocyclic ring from the C-13 proprionic acid group. Grazing activity of meiofauna may also have contributed to increased production of phaeophor-bides in the oxic treatment without amphipods during the last weeks of the experiment. In another study, it was shown that pigment decay rates in experimental sediments with very few meiofauna were lower than in those with abundant meiofauna (Sun et al., 1993a). We did not measure meiofauna in the experimental sediments. Natural
22
meiofaunal biomass in the sediments of this region average 1.2 g m shell-free dry weight (Ankar and Elmgren, 1976), but previous experimental work has shown that meiofaunal content in sediment treated as in our experiment tends to be about half the field density. A field study in the Baltic showed that 1–2 months were required for meiobenthic populations to increase markedly after the settling out of the spring
´
phytoplankton bloom (Olafsson and Elmgren, 1997).
Estimates of sediment processing by M. affinis support the idea that meiofauna and bacteria played a significant role in the breakdown of pigments. Based on previous experimental measurements of M. affinis ingestion rates (same age- and size class, at 78C, Elmgren et al., 1986; Lopez and Elmgren, 1989), we calculated that the 40
3
amphipods in our treatments could only have ingested 400–800 mm of surface
21
sediment day . Lopez and Elmgren (1989) found that M. affinis feeds primarily on surface sediment. If only the top mm of sediment was eaten it would take amphipods 40–80 days to ingest the top millimeter of sediment in an experimental box. Since there was considerable amphipod mortality, and added algal material presumably was gradually worked down into the sediment, the amphipods could not have ingested all added algal material during the 57-day experiment, and could therefore not alone explain the breakdown of almost all added chlorophyll.
21
Fig. 9. Concentrations (nmol g dry sediment ) of total chlorophyll a (A) and phaeopigments (B) on the last 21
al., 1991). Chlorophyllide and phaeophytin are usually produced by processes other than metazoan grazing (i.e. cell lysis and senescence, microbial decay), and did not increase as dramatically as did total phaeophorbides in oxic treatments. There appeared to be a delay in production of total phaeopigments in relation to loss of chlorophyll a. A similar time lag was observed in Long Island Sound sediments, and was attributed to time-dependent growth patterns of water column phytoplankton followed by later inputs of zooplankton feces to the sediments (Sun and Wakeham, 1994; Sun et al., 1994). While most of the chlorophyll a was lost by day 23 in both oxic and anoxic treatments, the most rapid increase in total phaeopigments in oxic treatments occurred after day 23 or 30 (Figs. 3 and 7). The breakdown of chlorophyll a to colorless products (Klein et al., 1986) is not likely to explain this delay, since the total amount of phaeopigments produced agreed with total chlorophyll a loss during the entire experiment. There are, however, numerous unidentified breakdown products from chlorophyll a digestion via copepod grazers (Head and Harris, 1992). The delayed appearance of phaeopigments may therefore have been due to intermediate pigment decay products, not resolved with the HPLC method used.
4.3. Anoxia and macrofaunal bioturbation effects on organic matter preservation in
the Baltic
Field studies have demonstrated increased preservation of sedimentary organic matter in laminated sediments during anoxic periods in the Baltic (Jonsson et al., 1990). The role of productivity and oxygen in preservation of sedimentary organic matter has been intensely debated in recent years, but no consensus has been reached (Henrichs and Reeburgh, 1987; Canfield, 1989; Fenchel and Finlay, 1995). Different decay rate constants of individual molecular biomarkers and bulk organic matter may explain some dissimilarity between oxic and anoxic treatments. In a recent study by Harvey et al. (1995), decay rates and overall turnover rates of different biochemical fractions in decomposing phytoplankton organic matter were significantly higher in oxic than in anoxic conditions. Harvey et al. (1995) also demonstrated that despite greater losses of organic matter in oxic treatments, bacterial abundance and metabolism were similar under anoxic and oxic conditions, indicating that oxygen increased rates of organic matter decomposition. Conversely, decomposition of individual carbon compounds in a stratified water column was not affected by oxygen in another study (Lee, 1992). Numerous studies have shown that there is little or no effect of oxygen on decay of some easily decomposed substrates derived from phytoplankton, such as sugars and amino acids (see discussion paper by Hedges and Keil, 1995). Hydrolysis-resistant, usually oxygen-poor substrates such as lignin, lipids, and carotenoid pigments are major exceptions (Hedges and Keil, 1995). The greater loss of chlorophyll a in higher oxygen treatments by day 57 shows that oxygen increased pigment decay. Thus, slower rate of pigment decay observed at low oxygen concentrations in our experiment supports the idea that preservation of sedimentary organic matter is likely to be enhanced during periods of anoxia, at least in the short-term (months to years).
may have oxygen levels where macrofauna can survive but recruitment has not yet occurred. Our experiments demonstrate that hypoxic and anoxic events, severe enough to eliminate or greatly reduce macrofauna, may have effects on pigment decay well beyond those caused by elimination of oxic break-down pathways during the period of acute oxygen deficiency. Even after high oxygen levels return to bottom water, the macrobenthos is gone, along with its stimulatory effect on pigment decay. Pigments will be less exposed to decay agents, and anaerobic breakdown pathways will continue to prevail in all but the thinnest surface layer. When oxygen was introduced by switching from anoxic to oxic conditions in experimental sediments, chlorophyll a concentrations remained constant, indicating that oxygen alone is not sufficient for pigment decay (Sun et al., 1993a). In deep Baltic sediments, macrofaunal recolonization is slow, and it may take 1–2 years before significant macrofauna activity returns. In recent years, hypoxia and anoxia have been frequent enough in the Baltic proper to keep very large areas free from macrobenthos, either continuously, or for very long periods (Andersin et al., 1978; Rumohr et al., 1996). Our experiments support the idea that, at least in the short term, this will enhance organic matter preservation in general, and that of plant pigments in particular. The latter observation is important for efforts to use plant pigments in sediment cores for reconstructing paleoecological conditions in the Baltic Sea (Bianchi et al., 2000).
5. Conclusions
On the basis of chlorophyll a and fucoxanthin decay rates, and rates of production of chlorophyll breakdown products, in experimental oxic and anoxic sediments, with and without deposit-feeding amphipods, we conclude that:
1. Plant pigments decay more rapidly in oxic than anoxic sediments 2. The highest decay rates were found in oxic treatments with amphipods
3. Macrofauna appear to enhance (directly and indirectly) the breakdown of phyto-plankton inputs to Baltic sediments
4. Anoxic events reduce plant pigment decay rates while they last, and have a further long-term effect through their elimination of benthic macrofauna, which if present stimulate pigment decay
Acknowledgements
We thank M. Argyrou, A. Bennett, E. Engelhaupt, and C. Lambert for assistance with ¨
graphics and HPLC analyses. B. Soderlund helped collect animals and start experiments.
¨ ¨
Anders Bjorkstrom of the Department of Mathematics, Stockholm University, gave ´
Science Research Council and the Swedish Environment Protection Agency to
RE. [SS]
References
Aller, R.C., 1982. The effect of macrobenthos on chemical properties of marine sediments and overlying water. In: McCall, P.L., Tevesz, M.S. (Eds.), Animal–Sediment Relations. Plenum Press, New York, pp. 1319–1337.
Alongi, D.M., 1995. Decomposition and recycling of organic matter in muds of the Gulf of Papua, northern Coral Sea. Cont. Shelf. Res. 15, 1319–1337.
Andersen, F.O., Kristensen, E., 1992. The importance of benthic macrofauna in decomposition of microalgae in a coastal marine sediment. Limnol. Oceanogr. 37, 1392–1403.
Andersin, A.B., Parkkonen, L., Sandler, H., 1978. The decline of macrofauna in the deeper parts of the Baltic proper and the Gulf of Finland. Kieler Meeresforsch. 4, 23–52.
¨
Ankar, S., Elmgren, R., 1976. The benthic macro- and meiofauna of the Asko-Landsort area (northern Baltic ¨
proper): A stratified random sampling survey. Contrib. Asko. Lab. Univ. Stockholm 11, 1–115. Berner, R.A., 1980. Early Diagenesis: A Theoretical Approach. Princeton University Press, Princeton, NJ. Bianchi, T.S., Findlay, S., 1991. Decomposition of Hudson estuary macrophytes: photosynthetic pigment
transformations and decay constants. Estuaries 14, 65–73.
Bianchi, T.S., Dawson, R., Sawanwong, P., 1988. The effects of macrobenthic deposit-feeding on the degradation of chloropigments in sandy sediments. J. Exp. Mar. Biol. Ecol. 122, 243–255.
Bianchi, T.S., Jones, C.G., Shachak, M., 1989. Positive feedback of consumer population density on resource supply. Trends. Ecol. Evol. 4, 234–238.
Bianchi, T.S., Findlay, S., Fontvieille, D., 1991. Experimental degradation of plant materials in Hudson River sediments. I. Heterotrophic transformations of plant pigments. Biogeochemistry 12, 171–187.
Bianchi, T.S., Lambert, C., Biggs, D.C., 1995. Distribution of chlorophylls and phaeopigments in the northwestern Gulf of Mexico: a comparison between fluorimetric and high-performance liquid chromatog-raphy measurements. Bull. Mar. Sci. 56, 25–32.
Bianchi, T.S., Demetropoulos, A., Hadjichristophorou, M., Argyrou, M., Baskaran, M., Lambert, C., 1996. Plant pigments as biomarkers of organic matter sources in sediments and coastal waters of Cyprus (eastern Mediterranean). Estuar. Coast. Shelf. Sci. 42, 103–115.
´
Bianchi, T.S., Engelhaupt, E., Westman, P., Andren, T., Rolff, C., Elmgren, R., 2000. Cyanobacterial blooms in the Baltic Sea: Natural or human-induced? Limnol. Oceanogr. 45, 716–726.
Bidigare, R.R., Kennicutt, M.C., Brooks, J.M., 1985. Rapid determination of chlorophylls and their degradation products by high-performance liquid chromatography. Limnol. Oceanogr. 30, 432–435. Canfield, D.E., 1989. Sulfate reduction and oxic respiration in marine sediments: implications for organic
carbon preservation in euxinic environments. Deep Sea Res. 36, 121–138.
Canuel, E.A., Martens, C.S., 1996. Reactivity of recently deposited organic matter: degradation of lipid compounds near the sediment–water interface. Geochim. Cosmochim. Acta 60, 1793–1806.
Cederwall, H., Elmgren, R., 1990. Biological effects of eutrophication in the Baltic Sea, particularly the coastal zone. Ambio 119, 109–112.
Diaz, R.L., Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33, 245–303.
Eglington, T.I., Aluwihare, L.I., Bauer, J.E., Druffel, E.R.M., McNichol, A.P., 1996. Gas chromatographic isolation of individual compounds from complex matrices for radiocarbon dating. Anal. Chem. 68, 904–912.
Elmgren, R., 1975. Benthic meiofauna as an indicator of oxygen conditions in the northern Baltic proper. Merentutkimuslait. Julk. 239, 265–271.
Elmgren, R., Hansson, S., Larsson, U., Sundelin, B., Boehm, P.D., 1983. The ‘Tsesis’ oil spill: acute and long-term impact on the benthos. Mar. Biol. 73, 51–65.
Elmgren, R., Ankar, S., Marteleur, B., Ejdung, G., 1986. Adult interference with postlarvae in soft sediment — the Pontoporeia –Macoma example. Ecology 67, 827–836.
Fenchel, T., Finlay, B.J., 1995. Ecology and Evolution in Anoxic Worlds. Oxford University Press, New York. Graf, G., Bengtsson, W., Diesner, U., Schulz, R., Theede, H., 1982. Benthic response to sedimentation of a
spring phytoplankton bloom: process and budget. Mar. Biol. 67, 201–208.
Harvey, H.R., Tuttle, J.H., Bell, J.T., 1995. Kinetics of phytoplankton decay during simulated sedimentation: Changes in biochemical composition and microbial activity under oxic and anoxic conditions. Geochim. Cosmochim. Acta 59, 3367–3377.
Hawkins, A.J.S., Bayne, B.L., Mantoura, R.F.C., Navarro, E., 1986. Chlorophyll degradation and adsorption throughout the digestive system of the blue mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 96, 213–223. Head, E.J.H., Harris, L.R., 1992. Chlorophyll and carotenoid transformation and destruction by Calanus
grazing on diatoms. Mar. Ecol. Prog. Ser. 86, 229–238.
Head, E.J.H., Harris, L.R., 1994. Feeding selectivity by copepods grazing on natural mixtures of phytoplankton determined by HPLC analysis of pigments. Mar. Ecol. Prog. Ser. 110, 75–83.
Head, E.J.H., Harris, L.R., 1996. Chlorophyll destruction by Calanus grazing on phytoplankton: kinetics, effects of ingestion rate and feeding history, and a mechanistic interpretation. Mar. Ecol. Prog. Ser. 135, 223–235.
Hedges, J.I., Keil, R.G., 1995. Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar. Chem. 49, 81–115.
Henrichs, S.M., Reeburgh, W.S., 1987. Anaerobic mineralization of marine sediment organic matter: rates and the role of anaerobic processes in the oceanic carbon economy. J. Geomicrobiol. 5, 191–237.
Hill, C., Elmgren, R., 1987. Vertical distribution in the sediment in the co-occurring benthic amphipods
Pontoporeia affinis and P. femorata. Oikos 49, 221–229.
Jonsson, P., Carman, R., 1994. Changes in deposition of organic matter and nutrients in the Baltic Sea during the twentieth century. Mar. Pollut. Bull. 28, 417–426.
Jonsson, P., Carman, R., Wulff, F., 1990. Laminated sediments in the Baltic — A tool for evaluating nutrient mass balances. Ambio 19, 152–158.
¨ ¨
Jarvekulg, A., 1973. Distribution and ecology of local populations of benthic glacial relicts. Oikos 15, 91–97. Kemp, P.F., 1990. The fate of bacterial production. Rev. Aquat. Sci. 2, 109–124.
Klein, B., Gieskes, W.W.C., Kraay, G.W., 1986. Digestion of chlorophylls and carotenoids by the marine protozoan Oxyrrhis marina studied by HPLC analysis of algal pigments. J. Plankton Res. 8, 827–836. Kohn, N.P., Word, J.Q., Niyogi, D.K., 1994. Acute toxicity of ammonia to four species of marine amphipods.
Mar. Environ. Res. 38, 1–15.
Larsson, U., Elmgren, R., Wulff, F., 1985. Eutrophication and the Baltic Sea: causes and consequences. Ambio 14, 9–14.
Larsson, U., Hobro, R., Wulff, F., 1986. Dynamics of a phytoplankton spring bloom in a coastal area of the ¨
northern Baltic proper. Contrib. Asko Lab. Univ. Stockholm 30, 1–32.
Leavitt, P.R., Carpenter, S.R., 1990. Aphotic pigment degradation in the hypolimnion — Implications for sedimentation studies and paleolimnology. Limnol. Oceanogr. 35, 520–534.
Lee, C., 1992. Controls on carbon preservation: The use of stratified water bodies to compare intrinsic rates of decomposition in oxic and anoxic systems. Geochim. Cosmochim. Acta 56, 3323–3335.
Lopez, G.R., Levinton, J.S., 1987. Ecology of deposit-feeding animals in marine sediments. Q. Rev. Biol. 8, 283–289.
Lopez, G., Elmgren, R., 1989. Feeding depths and organic absorption for deposit-feeding benthic amphipods
Pontoporeia affinis and P. femorata. Limnol. Oceanogr. 34, 982–991.
Mantoura, R.F.C., Llewellyn, C.A., 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reversed-phase high-performance liquid chromatography. Anal. Chim. Acta 151, 297–314.
´
Olafsson, E., Elmgren, R., 1997. Seasonal dynamics of sublittoral meiobenthos in relation to phytoplankton sedimentation in the Baltic Sea. Estuar. Coast. Shelf Sci. 45, 149–164.
Rhoads, D.L., 1974. Organism–sediment relations on the muddy seafloor. Oceanogr. Mar. Biol. 12, 263–300. Rice, D.L., 1986. Early diagenesis in bioadvective sediments: relationships between the diagenesis of Be-7, sediment reworking rates, and the abundance of conveyor-belt deposit-feeders. J. Mar. Res. 44, 149–184. Rosenberg, R., Elmgren, R., Fleischer, S., Jonsson, P., Persson, G., Dahlin, H., 1990. Marine eutrophication
case studies in Sweden. Ambio 19, 102–108.
Rumohr, H., Bonsdorff, E., Pearson, T.H., 1996. Zoobenthic succession in Baltic sedimentary habitats. Arch. Fish. Mar. Res. 44, 179–214.
Sanger, J.E., Gorham, E., 1970. The diversity of pigments in lake sediments and its ecological significance. Limnol. Oceanogr. 15, 59–69.
˚
Segerstrale, S.G., 1950. The amphipods on the coast of Finland — some facts and problems. Soc. Sci. Fenn. Comment. Biol. 14, 2–28.
˚
Segerstrale, S.G., 1959. Synopsis of data on the Crustaceans Gammarus locusta, G. oceanicus, Pontoporeia
affinis and Corophium volutator (Amphipoda Gammaridea). Soc. Sci. Fenn. Comment. Biol. 5, 7–23.
Shuman, F.R., Lorenzen, C.J., 1975. Quantitative degradation of chlorophyll by a marine herbivore. Limnol. Oceanogr. 20, 580–586.
Skopintsev, B.A., 1981. Decomposition of organic matter of plankton, humification and hydrolysis. In: Duursma, E.K., Dawson, R. (Eds.), Marine Organic Chemistry. Elsevier, Amsterdam, pp. 125–177. Solorzano, L., 1969. Determination of ammonia in natural waters by the phenylhypochlorite method. Limnol.
Oceanogr. 14, 799–801.
Stigebrandt, A., 1991. Computations of oxygen fluxes through the sea surface and net production of organic matter with application to the Baltic and adjacent seas. Limnol. Oceanogr. 36, 444–454.
Sun, M.Y., Wakeham, S.G., 1994. Molecular evidence for degradation and preservation of organic matter in the anoxic Black Sea basin. Geochim.Cosmochim. Acta 58, 3395–3406.
Sun, M.Y., Aller, R.C., Lee, C., 1991. Early diagenesis of chlorophyll a in Long Island Sound sediments: A measure of carbon flux and particle reworking. J. Mar. Res. 49, 379–401.
14
Sun, M.Y., Lee, C., Aller, R.C., 1993a. Anoxic and oxic degradation of C-labeled chloropigments and a 14
C-labeled diatom in Long Island Sound sediments. Limnol. Oceanogr. 38, 1438–1451.
Sun, M.Y., Lee, C., Aller, R.C., 1993b. Laboratory studies of oxic and anoxic degradation of chlorophyll a in Long Island Sound sediments. Geochim. Cosmochim. Acta 57, 2311–2323.
Sun, M.Y., Aller, R.C., Lee, C., 1994. Spatial and temporal distributions of sedimentary chloropigments as indicators of benthic processes in Long Island Sound. J. Mar. Res. 52, 149–176.
Watts, C.D., Maxwell, K., Kjosen, L., 1977. The potential of carotenoids as environmental indicators. In: Campos, R., Goni, J. (Eds.). Advances in Organic Geochemistry. Pergamon Press, New York.
Webb, D.G., Montagna, P.A., 1993. Initial burial and subsequent degradation of sedimented phytoplankton-relative impact of macrobenthos and meiobenthos. J. Exp. Mar. Biol. Ecol. 166, 151–163.
Webster, G., Benfield, E.F., 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Syst. 17, 567–594.
Welschmeyer, N.A., Lorenzen, C.J., 1985. Chlorophyll budgets: Zooplankton grazing and phytoplankton growth in a temperate fjord and the Central Pacific gyres. Limnol. Oceanogr. 30, 1–21.
Westrich, J.T., Berner, R.A., 1984. The role of sedimentary organic matter in bacterial sulfate reduction: The G model tested. Limnol. Oceanogr. 29, 236–249.