Aldose reductase in rice (
Oryza sati
6
a
L.): stress response and
developmental specificity
B. Karuna Sree, Chadalavada S.V. Rajendrakumar, Arjula R. Reddy *
Department of Plant Sciences,School of Life Sciences,Uni6ersity of Hyderabad,Hyderabad,500 046,India
Received 28 March 2000; received in revised form 11 August 2000; accepted 4 September 2000
Abstract
Aldose reductase (AR) protein and enzyme (alditol: NAD (P)+1-oxidoreductase, EC 1.1.1.21) activity have been identified in
mature seeds ofindicarice cultivars. The protein begins to accumulate 15 days after pollination, reaches a peak at seed maturity and disappears upon imbibition. Furthermore, AR is induced in vegetative tissues in response to exogenous ABA application and other stress conditions, such as PEG mediated water stress and salinity. Increase in AR protein levels upon stress are in close agreement with a similar increase in enzyme activity. Varietal differences in AR levels have been demonstrated. Interestingly, all tested tolerant cultivars (as denoted by breeders) accumulate AR in vegetative tisssue in response to ABA application, while the sensitive line, Hamsa, does not do this under similar stress conditions, suggesting that AR may be associated with stress tolerance. Furthermore, AR protein has been identified in mature seeds of some selected cereals indicating the conserved nature of AR across grasses. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Abscisic acid; Days after pollination; Days after germination; Desiccation tolerance; Aldose reductase; Water stress
www.elsevier.com/locate/plantsci
1. Introduction
Aldose (aldehyde) reductases (AR) belong to the well-conserved aldo – keto reductase super family of enzymes in plants and animals [1 – 3]. They (alditol: NAD (P)+1-oxidoreductase, EC 1.1.1.21)
are monomeric, cytosolic proteins that catalyze the NADPH dependent reduction of a variety of car-bonyl metabolites including the aldehyde form of glucose which is subsequently reduced to the cor-responding sugar alcohol, sorbitol [4]. They are reported to have broad substrate specificities. For instance, AR from Euonymus japonica was re-ported to reduce a range of substrates including
aldoses, aliphatic and aromatic aldehydes [5]. AR have been implicated in different cellular processes such as desiccation tolerance of maturing seed and vegetative tissues [2,6], and detoxification of bio-genic and xenobiotic aldehydes to their corre-sponding alcohols in plant and mammalian tissues [3,7 – 9]. AR in mung bean detoxifies the fungal toxin eutypine [3] and a carbonyl reductase metab-olizes HC toxin that affects sensitive maize culti-vars [7]. In mammalian cells, AR are reported to be involved in the inactivation of various toxic aldehydes such as chlordecone [8] and acreoline [9]. Similarly, aldo – keto reductase of rat liver metabolizes aflatoxin secreted byAspergillus fla6us
[10].
In humans, AR activity is associated with dia-betic complications such as hyperglycemia [1,4,11]. In mammalian renal medullary cells, AR activity appears to be associated with osmoregulation [11]. AR is considered as a key enzyme of the polyol pathway leading to the accumulation of sorbitol [12] in stress situations, which is associated with
Abbre6iations: ABA: abscisic acid; DAP: days after pollination;
DAG: days after germination; AR: aldose reductase; PEG: polyethylene glycol; HRPO IgG: Horse radish peroxidase im-munoglobulin conjugate; SDS: sodium dodecyl sulphate; PAGE: polyacrylamide gel electrophoresis; TCA: trichloroacetic acid.
* Corresponding author. Tel.: +91-40-3010265; fax: + 91-40-3010120 or 3010145.
E-mail address:[email protected] (A.R. Reddy).
maintenance of osmotic balance of the cytoplasm and protection of the function of macromolecules in both animals and plant systems [12,13].
In plants, not much is known about AR, partic-ularly their role in stress response processes. AR protein was reported as one of the desiccation responsive-proteins of barley embryos showing se-quence similarities with that of human AR, frog eye lens rho crystallin and bovine prostaglandins [2]. In barley, AR accumulates during a specific stage of embryogenesis where embryos acquire desiccation tolerance [2]. It was the first report suggesting a functional role for this protein in the desiccation tolerance processes of embryos. Stud-ies on AR activity in other crop plants are spo-radic. Of the testedGramineaemembers, only wild oats [6] and bromegrass [14] were reported to accumulate AR protein. Further AR accumulation was also reported in desiccated leaves of an African resurrection plant, Craterostigma plan -tagineum suggesting an osmoprotective function [2].
The present paper deals with the identification of AR protein and enzyme in mature seeds of rice and some members of Gramineae. We report that this protein/ enzyme is induced in vegetative tis-sues of rice lines by water stress, salt stress and exogenous ABA application, and its inducibility is genotype dependent. We further show that AR is developmentally regulated in rice.
2. Methods
2.1. Plant material
Rice lines used in the present study include both cultivars and land races. Annada, Hamsa, and Tulasi are well known cultivars belonging toindica subspecies which were originally obtained from DRR, Hyderabad, India. Hamsa is a known drought sensitive line while other lines exhibit a varying degree of tolerance. K39 is a cold tolerant cultivar grown in the North Eastern regions of India. The plants were grown in the green house or field for several generations. Other cereals used in the study include Zea mays, A6ena sati6a,
Eleucine coracana, Hordeum 6ulgare, Triticum
aes-ti6um, Sorghum bicolor and Pennisitum
typhoidieum.
2.2. Plant growth and stress treatment
Seeds were surfaced sterilized with 5% sodium hypochlorite (NaOCl) for 5 min and rinsed with distilled water several times. They were grown on rough filter papers in see-through plastic germina-tion boxes in growth chambers with 12 h light/
dark cycle at 2892°C. The 8 day old seedlings were treated with 20% polyethylene glycol (PEG 6000), ABA (100mM), NaCl (200 mM) or kept in the cold at 591°C for a specific number of days. Control plants were grown normally without any stress treatment. The seedlings were harvested at regular intervals and quick frozen in liquid nitro-gen or immediately processed.
2.3. Total soluble protein extraction
Leaf and developing seed (at different days after pollination, DAP) from young seedlings and pani-cles respectively, were collected, quickly frozen in liquid nitrogen and used for protein extraction. The total proteins from embryos, immature and mature seeds were isolated according to Rao et al. [15] with modifications. Samples were finely ground in liquid nitrogen and extracted with hot (95°C) buffer (1gm/2 ml) containing 0.25M Tris – HCl (pH 8.0), 0.4% SDS, 20 mM EDTA, 2 mM PMSF and 5% b-mercaptoethanol, centrifuged at 12 000×g for 10 min, and proteins in the super-natant were precipitated with 15% TCA at 4°C. The pellet was washed three times with 80% ace-tone and dissolved in Tris buffer (pH.8.0). The protein concentrations were determined by the Bradford method [16].
2.4. Partial purification of aldose reductase
di-alysed over night at 4°C in the same extraction buffer. Aliquots of the fractions were used for both enzyme activity and Western analysis.
2.5. Determination of AR acti6ity
AR activity was spectrophotometrically deter-mined by measuring the decrease in the concentra-tion of NADPH at 340 nm for 5 min at room temperature [2]. The assay mixture contained 100 mM sodium phosphate buffer, pH 6.9 and 0.15 mM NADPH with 25 mM DL-glyceraldehyde as
substrate. One unit of enzyme activity was defined as the amount of enzyme catalyzing the oxidation of 1 mmol of NADPH per min under the condi-tions mentioned above. Specific activity was ex-pressed as units per mg protein.
2.6. SDS-PAGE and Western analysis
SDS-PAGE was performed according to Laemmeli et al. [17]. Fifty – 100 mg of extracted proteins of control and treated samples were frac-tionated on 15% SDS-PAGE at 150V and then electrophoretically blotted onto nitrocellulose pa-per at a constant voltage of 30V at 4°C [18]. The membranes were stained with Ponceau-S solution and the molecular weight standards were marked. The blots were probed with the primary antibody (pG22-69) raised against the barley desiccation-re-sponsive AR protein from embryo [2]. The sec-ondary antibody, HRPO goat anti-rabbit IgG conjugate (Boehringer Mannheim) was used ac-cording to the manufacturer’s specifications. The blots were incubated in the presence of the sub-strate, 4-chloro-1-naphthol and hydrogen peroxide and the complex was visualized by the develop-ment of purple color.
3. Results
3.1. Identification of aldose reductase-related protein in rice seeds
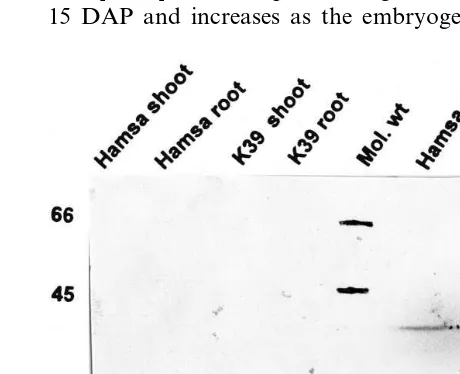
Rice genotypes were analyzed for the presence of AR like protein in both seeds and vegetative tissue. The Western blot data clearly showed that antibodies raised against the desiccation-respon-sive AR protein from barley embryo detected a 34-kDa protein in the mature seeds of Hamsa and
K39 (Fig. 1). On the contrary, this protein was not detected in shoots and roots of the seedlings grown under normal conditions. The AR activity in partially purified extracts from seeds was moni-tored using DL-glyceraldehyde as substrate in the
presence of NADPH as a co-factor. There was negligible activity when fructose and glucose were used as substrates (data not shown). Earlier re-ports indicated that purified aldose reductase from acidophilic and thermophilic red alga showed a high affinity towards DL-glyceraldehyde followed
by xylose [19].
3.2. De6elopmental profiles of AR-related protein
during embryogenesis and germination
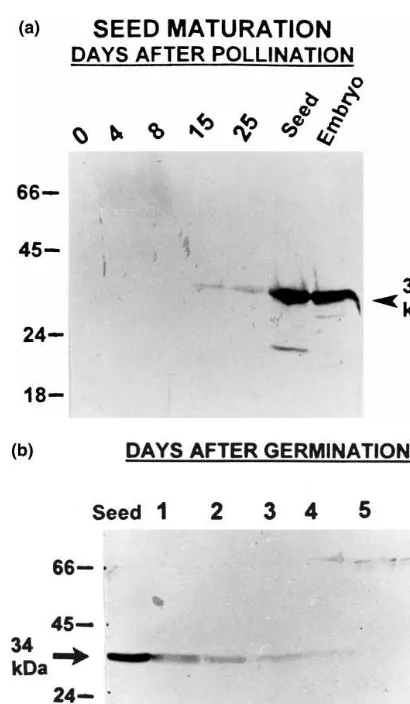
AR protein accumulation during embryogenesis was analyzed. Proteins isolated from immature seeds of K39 at different stages of maturation were fractionated on SDS-PAGE and the blots were probed with anti-AR antibodies. Western analysis revealed a stage specific accumulation of this protein, reminiscent of the typical LEA pat-tern [20,21]. AR-like protein begins to appear at 15 DAP and increases as the embryogenesis
Fig. 2. (a) Western blot showing the accumulation of the AR-related protein during seed maturation: Protein extracts from the panicles harvested at different days after pollination (DAP) were separated on SDS-PAGE and blotted onto nitro-cellulose filter paper and probed with pG22-69 antibodies raised against barley AR-protein. HRPO anti IgG conjugate was visualized in the presence of 4-chloro-1-naphthol and hydrogen peroxide. The standard molecular weight markers are shown on the left. On the right side of the panel the position of the 34-kDa aldose reductase-related protein is indicated by an arrowhead. Thirty microgram protein was loaded onto each lane. (b) Immunodetection of the AR-protein patterns during germination: Proteins from seeds at different days after germination (DAG) were separated on SDS-PAGE and blotted onto nitrocellulose filter paper and probed with pG22-69 antibodies for barley AR-protein. Lanes: Seed; 1, 1DAG; 2, 2DAG; 3, 3DAG; 4, 4DAG; 5, 5DAG; 6, 6 DAG. The standard molecular weight markers are shown on the left. The arrowhead points to the position of the 34-kDa aldose reductase-related protein. Thirty micro-gram protein was loaded onto each lane.
3.3. Induction of AR protein in rice shoots by ABA and other abiotic stresses
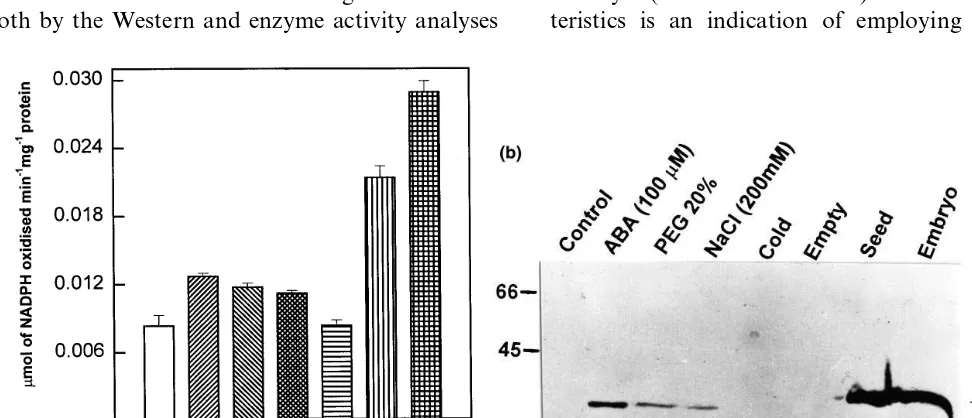
The effects of abiotic stress on AR protein and enzyme levels were investigated. Activity assays revealed a significant increase in AR activity in shoots exposed to ABA, PEG and NaCl treat-ments (Fig. 3a). The increase was maximum in response to ABA (53% increase) followed by PEG (40%) and NaCl (35%).
Western analysis revealed that this protein was induced in shoots of K39 seedlings treated with 100 mM of ABA, water stress (PEG 20%) and salinity (200 mM) stress (Fig. 3b). The protein fraction utilized for the immunoblotting and en-zyme analysis was the same. Moreover, quantita-tive induction of this protein in response to ABA and abiotic stresses was nearly proportional to the increase in the enzyme activity. However, there was no change in AR levels under cold stress (Fig. 3a, b).
AR protein and enzyme activity in seeds and embryos are relatively higher than that in stressed vegetative tissue (Fig. 3a, b). Further, embryo showed significantly more AR enzyme activity (35% more) than seed.
3.4. Varietal differences in the ABA-inducibility of AR-related protein
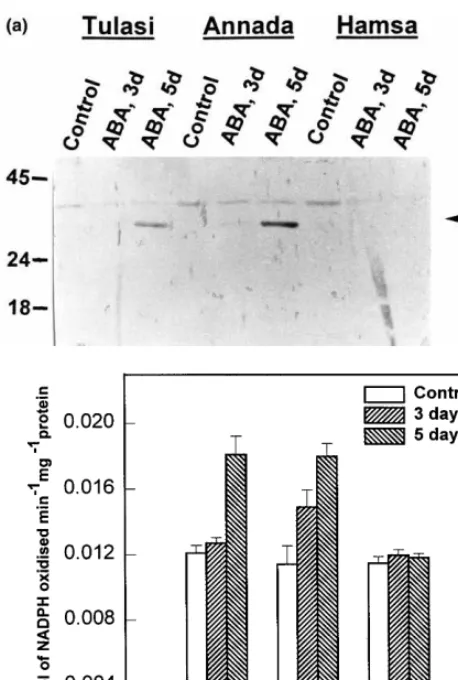
There were clear differences in the ABA induci-bility of AR-related protein among the rice vari-eties deploying different levels of drought tolerance. Application of 100 mM of ABA was found to induce this protein in drought tolerant varieties, Annada and Tulasi, but not in the sensi-tive cultivar, Hamsa, as revealed by Western data (Fig. 4a) and enzyme activity analysis (Fig. 4b). Interestingly, while Annada showed a detectable increase in enzyme levels 3 days after ABA treat-ment, Tulasi was conspicuously lacking such an increase. However, at 5 days after ABA treatment, both Annada and Tulasi showed 58 and 49% increases in enzyme levels, respectively (Fig. 4b).
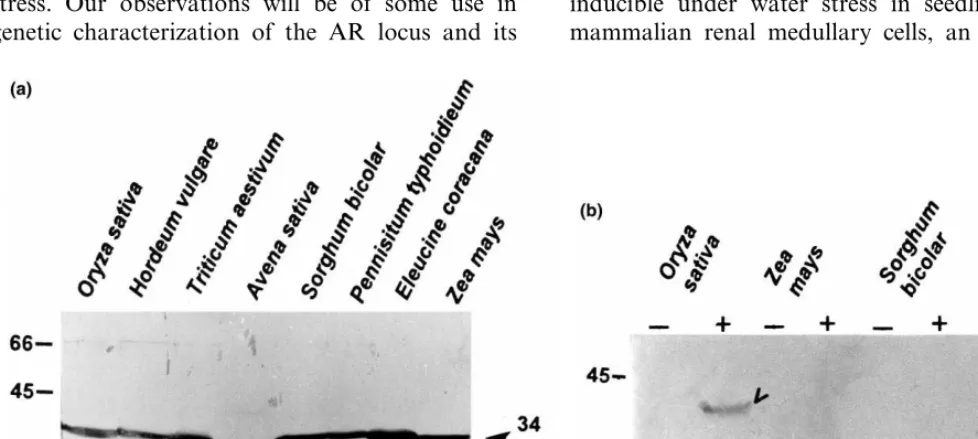
3.5. AR accumulation is e6olutionarily conser6ed
In view of the relevance of AR to biosynthesis of sorbitol, which has been reported in many higher plants [13], we have investigated the exis-tence of the AR-related protein in mature seeds of other cereals. SDS gels run from crude protein ceeds reaching the maximum at maturation (Fig.
extracts of mature seeds of several cereals such as rice, barley, Sorghum, Eleucine, pearl millet, oat, wheat and maize were blotted and probed with the same Anti-AR antibodies. A single protein of molecular weight of 34-kDa was detected in ma-ture seeds of all the tested members (Fig. 5a). Interestingly, this protein in rice, barley,Sorghum, Eleucine, pearl millet, maize and wheat appears to be of similar apparent molecular weight. However in oats, AR-related protein seems to be slightly smaller than the others. Significantly this protein was not detectable in the leaf tissue of any of these plants. Interestingly, AR protein is detected in ABA treated leaf tissues of rice seedlings but not in other cereals (Fig. 5b).
4. Discussion
Our results highlight the identification of AR in mature seeds of rice and other members of Gramineae. Further, the results demonstrate that the aldose reductase accumulation in rice is stress responsive and varietal specific. The AR begins to accumulate from 15 DAP and reaches maximum accumulation at mature seed stage as revealed both by the Western and enzyme activity analyses
(Fig. 2a and Fig. 3a, b). Such a developmental pattern of aldose reductase accumulation closely resembles that of endogenous ABA levels in ma-turing seeds of rice [22]. Further, the developmen-tal pattern of AR accumulation during late embryogenesis in rice resembles that of LEA proteins which are widely studied and implicated in desiccation tolerance in late embryogenesis in a variety of plant species [20,21]. LEA proteins have been implicated in conferring protection to cellular organelles through different ways based on their conserved domains [23]. LEA proteins are pre-dicted to have an enhanced water binding capac-ity, act like molecular chaperones, and some are predicted to play a role in the sequestration of ions that are concentrated during cellular dehydration [20,24]. In wheat seedlings during dehydration, a correlation was reported between organ survival and group 3 LEA protein accumulation [25].
Our studies on AR accumulation in late em-bryogenesis further support the probable utility of proteins with LEA characteristics in conferring stress tolerance during this desiccation phase. Par-ticularly, the identification of a stress/ABA re-sponsive enzyme involved in biosynthesis of an osmolyte (in this case sorbitol) with LEA charac-teristics is an indication of employing the
Fig. 4. (a) Western blot showing the differential accumulation of AR- related protein in rice cultivars by ABA application: Proteins from different rice cultivars treated with ABA were separated on SDS-PAGE and blotted onto nitrocellulose filter paper and probed with pG22-69 antibodies. The standard molecular weight markers are shown on the left. The arrow-head points to the position of the 34-kDa aldose reductase-re-lated protein. Thirty microgram protein was loaded onto each lane. (b) ABA-induced changes in aldose reductase activity in seedlings of different cultivars. Rice seedlings were treated with ABA (100mM) for 3 days and 5 days. The enzymatic activity represented inmmoles of NADPH oxidized per min per mg of protein. Each value represents mean9S.D of three replicates.
rice, and its possible role in desiccation tolerance processes. AR association in rice with the desicca-tion phase of seed maturadesicca-tion and its disappear-ance on germination is similar to the pattern of AR transcript accumulation and decline during seed maturation and germination respectively, in barley and wild oats. [2,6].
4.1. AR is responsi6e to exogenous ABA and
other abiotic stresses
AR protein is induced in the shoots of some rice genotypes by the exogenous application of ABA, water stress and salinity, but not by cold stress. Western data are in agreement with increased enzyme activity levels. The increase was maximum in response to ABA (53% increase) followed by PEG (40% increase) and NaCl (35% increase). However, under cold acclimation, there was no significant increase either in protein or enzyme activity levels leading to the conclusion that AR is primarily responsive to osmotic stress. The pres-ence of substantial AR enzyme activity but not immunodetectable AR protein (Fig. 3a as com-pared to Fig. 3b and Fig. 4a as comcom-pared to Fig. 4b) indicates the presence of other enzymes (proteins) having AR activity that are not recog-nized by the AR antibodies. Furthermore, both seeds and embryos showed higher levels of enzyme activity and protein levels (Fig. 3a, b) than vegeta-tive tissue. Among all the tested tissues, embryos accumulate highest levels of AR protein and en-zyme activity suggesting that AR is primarily syn-thesized in embryos under normal conditions and induced in other tissues under stress conditions. As AR protein accumulation in vegetative tis-sues is primarily associated with osmotic stress, it is not clear whether increased endogenous ABA is the cause for such an accumulation. Bartels et al. [2] showed that AR accumulation in desiccated leaves of Craterostigma, providing further evi-dence that it might play a role in osmoprotection. It is known that a majority of the LEA proteins from various crop plants are induced in response to ABA and abiotic stress conditions in vegetative tissues [20,21]. In bromegrass cell suspensions, ABA treatment induced significant levels of an AR mRNA and enzyme activity levels [14]. In wild oats, water stress leads to the induction of the AR transcript [6].
4.2. AR accumulation in 6egetati6e tissue under
stress is genotype dependent
An important observation in the present study is the absence of AR in ABA-treated shoots of a drought sensitive cultivar, Hamsa (Fig. 4a, b). All the other tested cultivars, which are relatively drought tolerant, accumulate this protein under stress. This could be due to the genetic back-ground differences that exist between the varieties. We propose that AR protein in rice is associated with desiccation tolerance processes both in seedlings as well as embryos. It is tempting to speculate that Hamsa plants are genetically defi-cient in a regulatory element that governs the expression of AR, particularly in vegetative tissues under stress or in response to ABA. It is to be noted that Hamsa seeds do accumulate AR as a normal developmental cue. Numerous earlier re-ports showed that stress induced proteins accumu-late both in sensitive and tolerant cultivars, though there are quantitative differences [29,30]. Our re-sults, however, clearly show that the tolerant lines selectively accumulate aldose reductase under stress. Our observations will be of some use in genetic characterization of the AR locus and its
role in drought tolerance in rice. However, further studies with AR are needed to unequivocally asso-ciate AR with stress tolerance processes and use it as a potential marker for the stress tolerance phenotype.
4.3. Occurrence in Gramineae members
Our results clearly demonstrate the occurrence of AR protein in mature seeds of other cereals, such as maize, barley, wheat, Sorghum, Eleucine, pearl millet and oats. With the exception of oats, all cereals tested show AR of similar apparent molecular weight. Li and Folay [6] reported that the AR clone (pAF30) from wild oats showed 90% homology in predicted amino acid sequence with respect to the putative AR gene from barley [2] and bromegrass suspension cells [14].
The stress responsive AR induction in vegetative tissues reveals that there are significant differences between cereals. Of all the cereals tested, only rice shows the induction of AR under ABA, water stress and salinity. In wild oats the AR gene is found to be ABA responsive in embryos and is inducible under water stress in seedlings [6]. In mammalian renal medullary cells, an increase in
AR activity was detected under salinity stress [1,11]. In response to osmotic stress, renal cells accumulate the osmolyte sorbitiol through in-creased transcription of the AR gene and its os-motic response element (ORE) has been identified [31]. These results further suggest that the os-moregulatory processes were evolutionarily conserved.
The accumulation of different osmolytes in cy-toplasm in response to various abiotic stress con-ditions is a well-established phenomenon in plants [32]. Among the osmolytes, proline, betaine and sugar alcohols have been implicated in a osmoreg-ulatory function in plants and have been thought to be of great adaptive value [33]. The identifica-tion of AR in rice and other cereals assumes importance in view of its role in the synthesis of the sugar alcohol, sorbitol. It plays an important role in osmotic adjustment in apple leaves during water stress [34], was also found in corn [35], Rosaceae members [36,37] and soybean [38]. Salt tolerant Plantago species accumulate significant levels of sorbitol [39]. In apples, sorbitol biosyn-thesis during water stress is mediated by an en-zyme aldose-6-phosphate reductase [34].
The transgenic approach seems to be the pre-ferred one for the elucidation of the role of AR in stress response pathways. The over production of osmolytes, proline, mannitol and ononitol in cyto-plasm of transgenic tobacco plants was proved to confer stress tolerance [40 – 42]. With the availabil-ity of reproducible and routine rice transformation techniques, it would soon be possible to test the role of AR in stress tolerance. The elaboration of the genetic and biochemical basis of osmolyte pathways, including the polyol pathway and the characterization of the regulatory elements that govern the expression of AR would help in the genetic improvement of the stress response in rice.
Acknowledgements
This work has been partly supported by the Grants from the Rockefeller Foundation Interna-tional Rice Biotechnology Project and European Economic Commission project to ARR. BKS is thankful to University Grants Commission and Council of Scientific and Industrial Research (CSIR-RA) for the Research fellowships. CRK is thankful to CSIR for financial support. The
au-thors specifically thank Dr Dorothea Bartels, MPI, Koln, Germany for the gift of anti pG22-69 antibodies.
References
[1] A. Garcia-Perez, B. Martin, H.R. Murphy, S. Uchida, H. Murer, B.D. Cowley, J.S. Handler, M.B. Burg, Molecular cloning of cDNA coding for kidney aldose reductase, J. Biol. Chem. 264 (1989) 16815 – 16821. [2] D. Bartels, K. Engelhardt, R. Roncarati, K. Schneider,
M. Rotter, F. Salamini, An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein, EMBO J. 10 (1991) 1037 – 1043.
[3] S. Colrat, A. Latche, M. Guis, J-C. Pech, M. Bouzayen, J. Fallot, J-P. Roustan, Purification and characterization of a NADPH-dependent aldehyde reductase from mung bean that detoxifies eutypine, a toxin fromEutypa lata, Plant Physiol. 119 (1999) 621 – 626.
[4] C. Nishimura, Y. Matsuura, Y. Kokai, T. Akera, D. Carper, N. Morjana, C. Lyons, T.G. Flynn, Cloning and expression of human aldose reductase, J. Biol. Chem. 265 (1990) 9788 – 9792.
[5] F.B. Negm, Purification and properties of an NADPH-aldose reductase (aldehyde reductase) from Euonymus japonicaleaves, Plant Physiol. 80 (1986) 972 – 977. [6] B. Li, M.E. Foley, Cloning and characterization of
differentially expressed genes in imbibed dormant and afterripened A6ena fatuaembryos, Plant Mol. Biol. 29
(1995) 823 – 831.
[7] R.B. Meeley, G.S. Johal, S.P. Briggs, J.D. Walton, A biochemical phenotype for a disease resistance gene of maize, Plant Cell 4 (1992) 71 – 77.
[8] C.J. Winters, D.T. Molowa, P.S. Guzelian, Isolation and characterization of cloned cDNAs encoding human liver chlordecone reductase, Biochemistry 29 (1990) 1080 – 1087.
[9] N.A. Kolb, L.A. Hunsaker, D.L. Vander Jagt, Aldose reductase catalyzed reduction of acrolein: implication in cyclophasphamide toxicity, Mol. Pharmocol. 45 (1994) 797 – 801.
[10] J.D. Hayes, D.J. Judah, G.E. Neal, Resistance to afla-toxin B1 is associated with the expression of a novel
aldo – keto reductase which has a catalytic activity to-wards a cytotoxic aldehyde containing metabolite of the toxin, Cancer Res. 53 (1993) 3887 – 3894.
[11] T. Moriyama, A. Garcia-Perez, M.B. Burg, Osmotic regulation of aldose reductase protein synthesis in renal medullary cells, J. Biol. Chem. 264 (1989) 16810 – 16814. [12] J. Jeffery, H. Jornvall, Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phos-phates in glucose metabolism, Proc. Natl. Acad. Sci. 80 (1983) 901 – 905.
[14] S.P. Lee, T.H.H. Chen, Expression of an aldose reduc-tase-related gene during the induction of freezing toler-ance in bromegrass cell suspension cultures, J. Plant Physiol. 142 (1993) 749 – 753.
[15] A.H. Rao, B. Karuna Sree, A.R. Reddy, Water stress-re-sponsive 23-kDa polypeptide from rice seedlings is boil-ing stable and is related toRAB16 family of proteins, J. Plant. Physiol. 142 (1993) 83 – 93.
[16] M.M. Bradford, A rapid and sensitive method for the quantification of microgram quantities of protein utiliz-ing the principle of protein-dye bindutiliz-ing kit, Anal. Biochem. 72 (1976) 248 – 254.
[17] U.K. Laemmli; Cleavege of structural proteins during the assembly head of the bacteriophage of T4, Nature, 227, (1970), 680-685.
[18] H. Towbin, T. Staehelin, T. Gordon, Electrophoretic transfer of proteins from polyacrylamide gels to nitrocel-lulose sheets: procedure and some applications, Proc. Natl. Acad. Sci. 76 (1979) 4350 – 4354.
[19] W. Gross, P. Seipold, C. Schnarrenberger, Characteriza-tion and purificaCharacteriza-tion of an aldose reductase from the acidophilic and thermophilic red alga Galdieria sul
-phuraria, Plant Physiol. 114 (1997) 231 – 236.
[20] J. Baker, C. Steele, L. DureIII, Sequence and character-ization of six Lea proteins and their genes from the cotton, Plant Mol. Biol. 11 (1988) 277 – 291.
[21] T.J. Close, A.A. Kortt, P.M. Chandler, A cDNA based comparison of dehydration induced proteins (dehydrins) in barley and corn, Plant Mol. Biol. 13 (1989) 95 – 108. [22] K. Skriver, J. Mundy, Gene expression in response to abscisic acid and osmotic stress, Plant Cell 2 (1990) 503 – 512.
[23] L. Dure III, M. Crouch, J. Harada, T-H.D. Ho, J. Mundy, R.S. Quatrano, T. Thomas, Z.R. Sung, Com-mon amino acid sequence domains aCom-mong the LEA proteins of higher plants, Plant Mol. Biol. 12 (1989) 475 – 486.
[24] J. Ingram, D. Bartels, The molecular basis for dehydra-tion tolerance in plants, Annu. Rev. Plant Physiol. and Plant Mol. Biol. 47 (1996) 377 – 403.
[25] J.L. Reid, M.K. Walker-Simmons, Group 3 Late em-bryogenesis abundant proteins in desiccation-tolerant seedlings of wheat (Triticum aesti6umL.), Plant Physiol.
102 (1993) 125 – 131.
[26] J.P. Hendrick, F-U. Hartl, Molecular chaperone func-tions of heat shock proteins, Annu. Rev. Biochem. 62 (1993) 349 – 384.
[27] D. Bartels, M. Singh, F. Salamini, Onset of desiccation tolerance during development of the barley embryo, Planta 175 (1988) 485 – 492.
[28] R. Roncarati, F. Salamini, D. Bartels, An aldose reduc-tase homologous gene from barley: regulation and
func-tion, Plant J. 7 (1995) 809 – 822.
[29] A. Moons, G. Bauw, E. Prinsen, M. Van Montagu, D. Van Der Straeten, Molecular and physiological re-sponses to abscisic acid and salts in roots of salt-sensi-tive and salt-tolerant indica rice varieties, Plant Physiol. 107 (1995) 177 – 186.
[30] F. Cellier, G. Conejero, J-C. Breitler, F. Casse, Molecu-lar and physiological responses to water deficit in drought-tolerant and drought-sensitive lines of sunflower, Plant Physiol. 116 (1998) 319 – 328.
[31] J.D. Ferraris, C.K. Williams, A. Ohtaka, A. Garcia-Perez, Functional consensus for mammalian osmotic response elements, Am. J. Physiol. 276 (1999) 667 – 673. [32] A.J. Delauney, D.P.S. Verma, Proline biosynthesis and
osmoregulation in plants, Plant J. 4 (1993) 215 – 223. [33] L. Csonka, Physiological and genetic responses of
bacte-ria to osmotic stress, Microbiol. Rev. 53 (1989) 121 – 147. [34] Z. Wang, B. Quebedeaux, G.W. Stutte, Partitioning of [14C] glucose into sorbitol and other carbohydrates in apple under water stress, Aust. J. Plant Physiol. 23 (1996) 245 – 251.
[35] J.R. Shaw, D.B. Dickinson, Studies of sugars and sor-bitol in developing corn kernels, Plant Physiol. 75 (1984) 207 – 211.
[36] R.L. Bieleski, R.J. Redgwell, Sorbitol metabolism in nectaries from flowers of Rosaceae, Aust. J. Plant Phys-iol. 7 (1980) 15 – 25.
[37] T.G. Ranney, N.L. Bassuk, T.H. Whitlow, Osmotic adjustment and solute constituents in leaves and roots of water stressed cherry (Prunus) trees, Journal of the American Society for Horticultural Science 116 (1991) 684 – 688.
[38] T.M. Kuo, D.C. Doehlert, C.G. Crawford, Sugar metabolism in germinating soybean seeds, Plant Physiol. 93 (1990) 1514 – 1520.
[39] M. Breins, F. Larher, Sorbitol accumulation inPlantag
-inaceae; further evidence for a function in stress toler-ance, Zeitschrift fur Pflanzenphysiologie 110 (1983) 447 – 458.
[40] P.B. Kavi Kishore, Z. Hong, G-H Miao, C-A.A. Hu, D.P.S. Verma, Overexpression of D1
-Pyrroline-5-car-boxylate synthetase increases proline production and confers osmotolerance in transgenic plants, Plant Phys-iol. 108 (1995) 1387 – 1394.
[41] M.C. Tarczynski, R.G. Jensen, H.J. Bohnert, Stress protection in transgenic tobacco producing a putative osmoprotectant mannitol, Science 259 (1993) 508 – 510. [42] E. Sheveleva, W. Chmara, H.J. Bohnert, R.G. Jensen,
Increased salt and drought tolerance byD-ononitol pro-duction in transgenic Nicotiana tabacumL, Plant Phys-iol. 115 (1997) 1211 – 1219.