Expression of adhesion molecules by human endothelial cells
exposed to oxidized low density lipoprotein
Influences of degree of oxidation and location of oxidized LDL
Akira Takei
a, Yan Huang
b,c, Maria F. Lopes-Virella
b,c,*
aDepartment of Medicine,Kgusha Uni6ersity,Fukuoka,JPN
bRalph H.Johnson Veterans Administration Medical Center,Charleston,SC,USA
cDepartment of Medicine,Di6ison of Endocrinology,Diabetes and Medical Genetics,Medical Uni6ersity of South Cardina,Charleston,
SC29403,USA
Received 5 August 1999; received in revised form 20 January 2000; accepted 2 March 2000
Abstract
The main objective of this study was to determine the influence of the degree of low density lipoprotein (LDL) oxidation and the location of oxidized LDL (oxLDL) on expression of adhesion molecules on endothelial cells (EC). OxLDL preparations 1 – 4 with different degrees of oxidative modification were studied. All preparations of oxLDL, after addition to the medium, stimulated the expression of intercellular adhesion molecule-1 (ICAM-1) by human umbilical vein endothelial cells (HUVEC) as determined by cell-ELISA. Concentration-dependent studies examining ICAM-1 expression by HUVEC showed that the minimal concentration of oxLDL which significantly stimulated ICAM-1 expression was 5mg/ml, suggesting that the predicted physiolog-ical concentration of oxLDL in plasma may be not high enough to elicit a substantial increase of ICAM-1 expression in EC. In contrast, very small amounts (0.15mg/well) of oxLDL-3 and 4, the more heavily oxidized LDL preparations, stimulated effectively ICAM-1 expression by HUVEC when located below the endothelial cell monolayer by immobilizing to type I collagen. The results suggest that the increased expression of ICAM-1 induced by accumulated oxLDL may be one of the mechanisms by which oxLDL contributes to atherogenesis. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Oxidized low-density lipoproteins; Intercellular adhesion molecule-1; Endothelial cells; Atherosclerosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
During the last decade, evidence that oxidized low density lipoproteins (oxLDL) play a critical role in atherogenesis has been steadily accumulating [1,2]. OxLDL contributes to the development of atheroscle-rosis by several mechanisms. One of them is the stimu-lation of endothelial cells (EC) to release/express leukocyte chemoattractants and adhesion molecules [3 – 6]. Adhesion molecules mediate the attachment of cir-culating mononuclear cells to the endothelium and facilitate their migration into the subendothelial space thus contributing to accumulation of mononuclear cells in the vascular wall, one of the initial steps in the
development of atherosclerosis [7]. Thus it is not sur-prising that several studies have shown increased ex-pression of adhesion molecules in the endothelium overlaying atherosclerotic lesions but not in the normal endothelium [8 – 11].
Thus far, all in vitro studies investigating the effect of oxLDL on the expression of adhesion molecules by endothelial cells have been performed by adding oxLDL to the culture medium [3 – 6]. However, the presence of circulating plasma oxLDL, even in small amounts, is debatable and the concentrations of oxLDL used in the above studies (25 – 100 mg/ml) have always been higher than the levels of oxLDL reported/
predicted to be present in human plasma [12,13]. The expression of adhesion molecules in the endothelium may however be induced by oxLDL located in the subendothelial space, likely in atherosclerotic lesions. It is well accepted that oxLDL becomes trapped in the * Corresponding author. Tel.: +1-843-5775011, ext. 6823; fax:
+1-843-9536480.
E-mail address:[email protected] (M.F. Lopes-Virella).
extracellular matrix during the formation of atheroma-tous plaques and, therefore, it is likely that larger amounts of oxLDL than those circulating in plasma accumulate in these regions [14]. Furthermore the LDL is likely more heavily oxidized since the entrapment of LDL by the extracellular matrix together with the presence of microenvironment conditions excluding plasma soluble anti-oxidants in the subendothelial space creates ideal conditions for oxidation of LDL. Studies showing that LDL eluted from atherosclerotic lesions has characteristics similar to those of copper-ox-idized LDL support this hypothesis [14].
In this study it was proposed to determine whether or not the expression of adhesion molecules by endothelial cells is influenced by the degree of LDL oxidation. It was also determined whether or not the expression of adhesion molecules by endothelial cells can be elicited by oxLDL located in the subendothelial space by im-mobilizing oxLDL to type I collagen, one of the main components of the subendothelial matrix.
2. Material and methods
2.1. Endothelial cells
Human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics (San Diego, CA) and grown on 0.1% gelatin-coated flasks in Medium 199 (M199, Life Technologies, Gaithersburg, MD) supple-mented with 20% fetal bovine serum (Sigma, St. Louis, MO), endothelial cell growth supplement (30 mg/ml, Sigma), heparin (1 U/ml), antibiotics and antimycotics (Penicillin G 100 U/ml, Streptomycin 100 mg/ml, Am-photericin B 0.25mg/ml, Sigma). HUVEC were used in their fifth or sixth passage in all experiments.
2.2. Isolation of nati6e LDL and lipoprotein deficient serum
Human native LDL (nLDL, 1.019BdB1.063 g/ml) was isolated from a plasma pool of healthy volunteers by sequential ultracentrifugation at 60 000 rpm for 24 h at 10°C on a Beckman L-80 ultracentrifuge using a type 60-Ti rotor (Beckman, Palo Alto, CA) as previously described [15]. The isolated LDL was washed by ultra-centrifugation, dialyzed against a 0.16 mol/L NaCl solution containing 300 mmol/l EDTA, pH 7.4, passed through a 0.45mm filter (Gelman Sciences, Ann Arbor, MI) in order to sterilize and remove aggregates and stored under nitrogen in the dark at 4°C.
Lipoprotein deficient serum (LPDS) was prepared from pooled plasma of healthy volunteers by ultracen-trifugation at 100 000×g, 10°C for 36 h on a Beckman L-80 ultracentrifuge, using a type 60-Ti rotor, after adjusting density to 1.25 g/ml with KBr [15]. After ultracentrifugation the lipoprotein deficient plasma was washed by ultracentrifugation and clotted with thrombin (20 U/ml) and CaCl2(2.5 mg/ml). Afterwards the serum was dialyzed against 0.16 M NaCl solution containing 300mmol/L EDTA, pH 7.4 and sterilized by passage through a 0.45 mm filter.
2.3. Oxidation of LDL
Native LDL (1.5 mg/ml) was passed through a PD-10 column containing Sephadex G-25 M (Pharmacia Biotech, Piscataway, NJ) to remove EDTA and then incubated at 37°C in the presence of 40 mM CuCl2. Oxidation of LDL was continuously monitored by measuring the formation of conjugated dienes [16] and fluorescent compounds [17]. The oxidation was stopped
Table 1
Relative electrophoretic mobility of oxidatively modified low density lipoprotein (LDL)a
Oxidation time
OxLDL Relative electrophoretic (h)
preparations mobility(fold of mobility for native LDL)
OxLDL-4 24 2.8290.10
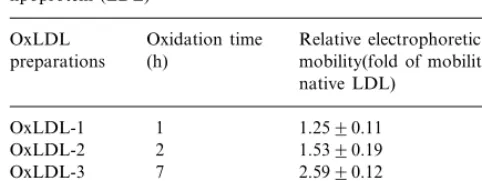
aLDL was oxidized with 40 mM of Cu2+ at 37°C for different times as indicated in Fig. 1. Levels of the relative electrophoretic mobility are expressed as percent of the mobility of native LDL (nLDL). The values represent mean9S.D. of the measurements of three oxidized LDL (oxLDL) pools.
incubation time (24 h) was determined, in pilot experi-ments, to allow maximal stimulation of ICAM-1 expression.
The second experimental system was designed to study the effects of sub-endothelial oxLDL on the expression of adhesion molecules. To perform these studies, 96-well plates were incubated with rat tail type I collagen (1 mg/ml) (Becton Dickinson Labware, Bed-ford, MA) and 0.02 N NaOH at 37°C for 30 min to allow collagen polymerization to occur. After polymer-ization of collagen, the plates were washed with PBS. After washing, culture medium containing either 10 mg/ml of (LPDS) or 10 mg/ml of LPDS to which 200
mg/ml of native or oxLDL were added was added to the wells, according to protocol. Incubation was then car-ried out at 37°C for 24 h, which has been shown to be the optimal time to achieve maximal diffusion of oxLDL into collagen [20]. After the incubation, the medium was removed and 3×104 cells were seeded in each well and grown at 37°C for 24 h. Since the cells were seeded at high density (93 750/cm2), a confluent monolayer was obtained within 24 h.
To determine the amount of oxLDL and native LDL immobilized to collagen radiolabeled oxLDL and na-tive LDL were used. It was found that the amount of immobilized oxLDL or immobilized native LDL, at the concentrations tested (1 – 400mg/ml), had a linear corre-lation with the concentrations of oxLDL/native LDL used. An average of 1.5% of the total oxLDL or native LDL added to the wells coated with type I collagen was immobilized. Therefore, 0.15 mg oxLDL/native LDL were immobilized after 50 ml of a 200mg/ml of oxLDL or native LDL were incubated with collagen at 37°C for 30 min. There was no difference, in the experimen-tal conditions, between the percentage of binding of oxLDL and native LDL to collagen.
2.5. Cell ELISA
The expression of adhesion molecules on HUVEC was determined by cell ELISA. After stimulation with oxLDL, HUVEC were washed with Dulbecco’s phos-phate-buffered saline (PBS) twice and fixed with 0.025% glutaraldehyde for 10 min. After fixation, the cells were washed once with PBS containing 0.05% Tween 20 (PBS/Tween). Afterwards the cells were incubated at room temperature for 1 h with 1 mg/ml of monoclonal antibody against ICAM-1, VCAM-1 or E-selectin (Pharmingen, San Diego, CA) or with 1 mg/ml of a non-specific mouse IgG1 (Sigma) in PBS containing 1% bovine serum albumin (PBS/BSA). The later was cho-sen as a control since the monoclonal antibodies against ICAM-1, VCAM-1 and E-selectin are IgG1. After incubation, the cells were washed three times with PBS/Tween and incubated at room temperature for 1 h with horseradish peroxidase-conjugated rabbit anti-at the times indicanti-ated in Fig. 1 by addition of 100mM
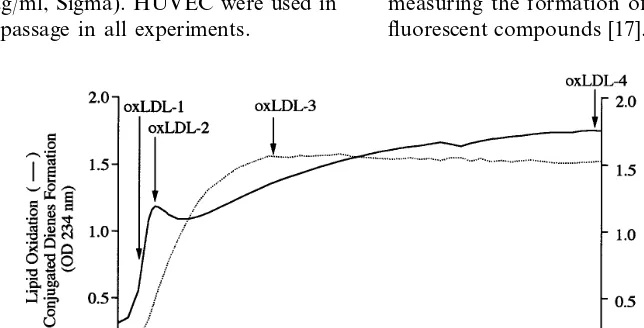
EDTA and 200mM butylhydroxytoluene. Two oxLDL preparations were collected during the period of conju-gated dienes formation (Fig. 1), one at the middle of conjugated dienes formation (oxLDL-1) and another when the formation of conjugated dienes reached its peak (oxLDL-2). Two more LDL preparations were collected during the formation of fluorescent com-pounds (Fig. 1), one immediately after the formation of the fluorescent compounds reached its peak (oxLDL-3), and another 24 h after the oxidation was started or 16 h after the peak in fluorescence was reached (oxLDL-4). Each oxLDL preparation was dialyzed against a solution containing 0.16 M NaCl and 300 mM EDTA and sterilized by passage through a 0.45mm filter. The electrophoretic mobility of all oxLDL preparations, as summarized in Table 1, was determined by agarose gel electrophoresis [18] in barbital buffer, pH 8.6. The protein content of native LDL and oxLDL prepara-tions was measured by the Lowry assay [19]. Endotoxin levels in the native and oxidized LDL preparations were measured using an endotoxin assay kit (Sigma). The endotoxin levels of the LDL preparations used in the studies were found to be below the lower limit of detection (0.015 U/ml).
2.4. Stimulation of HUVEC by nati6e and oxidized
LDL
To investigate the effects of native or oxidized LDL on the expression of adhesion molecules by HUVEC, two in vitro experimental systems were employed. In the first experimental system, HUVEC (2×104 cells/ well) were seeded in a 96-well plate coated with 0.1% gelatin and grown for 48 h to reach confluence. After reaching confluence the cells were washed with Dulbec-co’s phosphate buffered saline (PBS) and incubated, at 37°C for 24 h, with culture medium (M199) to which 10 mg/ml of LPDS and several concentrations (5 – 400
mouse IgG (Organon Teknika Corporation, Durham, NC) diluted at 1:5000 in PBS/BSA. The cells were washed again with PBS/Tween three times, and 2,2% -azin-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) with 0.003% H2O2 was added to each well and incu-bated for 30 min. The absorbance was measured at 414 nm in a microplate reader. The optical densities of the wells incubated with non-specific mouse IgG1 were subtracted from those of the wells incubated with mon-oclonal antibody. Each experiment was performed in triplicate and repeated at least three times.
2.6. Statistic analysis
Data was presented as mean9S.E.M. Comparison between treatments was performed using the one-way analysis of variance (ANOVA). The Tukey – Kramer multiple comparison test was used to compare the different treatments with each other. A value of PB
0.05 was considered significant.
3. Results
3.1. Characterization of the oxLDL preparations
Four oxLDL preparations (oxLDL-1 to oxLDL-4) were obtained by stopping the oxidative reaction cata-lyzed by copper chloride at different steps of the reac-tion, as shown in Fig. 1. Approximately 2 h after the addition of copper chloride, the formation of
conju-gated dienes, used as a surrogate for lipid oxidation, reached its peak (Fig. 1). Two LDL preparations were collected during the period of conjugated dienes forma-tion, one at the middle of conjugated dienes formation (oxLDL-1) and another when the formation of conju-gated dienes reached its peak (oxLDL-2). The forma-tion of fluorescent compounds reached its peak approximately 7 h after addition of copper chloride. A LDL preparation was collected at this stage (oxLDL-3). Another preparation was obtained 24 h after starting the oxidation reaction (oxLDL-4). For all the experi-ments performed LDL obtained from three different plasma pools was used. The pattern of oxidation of the three LDL preparations was similar although the maxi-mum values of O.D. and fluorescence at peak levels were slightly different for each pool. Representative results are shown in Fig. 1. Electrophoretic mobility of all oxLDL preparations used was also determined and the data is summarized in Table 1.
3.2. Concentration-dependent effect of oxLDL-3 on
ICAM-1 expression by HUVEC
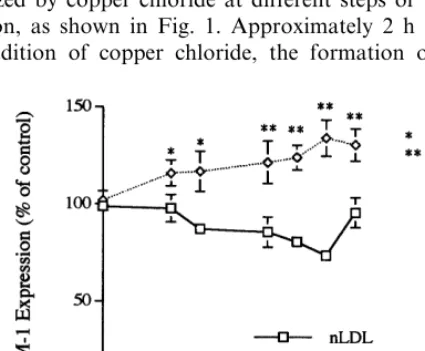
To determine the concentration of oxLDL needed to elicit maximal stimulation of ICAM-1 expression by HUVEC the cells were incubated with medium contain-ing different concentrations of oxLDL-3. As depicted in Fig. 2, oxLDL-3 stimulated ICAM-1 expression in a concentration-dependent manner. ICAM-1 expression was significantly increased (PB0.01) in the cells stimu-lated with 50-200 mg/ml of oxLDL. The minimum concentration of oxLDL-3 that stimulated ICAM-1 expression was 5mg/ml. Native LDL added at the same concentrations to the medium did not show a concen-tration-dependent stimulation of ICAM-1 expression. Time course experiments (from 2 to 48 h) revealed that oxLDL preparations led to maximal stimulation of ICAM-1 at 24 h. Thus all experiments thereafter were performed with oxLDL concentrations above 50 mg/ml and an incubation time of 24 h.
3.3. Influence of the degree of LDL oxidation on
ICAM-1 expression by HUVEC
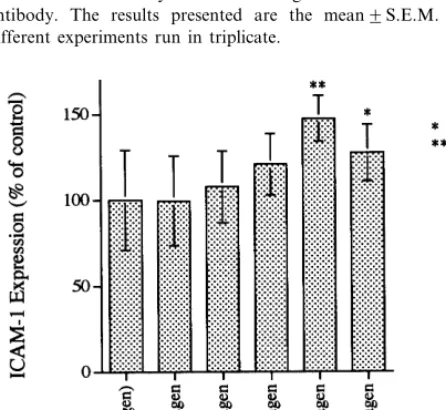
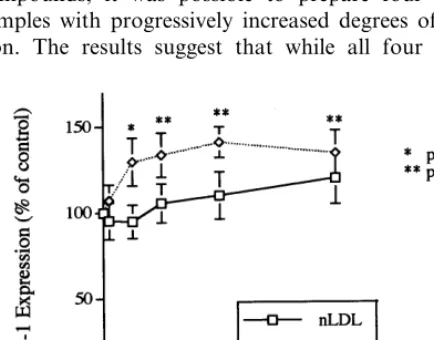
As depicted in Fig. 3, ICAM-1 expression was slightly but significantly increased in cells incubated with medium containing any of the four oxLDL prepa-rations. The levels observed, expressed as percentages of those obtained in cells incubated with medium alone, were 113.897% for oxLDL-1, 132.394% for oxLDL-2, 147.795% for oxLDL-3 and 123.097% for oxLDL-4. Of the four oxLDL preparations, oxLDL-3 was the most effective in stimulating the expression of ICAM-1.
Fig. 3. Effects of oxidized low density lipoprotein (oxLDL), at different stages of oxidation, on intercellular adhesion molecule-1 (ICAM-1) expression by human umbilical vein endothelial cells (HU-VEC). HUVEC were incubated at 37°C for 24 h with medium alone or medium containing 100mg/ml of native LDL or oxLDL-1, -2, -3 or -4 (see Fig. 1). After the incubation the cells were washed with Dulbecco’s PBS and fixed with glutaraldehyde. ICAM-1 expression was then determined by cell ELISA using a monoclonal anti-ICAM-1 antibody. The results presented are the mean9S.E.M. of three different experiments run in triplicate.
molecules other than ICAM-1 was increased when VEC were incubated in the presence of oxLDL, HU-VEC were incubated with medium containing 100
mg/ml of oxLDL-3 for 24 h and the expression of VCAM-1 and E-selectin was measured. E-selectin ex-pression by HUVEC was significantly increased by exposure to oxLDL-3 (188.6955.1% of control, PB
0.05). Expression of VCAM-1 was not stimulated by oxLDL-3. Native LDL at the same concentrations and using the same experimental conditions did not stimu-late the expression of E-selectin or VCAM-1.
3.5. ICAM-1 expression by HUVEC induced by
oxLDL immobilized by type I collagen
In order to determine whether oxLDL located in the subendothelial space was able to stimulate the expres-sion of ICAM-1, a novel experimental model was de-signed in which oxLDL (50 ml of 200 mg/ml oxLDL preparation) was added to type I collagen-coated wells prior to the seeding of HUVEC cells. Only 150 ng from the 10 mg of oxLDL added to each well bound to collagen (1.5% of total oxLDL added). As shown in Fig. 4, the expression of ICAM-1 by HUVEC exposed to type I collagen-bound oxLDL-3 or -4 was signifi-cantly increased (147.2%, PB0.01, 127.0%, PB0.05, respectively). The stimulatory effect of oxLDL-4 was less than that of oxLDL-3 (PB0.05). OxLDL-1 and -2, which stimulated ICAM-1 expression when added to medium, did not significantly increase ICAM-1 expres-sion in these experiments. Also, none of the different preparations of oxLDL (oxLDL-1, -2, -3, and -4) when added to collagen stimulated the expression of VCAM-1 and E selectin by HUVECs (data not shown). As expected, native LDL did not stimulate the expression of any of the adhesion molecules studied.
3.6. Concentration-dependent effect of collagen-bound
oxLDL-3 on ICAM-1 expression by HUVEC
Type I collagen-bound oxLDL-3 stimulated ICAM-1 expression on HUVEC in a concentration dependent manner as depicted in Fig. 5. Maximal ICAM-1 levels were observed when the HUVEC were seeded and grown on type I collagen gel pre-incubated in presence of 200 mg/ml of oxLDL-3 for 24 h (140% of levels observed in cells incubated in presence of medium alone). Type I collagen-bound native LDL did not significantly change ICAM-1 expression by HUVEC (Fig. 5). On average, as previously stated, 1.5% of oxLDL and native LDL remained attached to type I collagen, as determined by incubation of radiolabeled LDL and oxLDL with type I collagen in the same experimental conditions. Thus the amount of oxLDL, once immobilized to collagen, needed to stimulate Fig. 4. Effect of oxidized low density lipoprotein (oxLDL)
immobi-lized subendothelially on the expression of intercellular adhesion molecule-1 (ICAM-1). Two hundredmg/ml of native or oxLDL were incubated with type I collagen gel adhered to 96-well plates at 37°C for 24 h. Human umbilical vein endothelial cells (HUVEC) were then seeded and incubated at 37°C for 24 h. After the incubation the cells were washed with Dulbecco’s PBS and fixed with glutaraldehyde. ICAM-1 expression was then determined by cell ELISA using a monoclonal antibody against ICAM-1. The data presented are the mean9SEM of three different experiments run in triplicate.
3.4. Effects of oxLDL-3 on VCAM-1 and E-selectin expression by HUVEC
ICAM-1 expression by HUVEC is in the nanogram range.
3.7. The stimulation of ICAM-1 expression by oxLDL
is not dependent on cytokine release
Since it has been shown that cytokines such as TNFa
and IL-1bare potent stimulators for adhesion molecule expression in endothelial cells [4], it is possible that oxLDL induces release of cytokines that stimulate ICAM-1 expression in HUVECs. Thus, the effect of oxLDL on secretion of TNFa and IL-1b from HU-VECs was examined. HUHU-VECs were incubated with or without oxLDL for 24 h and secreted TNFaand IL-1b
in culture medium was measured by ELISA. The data showed that both TNFa and IL-1b in control medium were undetectable by ELISA, and oxLDL did not stimulate TNFa and IL-1b secretion. This study indi-cates that oxLDL-stimulated ICAM-1 expression is not cytokine-dependent.
4. Discussion
In the present study, the influence of the degree of LDL oxidation on ICAM-1 expression was demon-strated by endothelial cells. By continuously monitoring the formation of conjugated dienes and the fluorescent compounds, it was possible to prepare four oxLDL samples with progressively increased degrees of oxida-tion. The results suggest that while all four oxLDL
preparations stimulated ICAM-1 expression, oxLDL-3 stimulated it more effectively than the other oxLDL preparations. Thus, the data seem to indicate that the oxidation of not only the lipid components but also the protein moiety of LDL contributes to stimulation of ICAM-1 expression by HUVEC. A previous study by Granger and coworkers showed that neutrophil-en-dothelial cell adhesion elicited by different forms of oxLDL was related to the degree of lipid oxidation but not to protein oxidation [21]. The discrepancy between this and Granger’s data may be due to the different cell used (neutrophils instead of monocytes) or more likely to the different methods used for measuring the degree of protein oxidation. The formation of fluorescent com-pounds in LDL preparations were measured [17] while Granger’s group measured the relative electrophoretic mobility and TBARS as the parameters for protein oxidation. These two methods are too insensitive and they do not have a relationship with the formation of the fluorescent protein adducts measured by continuous monitoring of fluorescence. Recently, Granger and coworkers reported that ICAM-1 played an important role in oxidized LDL-induced leukocyte-endothelial cell adhesion [22]. The data is in agreement with their observations.
The maximal stimulation of ICAM-1 expression was induced by oxLDL-3. Interestingly, oxLDL-4, the more heavily modified oxLDL preparation, was less effective in stimulating ICAM-1 expression. Since heavily oxi-dized LDL has been shown to be cytotoxic to endothe-lial cells [23], it could lead to a decrease in ICAM-1 expression. Although the potential cytotoxicity induced by oxLDL-4 was largely excluded by the trypan blue exclusion and LDH-releasing assays, subtle cytotoxicity that could not be detected by LDH-releasing assay may contribute to the decreased expression of ICAM-1. Another possibility is that heavy oxidation of LDL may lead to the formation of oxLDL aggregates [24] which were less effective in stimulating ICAM-1 expression. Other investigators have also shown that some heavily oxidized forms of LDL may not have the ability to stimulate ICAM-1 expression by HUVEC. For in-stances, a study by Cominacini et al. showed that LDL exposed to 1mM of Cu2+ for 24 h stimulated ICAM-1
expression by HUVEC whereas LDL exposed to 2.5 – 5
mM of Cu2+ for 24 h did not [6].
Previous studies showed that the minimal concentra-tions of oxLDL required to achieve significant stimula-tion of ICAM-1 are between 50 and 100 mg/ml [3,5,6], which are considerably higher than those found in plasma (100 ng/ml to 5.9mg/ml) [12,13]. In reality, it is not even clear whether or not oxLDL circulates in plasma, since antioxidants in plasma may preclude its formation in the intravascular compartment, and when oxLDL is formed it is rapidly cleared by the liver [25]. A small amount of oxLDL may be circulating in Fig. 5. Concentration-dependent stimulation of intercellular adhesion
plasma only if oxLDL located in atherosclerotic plaques regurgitates to plasma. Since oxLDL is accu-mulated in atherosclerotic lesions [26] and the concen-tration of oxLDL in these lesions is likely higher than that reported in plasma, a novel experimental model was designed in which oxLDL was immobilized by type I collagen, a major matrix component in atherosclerotic lesions [27]. In contrast to the conven-tional model in which oxLDL contacts with the api-cal surface of the endothelial cells, oxLDL in this model interacts with endothelial cells basolaterally. The basolateral interaction of oxLDL with endothe-lial cells mimics the in vivo interaction between oxLDL accumulated in the subendothelium and en-dothelial cells, including the cells covering the lesions and the cells present in neovessels inside the lesions. Despite a small amount of oxLDL bound to collagen (approximately 150 ng/well), immobilized oxLDL stimulated ICAM-1 expression by approximately 150% of baseline level. To the authors’ knowledge, this is the first report showing that oxLDL immobi-lized by a matrix component stimulates ICAM-1 ex-pression in endothelial cells. In addition to oxLDL, recent studies also demonstrated that enzymatically degraded, non-oxidized LDL (E-LDL), which was lo-calized in early human atherosclerotic lesions [28], also stimulated the expression of endothelial adhesion molecules, including ICAM-1 [29]. These studies and the previous data indicate that multiple processes, in-cluding oxidation, glyco-oxidation [30], and enzymatic degradation, render LDL capable of stimulating the expression of adhesion molecules by vascular endothe-lial cells.
In summary, it has been shown that the degree of LDL oxidation had an impact on the oxLDL-stimu-lated expression of ICAM-1. Furthermore, the results showed that oxLDL immobilized by the subendothe-lial matrix components stimulated ICAM-1 expression more effectively than circulating oxLDL. Since adhe-sion molecules play an important role in the initiation and progression of atherosclerotic plaques, the results suggest that accumulation of oxLDL in the suben-dothelium is likely to play an important role in atherogenesis.
Acknowledgements
This work was supported by the National Institutes of Health grant HL-55782 and by the Research Ser-vice of the Department of Veterans Affairs. The au-thors wish to thank Ms Charlyne Chassereau for her skilled technical assistance.
References
[1] Witztum JL, Steinberg D. Role of oxidized low density lipo-protein in atherogenesis. J Clin Invest 1991;88:1785 – 92. [2] Steinberg D. Low density lipoprotein oxidation and its
pathobio-logical significance. J Biol Chem 1997;272:20963 – 6.
[3] Jeng JR, Chang CH, Shieh SM, Chiu HC. Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear stress-induced detachment. Biochim Biophys Acta 1993;1178:221 – 7.
[4] Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expres-sion in human vascular endothelial cells. J Clin Invest 1995;95:1262 – 70.
[5] Gebuhrer V, Murphy LF, Bordet JC, Reck MP, McGregor JL. Oxidized low-density lipoprotein induces the expression of P-se-lectin (GMP 140/PADGEM/CD62) on human endothelial cells. Biochem J 1995;306:293 – 8.
[6] Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, Pastorino AM, Cascio VL. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vas-cular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med 1997;22:117 – 27.
[7] Ross R. The pathogenesis of atherosclerosis: a prospective for the 1990s. Nature 1993;362:801 – 9.
[8] Poston RN, Haskard DO, Coucher JR, Gall NP, Johnson-Tidey RR. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol 1992;140:665 – 73. [9] Wood KM, Cadogan MD, Ramshaw AL, Parums DV. The
distribution of adhesion molecules in human atherosclerosis. Histopathology 1993;22:437 – 44.
[10] Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 1993;171:223 – 9.
[11] Johnson-Tidey RR, McGregor JL, Tayler PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol 1994;144:952 – 61.
[12] Itabe H, Yamamoto H, Imanaka T, Shimamura K, Uchiyama H, Kimura J, Sanaka T, Hata Y, Takano T. Sensitive detection of oxidatively modified low density lipoprotein using a mono-clonal antibody. J Lipid Res 1996;37:45 – 53.
[13] Holvoet P, Donck J, Landeloos M, Brouwers E, Luijtens K, Arnout J, Lesaffre E, Vanrenterghem Y, Collen D. Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure. Thromb Haemost 1996;76:663 – 9. [14] Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086 – 95.
[15] Lopes-Virella MF, Sherer GK, Lees AM, Wohltmann H, Mayfield R, Sagel J, leroy C, Colwell JA. Surface binding, internalization and degradation by cultured human fibroblasts of LDL isolated from type I (insulin-dependent) diabetic patients: change with metabolic control. Diabetologia 1982;22:430 – 6. [16] Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous
monitoring of in vitro oxidation of human low density lipo-protein. Free Radic Res Commun 1989;6:67 – 75.
[18] Kawamura M, Heinecke JW, Chait A. Pathophysiological con-centrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invest 1994;94:771 – 8.
[19] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1957;193:265 – 75.
[20] Jimi S, Sakata N, Matunaga A, Takebayashi S. Low density lipoproteins bind more to type I and III collagens by negative charge-dependent mechanisms than to type IV and V collagens. Atherosclerosis 1994;107:109 – 16.
[21] Liao L, Aw TY, Kvietys PR, Granger DN. Oxidized LDL-in-duced microvascular dysfunction. Dependent on oxidation pro-cedure. Arterioscler Thromb Vasc Biol 1995;15:2305 – 11. [22] Liao L, Starzyk RM, Granger DN. Molecular determinants of
oxidized low-density lipoprotein-induced leukocyte adhesion and microvascular dysfunction. Arterioscler Thromb Vasc Biol 1997;17:437 – 44.
[23] Thorne SA, Abbot SE, Winyard PG, Blake DR, Mills PG. Extent of oxidative modification of low density lipoprotein deter-mines the degree of cytotoxicity to human coronary artery cells. Heart 1996;75:11 – 6.
[24] Hoff HF, O’Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb 1991;11:1209 – 22.
[25] Ling WL, Lougheed M, Suzuki H, Buchan A, Kodama T, Steinbrecher UP. Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/II gene. J Clin Invest 1997;100:244 – 52.
[26] Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086 – 95.
[27] Morton LF, Barnes MJ. Collagen polymorphism in the normal and diseased blood vessel wall. Investigation of collagen types I, III and V. Atherosclerosis 1982;42:41 – 51.
[28] Seifert PS, Hugo F, Tranum-Jensen J, Zahringer U, Muhly M, Bhakdi S. Isolation and characterization of a complement-acti-vating lipid extracted from human atherosclerotic lesions. J Exp Med 1990;172:547 – 57.
[29] Klouche M, May AE, Hemmes M, Mebner M, Kanse SM, Preissner KT, Bhakdi S. Enzymatically modified, nonoxidized LDL induces selective adhesion and transmigration of cytes and T-lymphocytes through human endothelial cell mono-layes. Arterioscler Thromb Vasc Biol 1999;19:784 – 93.
[30] Takei A, Crawford A, Huang Y, Lopes-Virella MF. Advanced glycation end-products-LDL stimulates adhesion molecule ex-pression by human aortic endothelial cells. Diabetes 1999;48(Supplement 1):A31.