www.elsevier.com / locate / bres
Interactive report
Sodium channels and their genes: dynamic expression in the normal
1nervous system, dysregulation in disease states
a,b ,
*
a,b a,b a,bStephen G. Waxman
, Sulayman Dib-Hajj
, Theodore R. Cummins
, Joel A. Black
a
Department of Neurology and PVA /EPVA Neuroscience Research Center, Yale School of Medicine, 333 Cedar Street, New Haven, CT 06510, USA
b
Rehabilitation Research Center, VA Connecticut, West Haven, CT 06516, USA
Accepted 3 August 2000
Abstract
Although classical neurophysiological doctrine rested on the concept of the sodium channel, it is now clear that there are nearly a dozen sodium channel genes, each encoding a molecularly distinct channel. Different repertoires of channels endow different types of neurons with distinct transduction and encoding properties. Sodium channel expression is highly dynamic, exhibiting plasticity at both the transcriptional and post-transcriptional levels. In some types of neurons within the normal nervous system, e.g. hypothalamic magnocellular neurosecretory neurons, changes in sodium channel gene expression occur in association with the transition from a quiescent to a bursting state; these changes are accompanied by the insertion of a different set of sodium channel subtypes in the cell membrane, a form of molecular plasticity which results in altered electrogenic properties. Dysregulation of sodium channel genes has been observed in a number of disease states. For example, transection of the peripheral axons of spinal sensory neurons triggers down-regulation of some sodium channel genes, and up-regulation of other sodium channel genes; the resultant changes in sodium channel expression contribute to hyperexcitability that can lead to chronic pain. There is also evidence, in experimental models of demyelination and in post-mortem tissue from patients with multiple sclerosis, for dysregulation of sodium channel gene expression in the cell bodies of some neurons whose axons have been demyelinated, suggesting that an acquired channelopathy may contribute to the pathophysiology of demyelinating diseases such as multiple sclerosis. The dynamic nature of sodium channel gene expression makes it a complex topic for investigation, but it also introduces therapeutic opportunities, since subtype-specific sodium channel modulating drugs may soon be available. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Sodium channels
Keywords: Electrogenesis; Ion channel; Plasticity
1. Introduction sodium channels or directly discern their molecular
struc-ture, they were able to infer some important features of One of the features that makes neurons special is their these channels and to predict, e.g., that they possess ‘gates’ ability to produce regenerative electrical signals, a charac- which opened as the channels were activated by depolar-teristic that rests, in large part, on the presence of voltage- ization, allowing sodium ions to pass through. Nearly forty gated sodium channels within neurons. The seminal studies years later, Numa and his colleagues took another large of Hodgkin and Huxley [27] revealed that, in most step forward with the cloning of the first sodium channel mammalian neurons, ion flux through sodium channels [34].
underlies the regenerative upstroke of the action potential. The early electrophysiological and pharmacological Although Hodgkin and Huxley were not able to visualize experiments provided hints that there might be more than one sodium channel. However, the idea that there are multiple, molecularly distinct sodium channels had its
1
Published on the World Wide Web on 15 August 2000.
formal birth when Numa and his colleagues demonstrated *Corresponding author. Tel.: 11-203-785-6351; fax: 1
1-203-785-that, within the mammalian brain, there were (at least) 7826.
E-mail address: [email protected] (S.G. Waxman). three sodium channel genes, each encoding a different but
related molecule [35] Other genes encoding additional have other properties and other functions. The molecular sodium channels sharing a common overall motif, but with identities of some of these ‘non-conventional’ sodium subtlety different amino acid sequences, were discovered channels have recently been established.
soon thereafter. Some neurons co-express several different One example is provided by sodium channel NaN, sodium channel genes, indicating that their membranes which was cloned and sequenced by Dib-Hajj et al. [17]. contain several types of sodium channels. The expression NaN is preferentially expressed in small DRG neurons. of different repertoires of sodium channels in different The presence of a serine at position 355 in the amino acid types of neurons endows them with different transducing sequence of NaN suggests that NaN is TTX-resistant and encoding properties [49]. We now know that there are, [17,44]. The gene for NaN is localized, together with the in fact, nearly a dozen genes, each encoding a distinct genes encoding two other TTX-resistant sodium channels, sodium channel. Physiological signatures have been estab- SNS and SkM2, within a conserved linkage group at lished for only some of these sodium channels. Nonethe- 3p21–3p24 within human chromosome 3, suggesting that less, the available evidence makes it clear that, from a the three TTX-resistant sodium channels arose by duplica-functional point of view, sodium channels are not all the tion from a common ancestral gene [19].
same: different channels can have different physiological NaN and SNS are the only TTX-resistant sodium characteristics, and can play different roles in the physi- channels that are present within DRG neurons. Thus, when ology of excitable cells. DRG neurons are exposed to TTX and studied by patch Recent research has also made it apparent that the clamp, the NaN and SNS currents can be recorded in expression of sodium channels within neurons is not a isolation. SNS-knockout mutant mice provide a model static or fixed process. While neuronal plasticity has been system in which the SNS current is absent [2]. Using this most extensively studied with respect to synaptic potentia- model, Cummins et al. [13] identified a TTX-resistant tion and depression, sprouting and pruning of neurites, and (Ki539mM) persistent sodium current (Fig. 1A) attribut-the recruitment of pre-existing or new neurons into func- able to NaN. A similar TTX-resistant persistent current, tional circuits, it is now clear that plasticity in expression which appears to be produced by NaN, has been observed of the genes encoding voltage-gated sodium channels can in wild-type mouse and rat DRG neurons [13] and human result in plasticity in the deployment of sodium channels DRG neurons [20]. The NaN current is unique in ex-which, in turn, can produce significant changes in the hibiting a hyperpolarized dependence of activation electroresponsive properties of neurons. These changes (threshold(270 mV; midpoint of activation5241 mV in may be especially important in disease states characterized mouse DRG neurons) and steady-state inactivation by hypo- or hyper-excitability, or where altered impulse (midpoint5244 mV), with a substantial overlap between trafficking contributes to pathophysiology.
Gene expression is controlled at the transcriptional (DNA→mRNA), translational (mRNA→protein), and post-translational (protein→modified protein) levels. While regulation at all of these levels is important, transcriptional regulation plays a particularly important role in controlling the molecular architecture of cells. This article will first provide examples of some of the diverse physiological functions that are subserved by the distinct sodium chan-nels that are encoded by different genes. It will then discuss dynamic aspects of sodium channel gene expres-sion in normal neurons, and dysregulated expresexpres-sion of sodium channels in injured neurons.
2. Sodium channels can modulate resting potential and can amplify subthreshold inputs
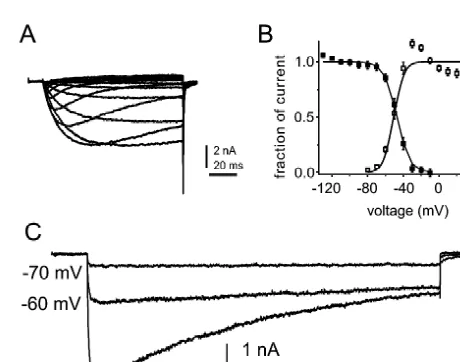
Fig. 1. Persistent TTX-resistant sodium currents are produced by NaN Given that all sodium channels share a common overall channels in small DRG neurons. (A) Representative TTX-resistant sodium structural motif [10], why should the expression of one currents recorded from a DRG neuron from a SNS-null mouse with 100 type of channel, rather than another, have functional ms test pulses. (B) Activation (unfilled squares) and steady-state inactiva-tion (filled squares) curves for the NaN current show significant overlap. significance? Although the concept of the ‘fast’,
rapidly-Steady-state inactivation was measured with 500 ms prepulses. (C) NaN activating and inactivating sodium channel as a mediator
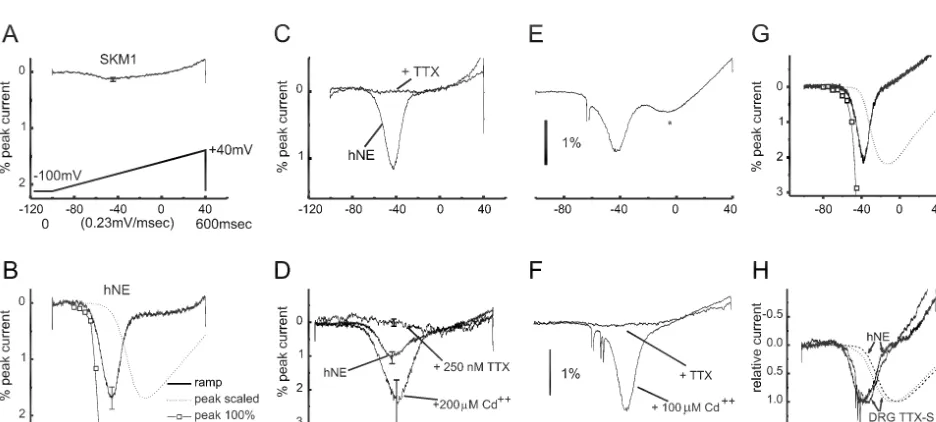
activation and steady-state inactivation curves (Fig. 1B) slow, small depolarizations (e.g. a ramp at 0.23 mV/ ms), which extends from 270 to 230 mV [13]. Because the the SkM1 channels do not generate a response (Fig. 2A). resting potential of small DRG neurons is close to255 mV PN1 / hNE channels display slow closed-state inactivation, [9], the properties of NaN suggest that it should generate a up to five-fold slower than SkM1 [12]. As a result of this, ‘window’ current close to resting membrane potential. PN1 / hNE channels activate and generate a sodium current Moreover, the low threshold for activation of NaN chan- in response to small and gradual depolarizations close to nels suggests that they should open in response to small resting potential (Fig. 2B). PN1 / hNE channels also display subthreshold depolarizations close to membrane potential, a unique physiological profile, including block by tet-thus contributing to subthreshold electrogenesis. Computer rodotoxin (Fig. 2C) and enhancement by cadmium (Fig. simulations, in fact, suggest that NaN channels contribute a 2D).
10–15 mV depolarizing influence to resting potential, and Having established the functional signature for PN1 / amplify small depolarizing inputs by more than 50%, in hNE channels using this bottom-up analysis in a hetero-small DRG neurons (Herzog, Cummins and Waxman, logous expression system, the next step was to use this unpublished results). NaN thus appears to regulate the information to determine, in a top-down approach, whether excitability of DRG neurons. these channels display similar properties in their native Another example of a channel that has functions other milieu within DRG neurons [12]. Because small, ramp-like than as a mediator of the action potential upstroke is depolarizations constitute an effective stimulus for the provided by the PN1 sodium channel. Like NaN, PN1 is PN1 / hNE sodium channel in HEK293 cells, these stimuli selectively expressed within dorsal root ganglion (DRG) were applied to intact DRG neurons, and were found to neurons [47]. To establish the physiological role of PN1, evoke depolarizing responses within these cells (Fig. 2E), Cummins et al. [12] used patch-clamp techniques to study similar to those seen in the HEK expression system (Fig. its human ortholog PN1 / hNE expressed in a model 2B). The current evoked by these ramp stimuli within expression system (HEK293 cells), in a ‘bottom-up’ intact DRG neurons displayed responses to TTX and analysis. For comparison, Fig. 2A shows patch-clamp cadmium (Fig. 2F) that are identical to those of PN1 / hNE recordings from muscle (SkM1) sodium channels, ex- channels within the heterologous expression system. The pressed in transfected HEK293 cells. Like most traditional convergence of the bottom-up and top-down analyses sodium channels, the SkM1 channels require sudden, suggests that, in intact DRG neurons, PN1 / hNE channels relatively large depolarizations for activation. These chan- respond to small, slow depolarizations close to resting nels do not open in response to slow depolarizations close potential, activating so as to produce inward (depolarizing) to resting potential. When stimulated, for example, by currents and thereby amplifying inputs such as generator
Fig. 2. ‘Bottom-up’ and ‘top-down’ analyses reveal similar properties of the PN1 / hNE sodium channel in HEK 293 cells and in DRG neurons. (A) SkM1 sodium channels, transfected into HEK293 cells, do not activate in response to slow ramp-like (0.23 mV/ ms) depolarizations. (B) In contrast, these slow ramp stimuli activate PN1 / hNE channels transfected into HEK293 cells, generating distinct inward currents which are evoked close to resting potential.
21
(C) PN1 / hNE currents are blocked by TTX. (D) PN1 / hNE currents are enhanced by Cd . (E) In a ‘top-down’ analysis, nearly identical stimuli yield a similar inward current in DRG neurons. (F) Ramp currents in DRG neurons display a similar pharmacologic profile, being blocked by TTX and enhanced
21
potentials. Consistent with this function, PN1 / hNE chan-nels are localized at the distal ends of neurites of spinal sensory neurons [47]. Given that NaN and PN1 both activate at voltages close to resting potential, it is possible that they cross-activate each other; cross-activation be-tween these two types of channels, however, remains speculative.
3. Sodium channel gene expression: a dynamic process in normal neurons
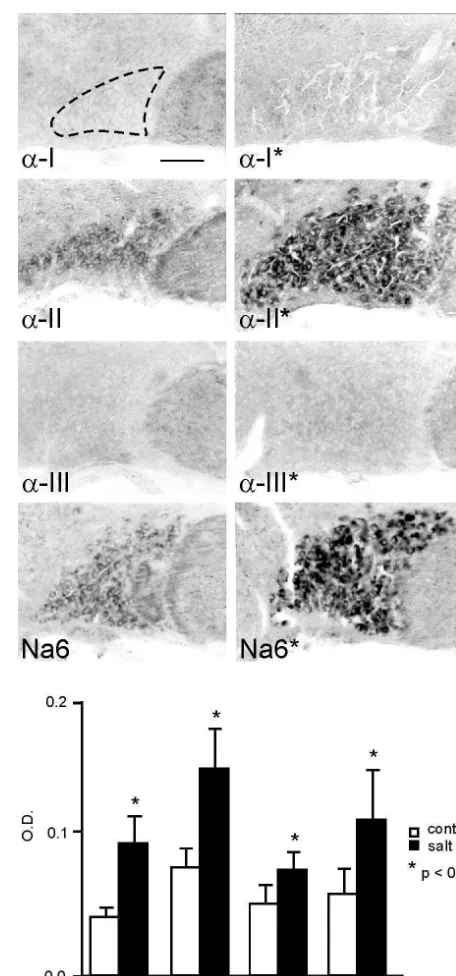
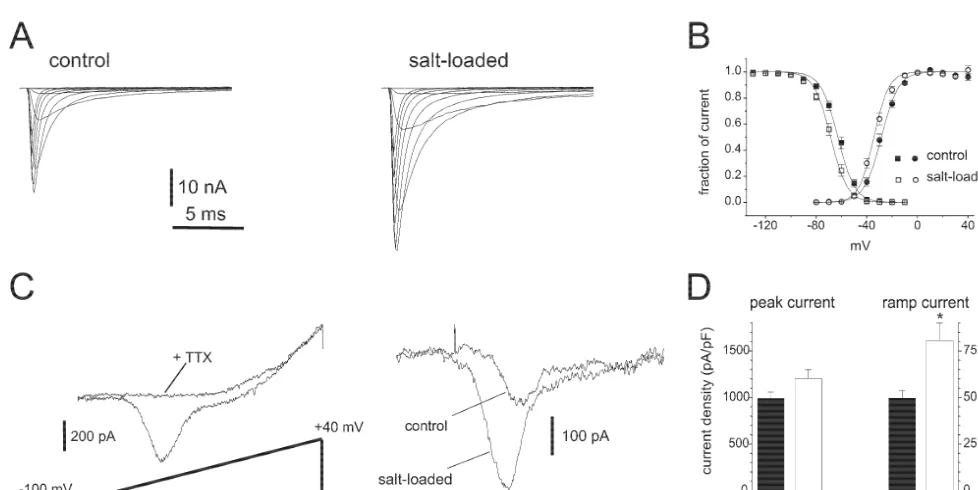
It is almost axiomatic that, as neurons pass from a quiescent state to a high-frequency firing state, they use their (pre-existing) sodium channels differently, i.e. they activate these channels repetitively. But do neurons deploy a different set of sodium channels when they make these state transitions? An example of such a change is provided by the magnocellular neurosecretory cells within the supraoptic nucleus [43]. These specialized neurons send their axons to the neurohypophysis where they release vasopressin, a response that is triggered by increases in plasma osmolality. In their basal state these cells are relatively quiescent, firing irregularly at low frequencies (,3 impulses / s) but, in response to changes in the osmotic milieu, these cells respond by generating high-frequency bursts of action potentials which elicit vasopressin release [31]. To test the hypothesis that the transition from the quiescent to the bursting state involves a change in the expression of sodium channel genes, Tanaka et al. [43] studied neurons under normal conditions and following salt-loading, which triggers a transition to a bursting state. As seen in Fig. 3, in situ hybridization reveals that salt-loading triggers an up-regulation of mRNAs for two sodium channels, a-II and Na6, in these cells.
Ion channel expression within the cell membrane is controlled at both the transcriptional and translational levels. Thus increased mRNA levels are not necessarily accompanied by increased synthesis of channel protein. To determine whether increased levels of sodium channel protein were produced as mRNA levels rose, sodium channel protein expression was measured in magnocellular neurons, using immunocytochemical and immunoblotting methods with an antibody directed against a conserved region of sodium channels. These studies demonstrated a
distinct increase in the level of sodium channel protein Fig. 3. a-II and Na6 sodium channel mRNA are up-regulated, together with sodium channel b1 andb2 mRNA, in supraoptic magnocellular within the magnocellular neurons of salt-loaded rats [43].
neurons following salt-loading. The micrographs, from control (left Sodium channel protein molecules become functional in
column) and salt-loaded (right column) rats, were digitally enhanced to an electrogenic sense when they are inserted (together with show in situ hybridization with subtype-specific riboprobes for Na accessary subunits) in the cell membrane, but they can also channel subunitsa-I,a-II,a-III and Na6.a-I anda-III mRNA are not be sequestered within intracellular pools. Thus, the next detectable, and low levels of a-II and Na6 mRNA are present in the control supraoptic nucleus (no asterisks). Expression of thea-II and Na6 question was whether the changes in sodium channel gene
transcripts is up-regulated following salt-loading (asterisks). Optical transcription are accompanied by the insertion of
these cells should produce two sodium currents. Studies on 4. Sodium channel gene expression: down-regulation
other neuronal cell types, such as Purkinje cells, indicated in axotomized neurons
that the Na6 sodium channel can produce a slow or
persistent TTX-sensitive sodium current [37,48]. Thea-II The dynamic nature of sodium channel gene expression channel, in contrast, has been shown to produce a fast in normal neurons raises the question: is sodium channel transient current [3,35]. Using patch clamp methods, gene expression altered in injured neurons? A hint that Tanaka et al. [43] showed that, consistent with the sodium channel expression might change in injured neu-expression of these two types of sodium channels, two rons was provided by early microelectrode observations of distinct sodium currents are present in control magnocellu- altered somato-dendritic excitability in axotomized motor lar neurons: (i) a fast transient sodium current which neurons [22,30]. Early pharmacological and ion-substitu-would be expected to contribute to the rapid depolarizing tion experiments suggested that this involves a sodium upstroke of the action potential; and (ii) a persistent conductance [41,44], but these studies did not examine the ‘threshold’ sodium current which activates closer to resting roles of sodium channels in this process, or the expression potential. Both currents were increased in magnocellular of sodium channel genes.
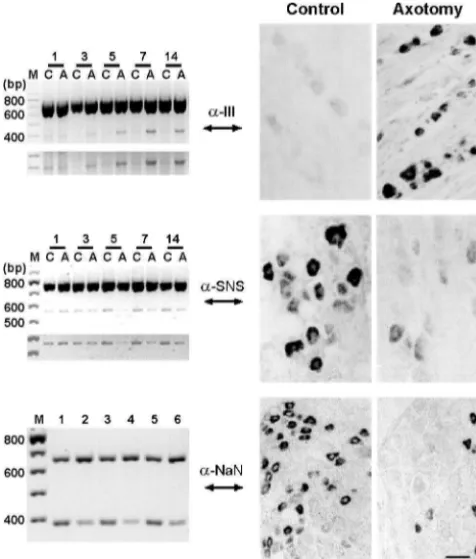
neurons from salt-loaded rats, but to different degrees. More recent studies on this question have used molecu-There was an increase of 20% in the density of the fast lar biological methods to study sodium channel gene transient sodium current, while the density of the threshold expression together with patch clamp methods to study the current was approximately 60% larger in salt-loaded rats currents produced by these channels. Dorsal root ganglion (Fig. 4). The disproportional increases in the two currents (DRG) neurons have been especially well-studied in this encoded by these two channels would be expected to lower respect. These studies have shown that, in these cells, the threshold for action potential generation. Thus, molecu- expression of some sodium channel genes is up-regulated, lar changes at the sodium channel gene transcription level and expression of others is down-regulated, following produce functional changes in electrogenesis in these axonal transection (Fig. 5).
neurons as they move from a quiescent to a bursting state. As illustrated in Fig. 5 (middle and bottom rows),
Fig. 6. In parallel with down-regulation of SNS and NaN channels, slow and persistent TTX-resistant sodium currents in small DRG neurons are down-regulated following axonal transection within the sciatic nerve. (A) patch clamp recordings showing SNS (left) and NaN (right) currents from representative control DRG neurons. (B) SNS and NaN currents are reduced in axotomized DRG neurons (B, 6 days post-axotomy).
Fig. 5. Sodium channel expression can change strikingly in neurons following injury. mRNA for sodium channelsa-III (top) is up-regulated,
and mRNA for SNS (middle) and NaN (bottom) are down-regulated, in vivo delivery of NGF [18] and GDNF (Cummins, Black, DRG neurons following transection of their axons within the sciatic
Dib-Hajj and Waxman, unpublished results) to axotomized nerve. The in situ hybridizations (right side) showa-III, SNS, and NaN
DRG neurons can rescue the expression of SNS and NaN mRNA in control DRG, and at 5–7 days post-axotomy. RT-PCR (left
in these cells. side) shows products of co-amplification ofa-III and SNS together with
b-actin transcripts in control (C) and axotomized (A) DRG (days post- In parallel with the down-regulation of SNS and NaN axotomy indicated above gels), with computer-enhanced images of mRNA and protein in DRG neurons following peripheral amplification products shown below gels. Co-amplification of NaN (392
axotomy, the slowly-inactivating and persistent TTX-resis-bp) and GAPDH (6076 TTX-resis-bp) shows decreased expression of NaN mRNA
tant sodium currents produced by these channels are at 7 days post-axotomy (lanes 2, 4, 6) compared to controls (lanes 1, 3,
reduced [11,42] (Fig. 6A, B). These electrophysiological 5). Top, middle panels modified from Dib-Hajj et al [15]. Bottom
modified from Dib-Hajj et al [17]. changes persist for at least 60 days post-axotomy [11]. As noted above, the persistent current produced by NaN appears to contribute a depolarizing influence to resting following transection of the peripheral axons of DRG potential (Herzog, Cummins and Waxman, unpublished neurons (by ligation within the sciatic nerve), expression results). Cummins and Waxman [11] suggested that down-of the SNS [15] and NaN [17] sodium channels is down- regulation of NaN channels shifts membrane resting regulated at the transcriptional level. The reductions in potential in axotomized DRG neurons in a hyperpolarizing SNS and NaN mRNA are accompanied by reduced levels direction, reducing resting inactivation of fast sodium of SNS and NaN sodium channel protein [42]. This change channels, and thereby producing hyperexcitability which in sodium channel gene expression is not a response to can contribute to pain and parasthesia [11]. Consistent with axotomy per se. In contrast to transection of the peripheral a contribution of these changes to neuropathic pain, Dib-axons of DRG neurons, which triggers a down-regulation Hajj et al. [21] observed down-regulation in SNS and NaN of SNS and NaN, transection of the centrally-directed channels and their currents in DRG neurons in the chronic branches of DRG neurons (within dorsal roots) does not constriction injury model of neuropathic pain.
alter the expression of these channels [42]. One explana-tion of this might be that down-regulaexplana-tion of SNS and NaN
is due to loss of access to a peripheral pool of trophic 5. Sodium channel gene expression: up-regulation in
factors. Consistent with this hypothesis, nerve growth axotomized neurons
factor (NGF) up-regulates SNS expression [4], while glial
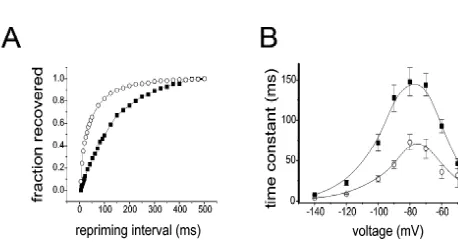
there are changes in excitability of motor neurons. Sub- in axotomized DRG neurons, of a TTX-sensitive sodium sequent studies established that this abnormal excitability current that recovers (reprimes) rapidly from inactivation is sodium-dependent [41,44]. Abnormal accumulations of [11]. Recovery from inactivation is accelerated by as much sodium channels were visualized in early immunohistoch- as four-fold in the axotomized neurons. Recordings from emical studies at injured axonal tips within experimental many neurons indicate that the change to a rapidly-reprim-neuromas, but these studies used generic antibodies that do ing current is a discrete, step-like transition, and not a not discriminate between different types of sodium chan- gradual shift as might be expected if a population of nels [14,24,25]. Delineation of the mechanism underlying pre-existing channels had been modulated. Thus axotomy aberrant sodium channel expression, and determination of appears to trigger a transition from expression of a slowly whether abnormal types of sodium channels are produced repriming TTX-sensitive sodium channel, to expression of in injured neurons, however, required the study of sodium a rapidly-repriming sodium channel. It has been suggested channel gene expression. The first studies were carried out that this contributes to hyperexcitability of these injured by Waxman et al. [50] and Dib-Hajj et al. [15] who used in neurons [11].
situ hybridization and RT-PCR to demonstrate that there is It has been proposed that type III channels produce the a an up-regulation of the previously silent a-III sodium rapidly-repriming TTX-sensitive current in axotomized channel gene in adult DRG neurons following transection neurons [11]. Several observations support this conclusion: of their axons within the sciatic nerve (Fig. 5, top). First, rapidly-repriming TTX-sensitive sodium current and Iwahashi et al. [28] observed a similar up-regulation of the expression of type III sodium channels show parallel a-III sodium channel gene following axotomy of adult patterns of up-regulation following the transection of facial motor neurons. These changes are not due to a peripherally-directed (sciatic nerve) axons of DRG neurons global increase in channel protein synthesis because, as but not following transection of the centrally-directed described above, other sodium channel genes are down- (dorsal root) axons of these cells [5]. Second, type III regulated in these axotomized neurons. Black et al. [5] sodium channels display rapid repriming when expressed have demonstrated that increased synthesis of type III in mammalian expression systems (HEK293 cells; Cum-sodium channel protein accompanies the up-regulation of mins, Dib-Hajj, and Waxman, unpublished observations). type III mRNA in axotomized DRG neurons. Third, abnormal accumulations of type III sodium channel In tandem with activation of the type III sodium channel proteins can be detected by immunocytochemistry with gene in axotomized DRG neurons, there is a switch in the subtype-specific antibodies, close to the tips of injured sodium currents that are expressed by these cells (Fig. 7A, axons within experimental neuromas [5], a site where B). Patch clamp studies have demonstrated the emergence, abnormal hyperexcitability has been demonstrated [8,33,39]. Axotomized DRG neurons provide an example of up-regulation of a sodium channel gene following axonal injury, and this change in gene expression appears to be maladaptive, contributing to the development of hyperexcitability.
6. Sodium channel gene expression: up-regulation in demyelinated neurons
While multiple sclerosis has classically been viewed as a ‘demyelinating’ disease in which myelin is the primary target, there is recent evidence for abnormal expression of Fig. 7. A rapidly repriming TTX-sensitive sodium current emerges in
sodium channel genes in several experimental models of DRG neurons following axonal injury. (A) The graph displays the time
demyelinating diseases, and in multiple sclerosis. Studies course for recovery from inactivation at280 mV for the peak currents,
for control (filled circles) and axotomized (open circles, 7 days post- in our laboratory have demonstrated abnormal up-regula-axotomy). Recovery is much faster for the currents in the axotomized tion of Sensory Neuron Specific sodium channel SNS neuron. Single exponential functions fitted to the data gave time constants within cerebellar Purkinje cells in these disorders [6,7]. of 160 ms for the control neuron and 41 ms for the axotomized neuron.
SNS is normally expressed in a highly specific manner (B) Time constants for recovery from inactivation for control neurons
within primary sensory neurons within DRG and trigemi-(closed squares) and peripherally axotomized neurons (open circles),
plotted as a function of voltage. Cells were prepulsed to220 mV for 20 nal ganglion, and is not normally expressed within the ms to inactivate all of the current, then brought back to the indicated brain [1,38]. This sensory neuron specific TTX-resistant recovery voltage for increasing recovery durations prior to a test pulse to sodium channel exhibits a depolarized voltage-dependence
220 mV. Time constants were estimated from single exponential fits.
and slow activation and inactivation kinetics [1,38], to-Repriming is significantly accelerated at all voltages between260 and
gether with a more rapid recovery from inactivation
2100 mV. Modified from Cummins and Waxman [11] and Black et al.
chan-nels. Because of the distinct electrophysiological charac- manner, the mis-expression of SNS sodium channels teristics of SNS-type channels, the presence of these within Purkinje cells in demyelinating diseases may alter channels can alter the firing properties of neurons [2,40]. the firing patterns of these neurons, thereby contributing to We first studied the taiep rat, a mutant model in which clinical abnormalities such as ataxia in these disorders [6]. myelin is initially formed normally, but then lost as a result The hypothesis that multiple sclerosis and related diseases of an oligodendrocyte abnormality, using in situ hybridiza- include an ‘acquired channelopathy’ has important func-tion and immunocytochemistry with subtype-specific so- tional and clinical implications.
dium channel antibodies. These studies demonstrated the abnormal expression of SNS sodium channel mRNA and protein in Purkinje cells after they have lost myelin (but
not prior to loss of myelin) in this model of demyelination 7. Dynamic aspects of sodium channel expression:
[7]. More recently, we have demonstrated that SNS mRNA conclusions and prospects
and protein, which are not present within the cerebellum of
control mice, are expressed within cerebellar Purkinje cells As outlined above, it is now clear that sodium channels in a mouse model of multiple sclerosis, chronic relapsing represent a diverse family of related proteins, encoded by experimental allergic encephalomyelitis [6]. We have also different genes, and with different physiological properties demonstrated the expression of SNS mRNA (Fig. 8a) and and functions. It has also become clear that the activation protein (Fig. 8d) within cerebellar Purkinje cells from of sodium channel genes is not a fixed, static process. On tissue obtained post-mortem from MS patients, but not in the contrary, the activation of sodium channel genes is controls with no neurological disease (Fig. 8e) [6]. These highly dynamic, changing in tandem with state-transitions new results provide evidence for dysregulation of sodium in normal neurons, and subject to dysregulation in a variety channel gene expression within Purkinje neurons in at least of disease states.
three ‘demyelinating’ disorders including two experimental At first glance, the multiplicity of sodium channel genes models and one human disease, multiple sclerosis. and the changing nature of their activation may appear to As illustrated by the studies on DRG neurons summa- make sodium channel expression a difficult subject for rized above, altered sodium channel gene expression can research. On the other hand, however, the complexity of result in significant changes in neuronal physiological sodium channel expression may present experimental and properties. Normal cerebellar functioning depends on the therapeutic opportunities. As selective sodium channel precise timing of impulses. Non-pathological Purkinje cells modulating drugs are developed, it may become possible produce multiple sodium currents which interact to de- to alter the activities of specific types of sodium channels, termine the firing properties of these cells [32,36]. Muta- without changing the activities of others. This will un-tions which alter the voltage-dependence of the sodium doubtedly help us to understand the specific roles of channels that are expressed in Purkinje cells produce various channel subtypes in shaping neuronal behavior. abnormal patterns of impulse activity in these cells, and More importantly, it may open up new avenues for the these physiological changes are associated with cerebellar treatment of neurological disorders in which there is hypo-ataxia [29,37]. We have hypothesized that, in a similar or hyperexcitability, or altered impulse trafficking.
recovery from inactivation of skeletal muscle voltage-gated Na
Acknowledgements
channelm1 in HEK293 cells, FEBS Letts. 416 (1997) 11–14. [17] S.D. Dib-Hajj, L. Tyrrell, J.A. Black, S.G. Waxman, NaN, a novel Research described in this article has been supported, in voltage-gated Na channel preferentially expressed in peripheral part, by grants from the National Multiple Sclerosis sensory neurons and down-regulated following axotomy, Proc. Natl. Society, and from the Rehabilitation Research Service and Acad. Sci. 95 (1998) 8963–8968.
[18] S.D. Dib-Hajj, J.A. Black, T.R. Cummins, A.M. Kenney, J.D. Medical Research Service, Department of Veterans Affairs.
Kocsis, S.G. Waxman, Rescue ofa-SNS sodium channel expression We also thank the Eastern Paralyzed Veterans Association
in small dorsal root ganglion neurons after axotomy by nerve growth and the Paralyzed Veterans of America for support. factor in vivo, J. Neurophysiol. 79 (1998) 2668–2676.
[19] S.D. Dib-Hajj, L. Tyrrell, A. Escayg et al., Coding sequence genomic organization and conserved chromosomal localization of mouse gene Scn11a encoding the sodium channel NaN, Genomics
References 59 (1999) 309–318.
[20] S.D. Dib-Hajj, L. Tyrrell, T.R. Cummins, J.A. Black, P.M. Wood, [1] A.N. Akopian, L. Sivilotti, J.N. Wood, A tetrodotoxin-resistant S.G. Waxman, Two tetrodotoxin-resistant sodium channels in human
voltage-gated sodium channel expressed by sensory neurons, Nature dorsal root ganglion neurons, FEBS Letts. 462 (1999) 117–120. 379 (1996) 257–262. [21] S.D. Dib-Hajj, J. Fjell, T.R. Cummins et al., Plasticity of sodium [2] A.N. Akopian, V. Souslova, S. England et al., The tetrodotoxin- channel expression in DRG neurons in the chronic constriction
resistant sodium channel SNS has a specialized function in pain injury model of neuropathic pain, Pain 83 (1999) 591–600. pathways, Nat. Neurosci. 2 (1999) 541–548. [22] J.C. Eccles, B. Libet, R.R. Young, The behavior of chromatolysed
1
[3] V.J. Auld, A.L. Goldin, D.S. Krafte et al., A rat brain Na channela motoneurons studied by intracellular recording, J. Physiol. 143 subunit with novel gating properties, Neuron 1 (1998) 449–461. (1958) 11–40.
[4] J.A. Black, K. Langworthy, A.W. Hinson, S.D. Dibb-Hajj, S.G. [23] A.A. Elliott, J.R. Elliott, Characterization of TTX-sensitive and 1
Waxman, NGF has opposing effects on Na channel lll and SNS TTX-resistant sodium currents in small cells from adult rat dorsal gene expression in spinal sensory neurons, NeuroReport 8 (1997) root ganglia, J. Physiol. Lond. 463 (1993) 39–56.
2331–2335. [24] J.D. England, F. Gamboni, M.A. Ferguson, S.R. Levinson, Sodium [5] J.A. Black, T.R. Cummins, C. Plumpton et al., Upregulation of a channels accumulate at the tips of injured axons, Muscle & Nerve
silent sodium channel after peripheral, but not central, nerve injury 17 (1994) 593–598.
in DRG neurons, J. Neurophysiol. 82 (1999) 2776–2785. [25] J.D. England, L.T. Happel, D.G. Kline et al., Sodium channel [6] J.A. Black, S. Dib-Hajj, D. Baker, J. Newcombe, M.L. Cuzner, S.G. accumulation in humans with painful neuromas, Neurology 47
Waxman, Sensory neuron specific sodium channel SNS is abnormal- (1996) 272–276.
ly expressed in the brains of mice with experimental allergic [26] J. Fjell, T.R. Cummins, S.D. Dib-Hajj, K. Fried, J.A. Black, S.G. encephalomyelitis and humans with multiple sclerosis, Proc. Natl. Waxman, Differential role of GDNF and NGF in the maintenance of Acad. Sci. (2000), in press. two TTX-resistant sodium channels in adult DRG neurons, Molec. [7] J.A. Black, J. Fjell, S. Dibb-Hajj et al., Abnormal expression of Brain Res. 67 (1999) 267–282.
SNS / PN3 sodium channel in cerebellar Purkinje cells following loss [27] A.L. Hodgkin, A.F. Huxley, A quantitative description of membrane of myelin in the taiep rat, NeuroReport 10 (1999) 913–918. current and its application to conduction and excitation in nerve, J. [8] K.L. Burchiel, Effects of electrical and mechanical stimulation on Physiol. Lond. 117 (1952) 500–544.
two foci of spontaneous activity which develop in primary afferent [28] Y. Iwahashi, T. Furuyama, S. Inagaki, Y. Morita, H. Takagi, Distinct neurons after peripheral axotomy, Pain 18 (1984) 249–265. regulation of sodium channel types I, II, and III following nerve [9] J.M. Caffrey, D.L. Eng, J.A. Black, S.G. Waxman, J.D. Kocsis, transection, Mol. Brain Res. 22 (1994) 341–345.
Three types of sodium channels in adult rat dorsal root ganglion [29] D.C. Kohrman, M.R. Smith, A.L. Goldin, J. Harris, M.H. Meisler, A neurons, Brain Res. 592 (1992) 283–297. missense mutation in the sodium channel Scn8a is responsible for [10] W.A. Catterall, Cellular and molecular biology of voltage-gated cerebellar ataxia in the mouse mutant jolting, J. Neurosci. 16 (1996)
sodium channels, Physiol. Rev. 72 (1992) 515–548. 5993–5999.
[11] T.R. Cummins, S.G. Waxman, Down-regulation of tetrodotoxin- [30] M. Kuno, R. Llinas, Enhancement of synaptic transmission by resistant sodium currents and up-regulation of a rapidly repriming dendritic potentials in chromatolysed motoneurons of the cat, J. tetrodotoxin-sensitive sodium current in small spinal sensory neu- Physiol. Lond. 210 (1970) 807–821.
rons following nerve injury, J. Neurosci. 17 (1997) 3503–3514. [31] Z. Li, G.I. Hatton, Oscillatory bursting of phasically firing rat
21 1
[12] T.R. Cummins, J.R. Howe, S.G. Waxman, Slow closed-state in- supraoptic neurones in low-Ca medium: Na influx, cytosolic
21
activation: a novel mechanism underlying ramp currents in cells Ca and gap junctions, J. Physiol. Lond. 496 (1996) 379–394. expressing the hNE / PN1 sodium channel, J. Neurosci. 18 (1998) [32] R. Llinas, M. Sugimori, Electrophysiological properties of in vitro 9606–9619. Purkinje cell somata in mammalian cerebellar slice, J. Physiol. 305 [13] T.R. Cummins, S.D. Dib-Hajj, J.A. Black, A.N. Akopian, J.N. (1980) 171–195.
1
Wood, S.G. Waxman, A novel persistent tetrodotoxin-resistant [33] O. Matzner, M. Devor, Na conductance and the threshold for sodium current in SNS-null and wild-type small primary sensory repetitive neuronal firing, Brain Res. 597 (1992) 92–98.
neurons, J. Neurosci. 19 (1999) 1–6. [34] M. Noda, S. Shimizu, T. Tanabe et al., Primary structure of [14] M. Devor, C.H. Keller, T.J. Deerinck, S.R. Levinson, M.H. Ellis- Electrophorus electricus sodium channel deduced from
complemen-1
man, Na channel accumulation on axolemma of afferent endings in tary DNA sequence, Nature 312 (1984) 121–127.
nerve end neuromas in Apteronotus, Neurosci. Lett. 102 (1989) [35] M. Noda, T. Ikeda, T. Kayano et al., Existence of distinct sodium
149–154. channel messenger RNAs in rat brain, Nature 320 (1986) 188–192.
[15] S. Dib-Hajj, J.A. Black, P. Felts, S.G. Waxman, Down-regulation of [36] I.M. Raman, B.P. Bean, Resurgent sodium current and action transcripts for Na channela-SNS in spinal sensory neurons follow- potential formation in dissociated cerebellar purkinje neurons, J. ing axotomy, Proc. Natl. Acad. Sci. 93 (1996) 14950–14954. Neurosci. 17 (1997) 4157–4536.
firing patterns in Purkinje neurons of Scn8a mutant mice, Neuron 19 membrane of hypothalamic neurons in response to changes in their (1997) 881–891. input, Proc. Natl. Acad. Sci. 96 (1999) 1088–1093.
[38] L. Sangameswaran, S.G. Delgado, L.M. Fish et al., Structure and [44] S. Tate, S. Benn, C. Hick et al., Two sodium channels contribute to function of a novel voltage-gated tetrodotoxin-resistant sodium the TTX-R sodium current in primary sensory neurons, Nat. channel specific to sensory neurons, J. Biol. Chem. 271 (1996) Neurosci. 1 (1998) 653–655.
5953–5956. [47] J.J. Toledo-Aral, P. Brehm, S. Halegoua, G. Mandel, A single pulse [39] J.W. Scadding, Development of ongoing activity, mechanosensitivi- of nerve growth factor triggers long-term neuronal excitability ty, and adrenalin sensitivity in severed peripheral nerve axons, Exp. through sodium channel gene induction, Neuron 14 (1995) 607–
Neurol. 73 (1981) 345–364. 611.
1
[40] J.H. Schild, D.L. Kunze, Experimental and modeling study of Na [48] E. Vega-Saenz de Miera, B. Rudy, M. Sugimori, R. Llinas, Molecu-current heterogeneity in rat nodose neurons and its impact on lar characterization of the sodium channel subunits expressed in neuronal discharge, J. Neurophysiol. 78 (1997) 3198–3209. mammalian cerebellar Purkinje cells, Proc. Natl. Acad. Sci. 94 [41] E. Sernagor, Y. Yarom, R. Werman, Sodium-dependent regenerative (1997) 7059–7064.
responses in dendrites of axotomized motoneurons in the cat, Proc. [49] S.G. Waxman, The neuron as a dynamic electrogenic machine: Natl. Acad. Sci. USA 83 (1986) 7966–7970. modulation of sodium channel expression as a basis for functional [42] A.A. Sleeper, T.R. Cummins, W. Hormuzdiar et al., Changes in plasticity in neurons, Phil. Trans. Roy. Soc. Lond. B 355 (2000)
expression of two tetrodotoxin-resistant sodium channels and their 199–213.
currents in dorsal root ganglion neurons following sciatic nerve [50] S.G. Waxman, J.D. Kocsis, J.A. Black, Type III sodium channel injury, but not rhizotomy, J. Neurosci., in press mRNA is expressed in embryonic but not adult spinal sensory [43] M. Tanaka, T.R. Cummins, K. Ishikawa, J.A. Black, Y. Ibata, S.G. neurons, and is re-expressed following axotomy, J. Neurophysiol. 72
![Fig. 8. Sensory Neuron Specific sodium channel SNS is not present within the normal cerebellum, but is expressed in cerebellar Purkinje cells withinbrains obtained at post-mortem from MS patients [6]](https://thumb-ap.123doks.com/thumbv2/123dok/3137948.1382482/8.612.135.467.514.663/sensory-specic-cerebellum-expressed-cerebellar-purkinje-withinbrains-patients.webp)