The secretory pathway plays a central role in plant
development and morphogenesis. Storage protein deposition, plant cell division and the expansion of the plasma membrane and extracellular matrix all require the synthesis and trafficking of membranes, proteins and polysaccharides through this network of organelles. Increasing evidence demonstrates that the plant secretory pathway is more complex than previously appreciated and that its formation and maintenance are guided/regulated by many different mechanisms.
Addresses
*Laboratory of Biochemistry, Graduate School of Bio-agricultural Sciences, Nagoya University, Chikusa-ku, Nagoya 464-8601, Japan; e-mail: [email protected]
†Department of Biochemistry, University of Wisconsin-Madison, 420
Henry Mall, Madison, WI 53706, USA; e-mail: [email protected]
Current Opinion in Plant Biology1998, 1:463–469 http://biomednet.com/elecref/1369526600100463 © Current Biology Ltd ISSN 1369-5266
Abbreviations
BiP endoplasmic reticulum binding protein
CCV clathrin coated vesicle
CTPP carboxy-terminal propeptide
ER endoplasmic reticulum
PACV precursor-accumulating vesicle
PB protein body
PSV protein-storage vacuole
ssVTS sequence-specific vacuolar targeting signal
Introduction
The basic secretory pathway is comprised of several dis-crete organelles, including the endoplasmic reticulum (ER) and the Golgi apparatus, that are involved in the assembly, post-translational modification, trafficking and correct localization of newly synthesized proteins to the plasma membrane and vacuole. Trafficking between the various subcompartments of these organelles is thought to be primarily mediated by small carrier vesicles. In the past 10 years or so many of the basic molecular components involved in the formation and consumption of these vesi-cles have been identified in yeast and mammalian cells and related components have been identified in plants. This conservation is not surprising given that the basic organization of the plant secretory system is morphologi-cally similar to that of other eukaryotes. There are a number of features, however, that distinguish the plant pathway. For example, the de novo assembly of the cell-plate during plant cell division has no counterpart in other systems. One cannot simply infer how a plant process functions, therefore, from studies in other systems.
In this review, we will highlight advances from the past year in our understanding of the complex nature of the plant secretory pathway and of how membrane and pro-teins necessary for the formation and maintenance of the endomembrane system are localized. For additional dis-cussions of the various topics touched upon here the reader is referred to several other recent reviews [1–4].
Sorting of storage protein mRNA to different
ER subdomains in cereal seeds
In cereals, two major classes of storage proteins accumulate in the cells of maturing seeds; prolamins are retained with-in protewith-in bodies (PBs) that are delimited by the ER membrane, whereas globulins are transported through the Golgi apparatus and deposited in vacuoles (see Figure 1). The different fates of these storage proteins may be linked to the differential distribution of mRNAs encoding these proteins and their translocation into distinct subdomains of the ER [2]. Interestingly, prolamin mRNA charged polysomes are not directly anchored to the PB membrane, rather they are found to be associated with the cytoskele-ton [5•]. Similarly, elongation factor-1, which is required for
synthesis of the protein, binds to the cytoskeleton sur-rounding PBs in maize seeds [6]. These observations suggest that the cytoskeleton plays a direct role in the seg-regation and localization of PB-ER-specific mRNAs from messages that are translated on other ER domains.
Storage protein deposition in the ER
The four structurally distinct prolamin-type storage pro-teins of maize, the zeins (α, β, δ, γ), are deposited and arranged in an orderly fashion within ER-derived PBs. Inter- and intramolecular interactions between these sub-units are necessary for proper PB formation. Indeed, coexpression studies have recently shown that γ-zein and β-zein are necessary for the efficient deposition of α-zein and δ-zein in ER-PBs, respectively [7,8•]. These
interac-tions, however, must be properly organized within the ER as demonstrated when a gene encoding a mutant α-zein that is defective in signal sequence cleavage was expressed, causing the aberrant PB morphology associated with the floury2 phenotype [9,10].
Deposition of wheat storage protein aggregates is also dependent on a retention/aggregation mechanism between different storage protein subunits in the ER. Expression in tobacco of only a single gliadin subtype, γ-gliadin, resulted in its degradation in a post-ER compartment [11]. In con-trast to the maize and rice prolamins, however, the dense gliadin/glutenin deposits are transported via a novel Golgi-independent mechanism from the ER to the protein-storage vacuole (PSV) [12]. Similarly, the globulin protein-storage proteins in developing pumpkin cotyledons initially
Protein transport within the plant cell endomembrane system: an
update
aggregate in the ER and are subsequently transported via electron-dense precursor-accumulating vesicles (PACV) to PSV [13•]. Little is known about how these protein
aggre-gates are distinguished from other secretory proteins and packaged into to-PSV vesicles. The formation of ER-to-Golgi transport vesicles in yeast and mammalian cells is
driven by the COPII (coat protein II) vesicle coat protein complex [14].Arabidopsis homologs of COPII components have been identified and localized to the ER, indicating that COPII also mediates ER-to-Golgi transport in plant cells [15]. The mechanisms involved in PACV and COPII-vesicle formation are likely to differ because PACVs are
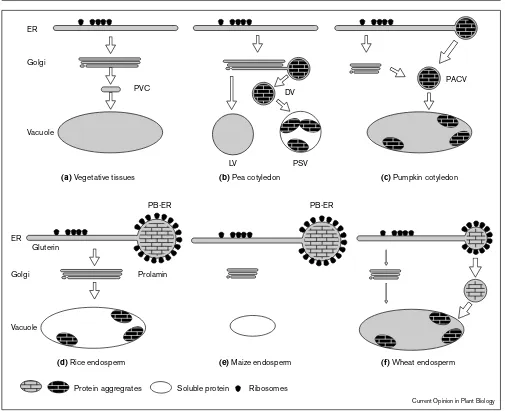
Figure 1
ER
Golgi
ER
Golgi Prolamin
Vacuole
Gluterin
Vacuole
PVC
PB-ER PB-ER
PSV LV
DV
PACV
(a) Vegetative tissues (b) Pea cotyledon (c) Pumpkin cotyledon
(f) Wheat endosperm
(d) Rice endosperm (e) Maize endosperm
Protein aggregrates Ribosomes
Current Opinion in Plant Biology
Soluble protein
Schematic representation of the different routes involved in the intracellular trafficking of vacuolar and storage proteins and some of their unique features that have been observed in various plant cells. (a)
Golgi-mediated transport of soluble proteins to the central vacuole in vegetative tissues and suspension-cultured cells. It is unknown if this step is mediated by one or more than one distinct vesicle class. The recently characterized prevacuolar compartment(s) (PVC) may exist in other plant cell types as well. (b)Transport of storage proteins in developing pea cotyledon. Storage protein aggregates form at the trans-side of the Golgi and are transported via dense vesicles (DV) to protein-storage vacuole (PSV). Hydrolases are transported from the Golgi apparatus to the lytic vacuole (LV), possibly by clathrin-coated vesicles. (c)Transport of proteins to the vacuoles in developing pumpkin cotyledons. The storage proteins that aggregate in the ER are packaged and exported from the ER to the PSV in precursor-accumulating vesicles (PACV). Precursor processing proteases are
transported through the Golgi apparatus and maybe directed to the PACV, which is consistent with the appearance of complex glycans in these vesicles [13•]. (d)Storage protein sorting in developing rice endosperm. Prolamins accumulate in the ER and remain in ER-membrane delimited protein bodies (PB-ER), whereas gluterin is transported to the vacuole via the Golgi apparatus. Gluterin mRNA is localized to the rER, whereas prolamin mRNA is segregated to the protein body forming region of the ER (PB-ER). (e)Deposition of storage proteins in developing maize endosperm. Zeins are deposited in ER-PB. (f)Storage protein transport in developing wheat
significantly larger (200–400 nm in diameter) than ER-to-Golgi vesicles (60–80 nm) and contain significant amounts of the ER resident molecular chaperone, binding protein (BiP) [13•].
ER chaperones and protein folding
BiP, a member of the Hsp70 family, has been strongly implicated in the assembly of storage protein deposits in the ER [8•,13•,16,17]. Analysis of the assembly of
phase-olin, the homotrimeric vacuolar storage glycoprotein from common bean, has also demonstrated that BiP associates with the monomeric protein before it trimerizes [18,19•]
and with misfolded protein subunits in the ER [20•]. In
addition to BiP, the folding and oligomerization of newly synthesized membrane and soluble secretory protein requires the coordinate action of the ER-resident molecu-lar chaperones, protein disulfide isomerase (PDI), calreticulin, and the type-I membrane protein, calnexin. Mutations that disrupt proper disulfide bond formation have been shown to affect the synthesis of 11S globulins, a class of seed storage proteins [21•] and the deposition of
gliadin monomers into PB [22,23]. This process is sensitive to the redox state of the ER [21•] and is likely to be
facili-tated by PDI. In tobacco, a large fraction of BiP is associated with calreticulin [24•]. This interaction maybe
important for the regulation of the level of free BiP in the ER and/or for the ER-retention of BiP. Calnexin and BiP have also recently been shown to interact with the vacuo-lar type-proton pump (V-ATPase) from oat seedlings [25•].
Binding of these molecular chaperones to the V-ATPase may facilitate the assembly of this large multimeric com-plex. Another possible function for these two chaperones could be to inhibit the activity of the V-ATPase in the ER by gating the opening of the proton-conducting channel. Indeed BiP has been shown to bind in vitroto the lumenal side of the Sec61p protein-translocation channel (translo-con), that transports nascent polypeptides across the ER membrane, thereby preventing the free movement of small molecules out of the ER [26]. Thus, it will be inter-esting to determine if the proton pump–chaperone complexes are active.
What is the fate of misfolded secretory proteins in the ER? Recent studies in yeast and mammalian cells have indicat-ed that the ER has a protein-degradation pathway that can recognize and selectively remove polypeptides from the secretory pathway. Misfolded proteins are retrotranslocat-ed through the Sec61p-translocon from the ER to the cytosol and degraded by the ubiquitin/proteasome system [27]. The existence of this pathway in plant cells can be inferred from a recent experiment showing that ER-targeted ricin A-chain, which hydrolyzes a phosphodiester band of rRNA and destroys ribosome activity, can inacti-vate protein synthesis in the cytosol [28]. It remains to be determined, however, whether this pathway is part of the general ‘quality control’ mechanism in plants for removing unfolded or misfolded secretory proteins such as assembly-defective phaseolin which was found to be slowly
degraded in a brefeldin A (BFA)-insensitive (i.e. Golgi-independent) manner [20•]. Alternatively, some misfolded
proteins may be exported from the ER and directly target-ed to the vacuole for degradation through the BFA-insensitive direct pathway(s) used to deliver pump-kin globulins and wheat gliadins to the PSV.
Golgi-mediated sorting to the different types
of plant vacuoles
Soluble vacuolar proteins that are not directly targeted from the ER to the vacuole are sorted away from secreted proteins within the trans-Golgi apparatus. Several inde-pendent vacuole sorting mechanisms have been identified in plants including sequence-specific vacuolar targeting signal (ssVTS) mediated targeting, carboxy-terminal propeptide signal (CTPP)-mediated transport, and aggre-gation-mediated targeting [29]. Interestingly, the ER retention signal HDEL has recently also been shown to function as a vacuolar targeting signal [30•] and some
pep-tide sorting sequences may behave as both ssVTS and CTPP [31]. Cargo proteins containing these different sig-nals are not sorted to the vacuole by the same machinery. First, wortmannin blocks the sorting of CTPP-containing proteins but does not affect ssVTS-mediated sorting [32]. Second, some plant cells, such as the parenchyma cells of developing seed cotyledons, root tip cells and barley aleu-rone cells, contain at least two distinct vacuolar compartments [33,34,35•]. In barely root tip cells, a
CTPP-containing protein, barley lectin, and a ssVTS-CTPP-containing protease, aleurain, are localized in separate functionally distinct vacuoles [33]. In addition, storage proteins that accumulate in PSV of developing pea cotyledons are not packaged into trans-Golgi derived clathrin coated vesicles (CCVs), rather they appear to be transported in smooth dense vesicles (DVs) that are distinct from the ER-derived PACVs of pumpkin cotyledons [36].
Recognition and packaging of proteins containing vacuolar sorting signals into Golgi-derived transport vesicles is like-ly to be mediated by their interaction with specific transmembrane cargo receptors. The saturable nature of phaseolin targeting to the vacuole in transgenic tobacco cells supports this idea [37•]. A putative plant vacuolar
sorting receptor, BP-80, that binds specifically to ssVTS
in vitro, was isolated from CCVs [38,39] and cloned [40•].
Related proteins have also been detected in Arabidopsis
(AtELP, [41]) and pumpkin (PV72/82, [42••]). These
CCVs and post-Golgi membranes (prevacuolar compart-ment; PVC) [38,39,41,45••]. Binding studies have
suggested that the Tyr-containing motif of the cytosolic tail of AtELP interacts with the Golgi-localized clathrin AP-1
adaptor complex [45••]. AtELP and BP-80 are, therefore,
likely to mediate the transport of cargo from the trans-Golgi to the prevacuolar compartment via CCVs, although the natural cargo for these receptors remains to be deter-mined. Interestingly, PV72/82 was isolated from ER-derived PACVs [13•,42••]. This protein has been
shown to recognize the NLPS amino acid sequence of 2S albumin in PACVs suggesting that PV72/82 is indeed a bona fide sorting receptor for 2S albumin [42••]. Many
questions about the role of PV72/82 and the formation of PACVs remain to be resolved, however, including why a protein that is packaged into ER-to-PSV vesicles has both a putative AP-1 binding motif and a di-acidic ER-export motif in its cytosolic tail.
Protein aggregation within the Golgi apparatus has been suggested to be another way in which proteins destined for the vacuole are segregated away from secreted proteins [36,46]. In developing legume seeds, electron-dense stor-age protein aggregates initially accumulate in the
trans-Golgi and are present in DVs of about 130 nm in diameter that are thought bud from the Golgi [36]. As these DVs are similar in function and morphology to pumpkin PACVs (although their origin is different), it is possible that receptor-like molecules also function in the concentration and sorting of storage proteins into DVs. Following formation of the DVs these putative receptors may be released and removed from the vesicles by CCVs that have been observed to bud from the surface of imma-ture DVs [36,47].
Targeting and fusion of Golgi-derived CCVs to
the prevacuolar compartment
Transport of proteins from the trans-Golgi to the vacuole is likely to occur through the recently identified PVC which is analogous to the endosomal compartment in yeast and mammalian cells. Like other vesicular transport steps in the secretory pathway, the targeting and fusion of the Golgi-derived CCVs with the PVC is thought to be medi-ated through the pairing of compartment specific integral membrane proteins, known as SNAREs, that reside on the two fusing membrane species (i.e. vesicle v-SNAREs pair with target membrane t-SNAREs) [48]. Their interaction
in vitro is modulated by a large host of regulatory factors (see Figure 2). In yeast, docking and fusion of Golgi-derived vesicles with the PVC is directed by the v-SNARE, Vti1p, and the t-SNARE, Pep12p, as well as the Sec1p-related protein, Vps45p. Another t-SNARE, Vam3p, is required for the transport step from the PVC to the vacuole [49] and for vacuole fusion [50]. Plant equiva-lents of these factors — AtPEP12p, AtVPS45p, AtVAM3p and AtVTI1p — have been characterized [51•,52,53•]
(Zheng H et al, Abstract 123, 9th International Conference on Arabidopsis Research, Madison, 24–29 June 1998). Their subcellular distribution closely approximates the location expected for the function of each of these proteins in vacuolar protein sorting. In particular, AtPEP12p and a fraction of AtELP were found to colocalize on PVCs
Figure 2
Schematic diagram of the critical components involved in NSF-dependent heterotypic or Cdc48p-NSF-dependent homotypic membrane fusion. (a)Targeting and fusion of Golgi-derived clathrin-coated vesicles (CCV) with the prevacuolar compartment. Heterotypic vesicle fusion at each step of the secretory pathway is mediated by the pairing of cognate compartment specific v- and t-SNAREs (e.g. AtVtilp and AtPep12p, respectively) and is modulated by a number of proteins including the general trafficking factors, the N-ethylmaleimide (NEM) sensitive factor (NSF), and soluble NSF-attachment proteins (SNAPs) [62] and specific members of the Rab/Ypt family of small GTP-binding proteins [63], the Sec1 protein family (including AtVps45p) [64] and putative ‘velcro’ factors such as p115/Uso1p [65,66]. Following fusion, NSF and SNAPs catalytically disassemble the energetically stable SNARE complex. (b)Cell-plate membrane fusion. SNAREs are not only involved in the heterotypic fusion of secretory vesicles with their appropriate acceptor compartment but also function in the homotypic fusion of like-like membranes such as vacuoles [50] and ER-membranes [67]. The recent identification and localization of KNOLLE, a putative cell-plate t-SNARE [59••] and AtCDC48 [68], a fusion factor closely related to NSF, may lend some insight into the mechanisms of membrane fusion involved in cell-plate formation. CDC48p has been shown to be required for the homotypic fusion of ER membranes in yeast [67] and is localized at the cell-plate in dividing cells [68]. In contrast to NSF-dependent homotypic and heterotypic membrane fusion, however, which operate through the pairing of v- and t-SNAREs, Cdc48p-dependent homotypic fusion is mediated by direct t-/t-SNARE interaction.
NSF/SNAP complex
Cytosol
Uso1p
Cytosol
Rab AtVPS45p AtVTI1p
AtPEP12p
AtCDC48p Prevacuolar compartment
Cell-plate membrane
Cell-plate membrane CCV
Knolle
Knolle
Current Opinion in Plant Biology
(a)
suggesting that this compartment serves to recycle plant vacuolar cargo receptors back to the Golgi apparatus. Similarly, BP-80 resides both in the Golgi apparatus and PVCs in pea root tips [40•].
Exocytosis and cell-plate formation
Plant cell growth and expansion is accomplished by the delivery and fusion of Golgi-derived exocytic vesicles car-rying membranes and cell wall components with the pre-existing plasma membrane. Similarly, the vesicles that fuse to form the cell-plate in dividing plant cells are thought to bud from the Golgi [54]. Very little is however known about the formation, trafficking and fusion of these vesicles. For example, it remains to be determined whether exocytosis and cell-plate formation are mediated by a single homogeneous vesicle population or whether multiple Golgi-derived vesicle populations exist, carrying distinct sets of cargo, that in the case of cell-plate forma-tion would converge and fuse to form a new plasma membrane and cell wall de novo. Recent studies in plants, yeast and mammalian cells have suggested the existence of multiple parallel vesicular routes that transport distinct sets of cargo from the Golgi to the plasma membrane [55–57]. In expanding cells these vesicles are likely to be targeted to the growing regions of the cell cortex. During cell-plate formation, one or more of these pathways may become polarized toward the plane of division, as they appear to do during cell division in yeast [58]. In this way, the time at which plasma membrane and secreted proteins are synthesized may dictate their deposition at the cell sur-face or the cell-plate. Consistent with this hypothesis is the observation that the putative t-SNARE, Knolle, which is expressed during mitosis, is localized specifically to the cell-plate of dividing Arabidopsiscells [59••].
To identify components involved in exocytosis from the Golgi apparatus, Nakano and coworkers have screened for
Arabidopsis cDNAs that would complement the yeast late secretory pathway mutant, sec15[60]. Sec15p is a compo-nent of the exocyst complex required for the Golgi-to-plasma membrane transport [61]. Interestingly, one gene that was identified, RMA1, does not encode a Sec15p homolog, rather it belongs to a new class of genes containing the RING-finger motif (a protein motif that binds two Zn2+ ions in a unique ‘cross-brace’ structure)
that is conserved in all multicellular organisms. Whether RMA1p is actually involved in secretion in plant cells requires further study.
Conclusions
A notable strength of the work highlighted above has been the integration of ideas and observations from a number of systems and different plant cell types over the last several years. Nevertheless caution needs to be exercised when it comes to deciding whether a particular secretory process is a general one or if it is restricted to only a limited set of cells (i.e. those from a specific tissue or developmental stage). For instance, is the direct ER-to-vacuole transport pathway
observed in developing wheat and pumpkin seeds utilized in other cell types? We would like to encourage frequent dis-cussions of such ideas through formal and informal venues such as the recently formed Secretory Pathway Group list-server ([email protected]). Morphological, biochemical and genetic studies of the plant secretory path-way are beginning to provide a detailed molecular description of how the elaborate endomembrane network of organelles in plant cells is established and how it functions in the growth and development of the whole organism.
Acknowledgements
We thank all the colleagues who provided us unpublished information. We apologize to those authors whose articles could not be cited due to space limitations.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Bassham DC, Raikhel NV: Transport proteins in the plasma membrane and the secretory system.Trends Plant Sci1996,
1:15-20.
2. Okita T, Rogers J: Compartmentation of proteins in the endomembrane system of plant cells.Annu Rev Plant Physiol Plant Mol Biol1996, 47:327-350.
3. Okita TW, Choi SB, Ito H, Muench DG, Wu Y, Zhang F: Entry into the secretory system — the role of mRNA localization.J Exp Bot 1998, 49:1081-1089.
4. Ceriotti A, Duranti M, Bollini R: Effects of N-glycosylation on the folding and structure of plant proteins.J Exp Bot1998.
49:1091-1103.
5. Muench DG, Wu Y, Coughlan SJ, Okita TW: Evidence for a
• cytoskeleton-associated binding site involved in prolamine mRNA localization to the protein bodies in rice endosperm tissue.Plant Physiol1998, 116:559-569.
Rice prolamin and glutelin mRNAs are localized to morphologically distinct parts of the ER. In this paper, PB associated polysomes were found to be resistant to detergent extraction and puromycin treatment suggesting that the majority of prolamine mRNA is not directly anchored to the ER mem-brane through ribosome binding sites nor through the binding of the nascent prolamin polypeptide. Rather, the prolamine polysome-binding activity was shown to be associated with cytoskeletal elements.
6. Clore AM, Dannenhoffer JM, Larkins BA: EF-1-alpha is associated with a cytoskeletal network surrounding protein bodies in maize endosperm cells.Plant Cell1996, 8:2003-2014.
7. Coleman CE, Herman EM, Takasaki K, Larkins BA: The maize g-zein sequesters a-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm.Plant Cell1996,
8:2335-2345.
8. Bagga S, Adams HP, Rodriguez FD, Kemp JD, Sengupta-Gopalan C:
• Coexpression of the maize d-zein and b-zein genes results in stable accumulation of d-zein in endoplasmic reticulum-derived protein bodies formed by b-zein.Plant Cell1997, 9:1683-1696. Zeins, the major seed storage proteins of maize, are synthesized on the rER and are deposited in ER-PB. In this paper β-zein and δ-zein were shown to be stably expressed and deposited in zein-specific ER-PB in transgenic tobacco leaves. Coexpression of β-zein and δ-zein together resulted in an increase in δ-zein accumulation relative to plants expressing d-zein alone and both proteins colocalized in the same PB. These results suggest that δ-zein interacts with and stabilizes the assembly of δ-zein into PB.
9. Gillikin JW, Zhang F, Coleman CE, Bass HW, Larkins BA, Boston RS:
A defective signal peptide tethers the floury-2 zein to the endoplasmic reticulum membrane.Plant Physiol1997, 114:345-352.
10. Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, Larkins, BA:
Expression of a mutant alpha-zein creates the floury2 phenotype in transgenic maize.Proc Natl Acad Sci USA1997, 94:7094-7097.
not contain an endoplasmic reticulum-retention signal.Planta 1997, 203:488-494.
12. Levanony H, Rubin R, Altschuler Y, Galili G: Evidence for a novel route of wheat storage proteins to vacuoles.J Cell Biol1992,
119:1117-1128.
13. Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M:
• Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles.Plant Cell 1998, 10:825-836.
Large 200–400 nm diameter vesicles that accumulate storage protein precur-sors were purified from pumpkin seeds. These vesicles contain an electron-dense core of storage proteins surrounded by an electron-translucent layer. Numerous storage protein aggregates were also found to be present in the ER suggesting that these vesicles are ER-derived and that they mediate the transport of the insoluble storage protein aggregates to vacuole.
14. Bednarek SY, Orci L, Schekman R: Traffic COPs and the formation of vesicle coats.Trends Cell Biol1996, 6:468-473.
15. Bar-Peled M, Raikhel NV: Characterization of AtSec12 and AtSAR1 proteins likely involved in ER and Golgi traffic.Plant Physiol1997,
114:315-324.
16. Muench DG, Wu Y, Zhang Y, Li X, Boston RS, Okita TW: Molecular cloning, expression and subcellular localization of a BiP homolog from rice endosperm tissue.Plant Cell Physiol 1997, 38:404-412.
17. Li X, Wu Y, Zhang D-Z, Gillikin JW, Boston RS, Franceschi VR, Okita TW: Rice prolamine protein body biogenesis: a BiP mediated process.Science1993, 262:1054-1056.
18. Vitale A, Bielli A, Ceriotti A, The binding protein associates with monomeric phaseolin.Plant Physiol1995, 107:1411-1418.
19. Lupattelli F, Pedrazzini E, Bollini R, Vitale A, Ceriotti A: The rate of
• phaseolin assembly is controlled by the glucosylation state of its N-linked oligosaccharide chains.Plant Cell1997, 9:597-609. In the ER nascent secretory proteins containing N-glycans are modified by the addition of an Glc3Man9GlcNAc2oligosaccharide unit. Subsequently, the three terminal glucose residues are removed by ER-resident glucosidas-es. In this paper, the role of glucose trimming on the folding and assembly of bean phaseolin was analyzed using an in vitrosystem. In the presence of specific inhibitors of the glucose trimming reaction, the assembly of phase-olin was found to be accelerated. In contrast, polypeptides bearing partially trimmed glycans were unable to properly trimerize. Glycan chains and their accessibility to glucosidases are, therefore, likely to modulate the assembly of phaseolin.
20. Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M,
• Faoro F, Bollini R, Ceriotti A, Vitale A: Protein quality control along the route to the plant vacuole.Plant Cell1997, 9:1869-1880. In this paper, the fate of an assembly-defective form of the trimeric storage protein, phaseolin, was analyzed in the leaves of transgenic tobacco. Assembly defective phaseolin was bound to BiP in the ER and slowly degraded by a Brefeldin-A insensitive process, suggesting that its turnover does not require Golgi-mediated secretory protein transport. These results provide strong evidence for the presence of a ‘quality control’ mechanism in the ER of plant cells that eliminates malfolded proteins.
21. Jung R, Nam YW, Saalbach I, Muntz K, Nielsen NC: Role of the
• sulfhydryl redox state and disulfide bonds in processing and assembly of 11S seed globulins.Plant Cell1997, 9:2037-2050. Seed legumins contain two conserved disulfide bonds that are important for the initial formation of 9S trimers in the ER and their assembly into 11S hexa-mers in the PSV. In this paper mutant subunits were constructed in which the critical cysteine residues for the interchain bond (IE) connecting the acidic and basic chains and the intrachain bond (IA) were disrupted. Whereas oxi-dized glutathione stimulated the trimerization of the wild-type protein no stimulation was detected for the assembly of IE mutant subunits and it was diminished for the IA mutant. IE mutant trimers were not capable of assem-bling into hexamers unless they were in the presence of wild-type subunits.
22. Shimoni Y, Galili G: Intramolecular disulfide bonds between conserved cysteines in wheat gliadins control their deposition into protein bodies.J Biol Chem1996, 271:18869-18874.
23. Shimoni Y, Blechl AE, Anderson OD, Galili G: A recombinant protein of two high molecular weight glutenins alters gluten polymer formation in transgenic wheat.J Biol Chem1997,
272:15488-15495.
24. Crofts AJ, Leborgne-Castel N, Pesca M, Vitale A, Denecke J: BiP and
• calreticulin form an abundant complex that is independent of endoplasmic reticulum stress.Plant Cell1998, 10:813-823. In this paper a large fraction of BiP was found to be in a stable complex with another soluble ER-resident molecular chaperone, calreticulin. Formation of this complex was not affected by either the presence or absence of stress
conditions that lead in an increase in the number of unfolded proteins in the ER. On the basis of antibody accessibility studies the binding of BiP to cal-reticulin and to unfolded proteins appears to be different. The BiP–calreticulin complex can be disrupted by low pH but not by divalent cation chelators and complex formation is independent of the ER retention signal on BiP.
25. Li X, Su RTC, Hsu HT, Sze H: The molecular chaperone calnexin
• associates with the vacuolar H+-ATPase from oat seedlings.Plant Cell1998, 10:119-130.
The V-ATPase complex consists of a peripheral sector (V1) and a membrane integral sector (V0). A 64 kDa polypeptide that copurifies with oat V-ATPase subunits was positively identified as the ER-membrane chaperone, calnexin, and shown to interact directly with the V-ATPase complex in purified ER membrane fractions by coimmunoprecipitation. Monoclonal antibodies against the catalytic subunit of the V1 complex precipitated the entire V-ATPase complex as well as calnexin and BiP.
26. Hamman BD, Hendershot LM, Johnson AE: BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation.Cell 1998, 92:747-758.
27. Suzuki T, Yan Q, Lennarz WJ: Complex, two-way traffic of molecules across the membrane of the endoplasmic reticulum.J Biol Chem 1998, 273:10083-10086.
28. Frigerio L, Vitale A, Lord JM, Ceriotti A, Roberts LM: Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts.J Biol Chem1998,
273:14194-14199.
29. Matsuoka K, Neuhaus JM: Cis-elements of protein transport to the vacuole.J Exp Bot1999, in press.
30. Gomord V, Denmat LA, Fitchette-Laine AC, Satiat-Jeunemaitre B,
• Hawes C, Faye L: The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J 1997,11:313-325.
The carboxy-terminal tetrapeptides, HDEL and KDEL, serve as retention sig-nals for soluble ER resident proteins. In this paper the authors characterized a fusion protein containing a secreted form of sporamin and an HDEL C-ter-minal extension. Addition of the HDEL extension resulted in the accumulation of sporamin in the ER. In addition, a significant amount of the fusion protein that escaped from the ER was transported to the vacuole.
31. Koide Y, Hirano H, Matsuoka K, Nakamura K: The N-terminal propeptide of the precursor to sporamin acts as a vacuole-targeting signal even at the C terminus of the mature part in tobacco cells.Plant Physiol1997, 114:863-870
32. Matsuoka K, Bassham DC, Raikhel NV, Nakamura K: Different sensitivity to Wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol1995, 130:1307-1318.
33. Paris N, Stanley CM, Jones RL, Rogers JC: Plant cells contain two functionally distinct vacuolar compartments.Cell1996,
85:563-572.
34. Hoh B, Hinz G, Jeong BK, Robinson DG: Protein storage vacuoles form de novo during pea cotyledon development.J Cell Sci1995,
108:299-310.
35. Swanson SJ, Bethke PC, Jones RL, Barley aleurone cells contain
• two types of vacuoles. Characterization of lytic organelles by use of fluorescent probes.Plant Cell1998, 10:685-698
Light microscopy was used to study the structure and function of vacuoles in living protoplasts of barley (Hordeum vulgare cv Himalaya) aleurone. Aleurone protoplasts contain two distinct types of vacuole: the protein stor-age vacuole and a lysosome-like organelle. In contrast to the two vacuole compartments in barley root tip cells, both of the vacuole types of aleurone cells contain the tonoplast marker protein α-TIP. The effects of the phyto-hormones, ABA and GA, on the formation and morphological change of these two vacuoles are reported.
36. Hohl I, Robinson DG, Chrispeels MJ, Hinz G: Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat.J Cell Sci1996, 109:2539-2550.
37. Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A: Sorting of
• phaseolin to the vacuole is saturable and requires a short C-terminal peptide.Plant Cell1998, 10:1031-1042.
resulted in the secretion of the mutant protein. These results provide strong evidence that phaseolin to the vacuole is signal-mediated and receptor dependent process.
38. Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC: Purification and initial characterization of a potential plant vacuolar targeting receptor.Proc Natl Acad Sci USA1994, 91:3403-3407.
39. Kirsch T, Saalbach G, Raikhel NV, Beevers L: Interaction of a potential vacuolar targeting receptor with amino- and carboxyl-terminal targeting determinants.Plant Physiol 1996, 111:469-474.
40. Paris N, Rogers SW, Jiang L, Kirsch T, Beevers L, Phillips TE, Rogers
• JC: Molecular cloning and further characterization of a probable plant vacuolar sorting receptor.Plant Physiol1997, 115:29-39. A cDNA encoding BP-80, an abundant type I integral membrane protein in pea (Pisum sativum) from CCVs that binds with high affinity to peptides con-taining vacuole-targeting determinant of aleurain precursor was isolated. Sequence analysis indicated that BP-80 defines a novel family of plant-spe-cific proteins that are highly conserved in both monocotyledons and dicotyledons. Immunolocalization showed that the BP-80 protein is present in dilated ends of Golgi cisternae and in ‘prevacuoles’.
41. Ahmed SU, Bar-Peled M, Raikhel NV: Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol1997, 114:325-336.
42. Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I: A pumpkin
•• 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor.Plant Cell Physiol1997, 38:1414-1420.
Precursor-accumulating vesicles mediate the transport of pro2S albumin to protein-storage vacuoles in developing pumpkin cotyledons. Two homolo-gous membrane proteins of 72 kDa and 82 kDa were isolated from these vesicles and shown to bind to peptides derived from pro2S albumin. These proteins are predicted to be type I membrane protein containing lumenal EGF receptor-like motifs similar to those found in BP-80 and AtELP. This is the first report in plants showing that a receptor-like protein and its defined ligand are packaged into the same transport vesicle.
43. Hoflak B: Mechanisms of protein sorting and coat assembly: clathrin coated vesicle pathways.Curr Opin Cell Biol1998,
10:499-503.
44. Nishimura N, Balch WE: A di-acidic signal required for selective export from the endoplasmic reticulum.Science1997,
277:556-558.
45. Sanderfoot AA, Ahmed SU, Marty-Mazars D, Rapoport I, Kirchhausen T,
•• Marty F, Raikhel NV: A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in
Arabidopsisroots.Proc Natl Acad Sci USA1998, 95: in press. The putative vacuolar cargo receptor, AtELP, from Arabidopsis thaliana shows significant sequence similarity to BP-80. AtELP was previously shown to be present in two membrane fractions corresponding to a novel, undefined compartment and a clathrin coated vesicle fraction. In this paper, the undefined compartment was shown to correspond to a post-Golgi pre-vacuolar compartment (PVC) containing the t-SNARE, AtPEP12p, which is required for Golgi-to-vacuole transport. In addition, AtELP is also present in the trans-Golgi. The cytosolic tail of AtELP binds to the mammalian AP-1 adaptor complex which connects the clathrin-coat to Golgi-localized recep-tors. Thus AtELP and its homolog BP-80 are likely to mediate protein sorting into CCVs and to shuttle back and forth between the trans-Golgi and the PVC.
46. Robinson DG, Hinz G: Multiple mechanisms of protein body formation in pea cotyledons.Plant Physiol Biochem1996,
34:155-163.
47. Robinson DG, Baeumer M, Hinz G, Hohl I, Clark SE, Williams RW, Meyerowitz EM, Ultrastructure of the pea cotyledon Golgi apparatus: origin of dense vesicles and the action of brefeldin A. Protoplasma1997, 200:198-209.
48. Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE: SNAREpins: minimal machinery for membrane fusion.Cell1998, 92:759-772.
49. Wada Y, Nakamura N, Ohsumi Y, Hirata A: Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae.J Cell Sci1997, 110:1299-1306. 50. Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A:
Homotypic vacuolar fusion mediated by t- and v-SNAREs.Nature 1997, 387:199-202.
51. da Silva Conceicão A, Marty-Mazars D, Bassham D, Sanderfoot, AA,
• Marty F, Raikhel NV: The syntaxin homolog AtPEP12p resides on a late post-Golgi compartment in plants.Plant Cell1997, 9:571-582. The intracellular localization of an Arabidopsis thalianahomolog (AtPEP12p) of the yeast Golgi-to-vacuole t-SNARE, Pep12p, was analyzed. Subcellular fractionation and immunolocalization studies indicated that AtPEP12 is an inte-gral membrane protein and that it is localized to a post-Golgi compartment.
52. Bassham DC, Raikhel NV, An ArabidopsisVPS45p homolog implicated in protein transport to the vacuole.Plant Physiol1998,
117:407-415.
53. Sato MH, Nakamura N, Ohsumi Y, Kouchi H, Kondo M,
Hara-• Nishimura I, Nishimura M, Wada Y, The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in
Arabidopsis thaliana.J Biol Chem1997, 272:24530-24535. In this paper an Arabidopsis thalianacDNA, AtVAM3, that complements the yeast vam3mutation was isolated. AtVAM3p is expressed in various tissues and was found to co-fractionate with a vacuolar membrane protein H+ -translocating inorganic pyrophosphatase. Immunoelectron microscopy showed that AtVam3p was localized to restricted regions on the tonoplast in the cells at the shoot apical meristem.
54. Staehelin LA, Hepler PK: Cytokinesis in higher plants.Cell1996,
84:821-824.
55. Moore PJ, Swords KM, Lynch MA, Staehelin LA: Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants.J Cell Biol1991,
112:589-602.
56. Harsay E, Bretscher A: Parallel secretory pathways to the cell surface in yeast.J Cell Biol1995, 131:297-310.
57. Yoshimori T, Keller P, Roth MG, Simons K: Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol1996, 133:247-256.
58. Lew DJ, Reed SI: Morphogenesis in the yeast cell-cycle: Regulation by Cdc28 and cyclins.J Cell Biol1993, 120:1305-1320.
59. Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U,
•• Hwang I, Lukowitz W, Jürgens G: The ArabidopsisKNOLLE protein is a cytokinesis-specific syntaxin.J Cell Biol1997, 139:1485-1493. The Arabidopsisgene, KNOLLE, which is required for cytokinesis, encodes a putative t-SNARE related to the mammalian plasma membrane fusion pro-tein, syntaxin. KNOLLE is membrane associated and was shown by immunofluorescence microscopy to be specifically expressed during mitosis and to be localized to the plane of division during cytokinesis. Electron microscopic analysis indicates that cell-plate vesicle fusion is impaired in knolle mutant embryos. This is the first report of a syntaxin-like protein that appears to be involved specifically in the formation of the cell-plate.
60. Matsuda N, Nakano A: RMA1, an Arabidopsis thalianagene whose cDNA suppresses the yeast sec15mutation, encodes a novel protein with a RING finger motif and a membrane anchor.Plant Cell Physiol1998, 39:545-554.
61. Terbush DR, Maurice T, Roth D, Novick P: The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae.EMBO J1996, 15:6483-6494.
62. Rothman JE: Mechanisms of intracellular protein transport.Nature 1994, 372:55-62.
63. Novick P, Brennwald P: Friends and family: the role of the Rab GTPases in vesicular traffic.Cell1993, 75:597-601.
64. Pevsner J: The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal.J Neurosci Res1996, 45:89-95.
65. Cao X, Ballew N, Barlowe C: Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J1998, 17:2156-2165.
66. Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G: The vesicle docking protein p115 binds to GM130, a cis-Golgi matrix protein, in a mitotically regulated manner.Cell1998, 89:445-455.
67. Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M: Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion.Cell1998, 92:611-620.
68. Feiler HS, Desprez T, Santoni V, Kronenberger J, Caboche M, Traas J:
![Figure 2adaptor complex [45••]. AtELP and BP-80 are, therefore,likely to mediate the transport of cargo from the trans-](https://thumb-ap.123doks.com/thumbv2/123dok/1037997.929642/4.612.56.299.89.424/figure-adaptor-complex-atelp-likely-mediate-transport-cargo.webp)