Simulated patterns of litter decay predict patterns of
extracellular enzyme activities
Daryl L. Moorhead
∗, Robert L. Sinsabaugh
Department of Biological Sciences, University of Toledo, Toledo, OH 43606, USAReceived 5 March 1998; received in revised form 26 August 1999; accepted 20 September 1999
Abstract
Decomposition is a complex suite of processes that strongly affects the mineralization and immobilization of mineral nutrients. Thus, considerable research has focused on gaining a mechanistic understanding of litter decay. Models of decay vary with respect to detail, but most utilize decay rate coefficients for particular chemical constituents of litter, derived from empirical observations of turnover. Recent studies have shown that the activities of extracellular enzymes are correlated with decay, and represent instantaneous measures of biochemical processes responsible for the hydrolysis of particular chemical compounds. For these reasons, temporal patterns in turnover rates for particular litter constituents should correspond to activity levels of particular degradative enzymes. To test this hypothesis, we modified a general model of litter decay (GENDEC) to predict activities of extracellular enzymes. This was accomplished by viewing turnover rates for carbon fractions of litter (extractive, acid-soluble and acid-insoluble compounds) as surrogates for the activity levels of relevant extracellular enzymes (e.g., glucosidase, cellulases, oxidases). The resulting temporal patterns of litter turnover rates generated by the model were similar to observed patterns of enzyme activities. These results demonstrated that traditional modeling approaches may be used to predict patterns of enzyme activities, although existing data are not sufficient to conduct a rigorous quantitative test of this approach. Conversely, assays of extracellular enzymes could be used to test models of litter decay with a high degree of chemical and temporal resolution, because enzymes catalyze specific reactions and measures of activity levels represent instantaneous rates of degradation. ©2000 Published by Elsevier Science B.V. All rights reserved.
Keywords: Decomposition; Enzymes; Modeling
1. Introduction
Moorhead et al. (1996) recently discussed the strengths and limitations of common approaches used to model litter decay. Most mathematical models relate decay rates to measures of climate and litter quality, but these relationships often apply only to limited ranges of conditions (Whitford et al., 1981).
∗Corresponding author. Tel.:+1-419-530-2017;
fax:+1-419-530-7737.
E-mail address: [email protected] (D.L. Moorhead).
In theory, some of the limitations to models based on litter quality could be circumvented by simulating de-composition as a product of the biological activities of decomposer organisms (Bunnell et al., 1977; McGill et al., 1981). However, decomposition emerges as a composite process at the community level, with mechanistic controls varying among systems. While a major benefit of adding more mechanistic detail to models is the confidence with which they can be applied to novel situations, a major drawback is the added complexity of the resulting model. Com-plex models require more data to define parameters,
generate more uncertainties associated with parameter estimates, and are more difficult to interpret.
Recently, a new approach has been used to model litter decay, based on the activities of extracellular enzymes (Sinsabaugh and Moorhead, 1994). Because microorganisms produce enzymes that catalyze the degradation of substrates in their immediate environ-ment, decomposition rates should be related to the ac-tivities of enzymes associated with the degradation of key classes of compounds (Sinsabaugh et al., 1991). Measurements of particular enzyme activities provide a more precise insight to microbial activities than gen-eral determinations of biomass or bulk respiration. Par-nas (1975) was among the first to present a model of litter decay that was controlled by differential acqui-sition of macronutrients by decomposers. Sinsabaugh and Moorhead (1994) extended this approach with the development of an explicit model of microbial allo-cation of resources among community indicator en-zymes (MARCIE), which estimates timing and levels of activity for particular suites of enzymes, based on energy and nutrient availabilities.
The basic assumptions of the MARCIE model are that (1) enzymic degradation of complex molecules is the rate-limiting step in both microbial production and litter decay, and (2) activities of key enzymes are controlled by their rates of synthesis, determined by an allocation strategy that optimizes resource acqui-sition by decomposers. Sinsabaugh et al. (1991) have shown that temporally-integrated rates of enzymic ac-tivities correlate with mass loss patterns in litter, and Sinsabaugh and Moorhead (1994) used the MARCIE model to simulate overall patterns of litter decay. In contrast, more traditional models of decomposition utilize rate constants to calculate degradation of par-ticular litter constituents, derived from empirical ob-servations of changes in litter chemistry during decay. Conceptually, these two approaches should be compat-ible, but no study has determined if traditional models yield patterns of turnover for litter constituents that correspond to patterns of enzyme activities.
The objective of the current study was to compare patterns of litter decay, based on traditional model-ing approaches, to patterns of activities for extracel-lular enzymes responsible for the degradation of par-ticular litter constituents. We used a general model of litter decay (GENDEC; Moorhead and Reynolds, 1991) to estimate temporal patterns of degradation for
three major categories of litter constituents: (1) extrac-tives, (2) acid-solubles, and (3) acid-insolubles. These classes of chemical compounds frequently are moni-tored during studies of litter decay and often are in-corporated in models of decomposition. The activities of three groups of enzymes (glucosidases, cellulases and oxidases) are associated with the degradation of these litter constituents, respectively, and have been monitored during litter decay in a limited number of studies (e.g., Sinsabaugh et al., 1991). Results of sim-ulations are compared to observed chemical changes in decomposing litter and reported patterns of enzyme activities. This study serves as a novel evaluation of traditional modeling approaches and suggests means by which enzymic data can be used to refine models to more closely simulate patterns of microbial activities responsible for litter decay.
2. Experimental methods and modeling approach
Although few studies of decomposition have exam-ined simultaneous changes in litter mass, chemistry and enzyme activities, different aspects of decay can be compared between studies. Herein, we use a gen-eral model of litter decay (GENDEC; Moorhead and Reynolds, 1991) to simulate the decomposition of leaf litter under field and laboratory conditions. The first set of simulations are compared to observed changes in litter chemistry during a field study of litter decay (Aber et al., 1984). In addition, the general pattern of simulated turnover in litter constituents is compared to temporal patterns of enzyme activities obtained from other field studies (Kshattriya et al., 1992; Sinsabaugh et al., 1992; Joshi et al., 1993), which do not report lit-ter chemistry. The second set of simulations are com-pared to results of a laboratory experiment, in which litter mass loss and activities of cellulase enzymes were monitored (Linkins et al., 1990).
2.1. Modeling approach
Fig. 1. Carbon flow diagram for GENDEC.
holocellulose (acid-soluble), (3) resistant plant com-pounds (acid-insoluble), (4) live microbial biomass, (5) dead microbial cell walls (acid-solubles and acid-insolubles; see below), and (6) dead microbial cytoplasm (extractives). These categories of chemical compounds often are determined by chemical analy-ses for decaying litter (see review of analytical meth-ods by Ryan et al., 1990). Nitrogen flows are assumed to balance calculated carbon flows, given the N : C ratios of decomposing materials. The loss of carbon from each dead organic matter pool is a function of moisture and temperature conditions, and nitrogen limitations. The details of model structure and opera-tion are provided elsewhere (Moorhead and Reynolds, 1991, 1993, 1996), but one modification of GENDEC was made for the current study; dead microbial cell walls were assumed to consist of approximately equal fractions of acid-soluble and acid-insoluble materials. The decay rates for these pools of materials were set equal to those of litter pools for acid-soluble and acid-insoluble compounds. Climate drivers used for simulations are explained in the following descrip-tions of field and laboratory studies.
2.2. Field studies
The first step in our efforts to estimate activities of extracellular enzymes associated with litter decay was to ensure that behavior of the decomposition model (GENDEC) was consistent with expected patterns of decay, including the dynamics of particular chemical fractions of litter that could be related to enzyme ac-tivities. This required observations of mass loss and carbon fractions in decaying litter, in conjunction with some measure of climate (moisture and temperature) to drive simulations. Fortunately, Aber et al. (1984)
Table 1
Initial chemical fractions and total Kjeldahl nitrogen (TKN) content of litter (% dry mass) used in simulations of decomposition in field studies (Aber et al., 1984)
Litter type Extractives Acid solubles Acid insolubles TKN
Sugar maple 44.8 43.1 12.1 0.83
Aspen 31.1 47.5 21.4 0.83
White oak 32.4 47.4 20.2 0.84
White pine 32.8 44.7 22.5 0.44
Hemlock 35.8 39.6 20.6 0.83
Red oak 30.0 45.2 24.8 0.82
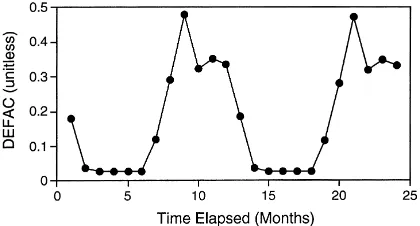
reported mass loss and carbon fractions (extractives, acid-solubles, acid-insolubles) of six litter types that were incubated in fine-mesh litterbags, over a period of 732 days, on the floor of a sugar maple stand in Wisconsin, USA. This suite of litters provided a rea-sonably broad range of initial litter quality character-istics for simulations (Table 1). The effects of climate on decomposition were estimated as a monthly scalar (DEFAC; Fig. 2), according to Parton et al. (1987), given local climate records (Parton, unpublished).
When simulated patterns of decay for particular categories of litter constituents are similar to obser-vations (see above), we may interpret turnover rates as surrogates for activity levels of various groups of enzymes. This is because Sinsabaugh et al. (1991) have shown that patterns of litter decay and enzyme activities are highly correlated, so the activity levels of enzymes responsible for the degradation of par-ticular litter factions might be predicted by assuming that they are proportional to the simulated turnover rates of these constituents. For example, the extrac-tives component of litter contains compounds that are
degraded by b-1,4-glucosidase and invertase (cel-lobiose and sucrose), cellulose (acid-soluble) is hy-drolyzed byb-1,4-endoglucanase andb-1,4-exoglucanase, and polyphenolic compounds (acid-insolubles) are degraded by phenol oxidase and peroxidase enzymes. Unfortunately, such relationships have seldom been examined in an integrated manner for decomposi-tion of bulk litter because few experiments have investigated both the patterns of litter chemistry and activities of extracellular enzymes. However, studies conducted in northeastern India (Kshattriya et al., 1992; Joshi et al., 1993) and northern New York, USA, (Sinsabaugh et al., 1992) can be used to evaluate model estimates of temporal patterns for some enzyme activities. Kshattriya et al. (1992) and Joshi et al. (1993) reported activities of invertase, cellulase and amylase during the decomposition of tree leaves, and Sinsabaugh et al. (1992) reported mass loss of birch wood in conjunction with the ac-tivities of b-1,4-glucosidase, b-1,4-endoglucanase,
b-1,4-exoglucanase, phenol oxidase and peroxidase.
2.3. Laboratory studies
In addition to field investigations, the enzyme activi-ties associated with litter decay have been examined in laboratory experiments. One of the few studies that si-multaneously measured both chemical characteristics of decaying litter and enzyme activities was performed by Linkins et al. (1990). In this investigation, litter bags containing senescent leaves of flowering dog-wood (Cornus florida), red maple (Acer rubrum) and chestnut oak (Quercus prinus) were placed in plastic basins containing forest floor material collected from a mixed deciduous forest site in southwestern Virginia, USA. These microcosms were maintained under con-stant temperature (ca. 20◦C) and moisture (holding capacity) conditions, over a 9 month period and litter was analyzed periodically for mass loss, fiber com-position and the activities of endocellulase and exo-cellulase. Simulations were conducted for these litter types using initial chemical characteristics (Table 2) and assuming no temperature or moisture limitations. As in comparisons with field studies, turnover rates of the acid-soluble fraction of litter were considered to be proportional to activities of cellulase enzymes, and compared to laboratory results.
Table 2
Initial chemical fractions and total Kjeldahl nitrogen (TKN) content of litter (% dry mass) used in simulations of decomposition in laboratory incubations (cf. LIDET, 1995)
Litter type Extractives Acid solubles Acid insolubles TKN
Dogwood 62.0 37.7 0.4 0.99
Chestnut oak 30.6 44.1 25.4 1.14
Red maple 54.9 27.5 16.6 0.87
3. Results and discussion
3.1. Field study
Overall relationships between observed and simu-lated patterns of litter decay were reasonably consis-tent for the field study conducted in Wisconsin (Aber et al., 1984). Herein, we illustrate the results of simu-lations for maple (Fig. 3) as representative of the en-tire suite of simulations (Table 3). Peak rates of mass loss corresponded to periods of favorable climate with rates slowing during winter (Figs. 2, 3a). For all lit-ter types, the extractives fraction of litlit-ter decreased over time (Fig. 3b), while the acid-insoluble fraction increased (Fig. 3d). However, patterns of degradation for the acid-soluble fraction of litter varied among litter types, and considerable differences existed be-tween observations and simulations (Fig. 3c). Even
so, estimated values were within±25% of
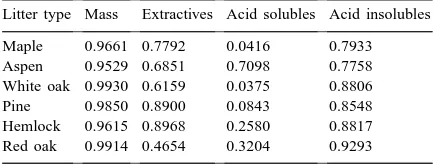
observa-tions throughout simulaobserva-tions. Simple correlaobserva-tions be-tween observations and simulations showed very high agreement for mass loss, and reasonably high corre-lations for extractives and acid-insoluble fractions of litter residues (Table 3).
The GENDEC model does not explicitly estimate activities of extracellular enzymes. We assumed that turnover rates of specific carbon fractions of litter
Table 3
Correlations (r2) between observed and simulated characteristics of litter residues during decomposition in field studies (N=11)
Litter type Mass Extractives Acid solubles Acid insolubles
Fig. 3. Decomposition of sugar maple leaf litter in the Wiscon-sin field study (Aber et al., 1984). (A) litter mass remaining, (B) extractives fraction of litter, (C) acid-soluble fraction, (D) acid-insoluble fraction.
(extractive, cellulose and lignin components) would be proportional to the corresponding activity levels of degradative enzymes. For example, the simulated pat-tern of enzyme activities in maple litter during the first full year of the decomposition study (Fig. 4), showed an early peak of activity associated with degradation of extractives (e.g., glucosidase or invertase), followed by a peak in activity of cellulase enzymes (e.g., endocel-lulase or exocelendocel-lulase) which, in turn, was followed by
Fig. 4. Simulated patterns of turnover rates (surrogates of enzyme activity) for chemical constituents of sugar maple leaf litter during decomposition, in the Wisconsin field study (Aber et al., 1984).
increasing activity of lignin-degrading enzymes (e.g., phenoloxidase or peroxidase). Results for other litter types revealed an overall similarity in general patterns of activity, with differences among litter types occur-ring as a result of differences in litter quality (Table 1). These temporal patterns in enzyme activities cor-respond to general patterns of fungal colonization of decaying litter in that species present in early stages of decay lack enzyme systems capable of degrading cel-lulose and lignin (Frankland, 1966, 1969, 1976), while species present in latter stages of decomposition have greater capacity to produce cellulolytic and lignolytic enzymes.
with these expectations: turnover rates for extractives were greater and peaked earlier in simulations than turnover rates for acid-soluble and acid-insoluble frac-tions (Fig. 4).
In contrast to invertase activity, Kshattriya et al. (1992) and Joshi et al. (1993) noted that activity lev-els of cellulase and amylase (hydrolyzes starch) in-creased more slowly and remained at higher rates for longer periods of time. Enzyme activities also showed seasonal variations that correlated with favor-able climatic conditions (Kshattriya et al., 1992; Joshi et al., 1993). These patterns of cellulase activities were consistent with simulations (Fig. 4) in that turnover rates of acid-soluble fractions of litter peaked more slowly than rates for extractives, and stayed at rela-tively higher levels for a longer period of time. Simula-tions also showed a strong seasonal pattern of turnover for litter fractions.
Kshattriya et al. (1992) and Joshi et al. (1993) noted that cellulase activity was not correlated with cellulose content of litter, which also was true for simulations. No clear trend in concentrations of the acid-soluble fraction was apparent from these field and simula-tion studies (Fig. 3c). Such inconsistencies in cellulose degradation may result from the interactions of mul-tiple controls. For example, simpler compounds are more readily utilized by microbiota than cellulose, and thus influence production of cellulase enzymes and concomitant cellulose turnover. Also, nitrogen avail-ability has been shown to affect cellulose decay (e.g., Berg et al., 1975) and should influence cellulase pro-duction, although we have no information on nitro-gen dynamics in these systems. Finally, Linkins et al. (1984) have shown that the activity of endocellu-lase sharply declines below a temperature threshold of about 10◦C, adding further complexity to interpreting seasonal responses of enzyme activities in the field studies. Thus, it is difficult to determine the controls on cellulase activity and cellulose turnover.
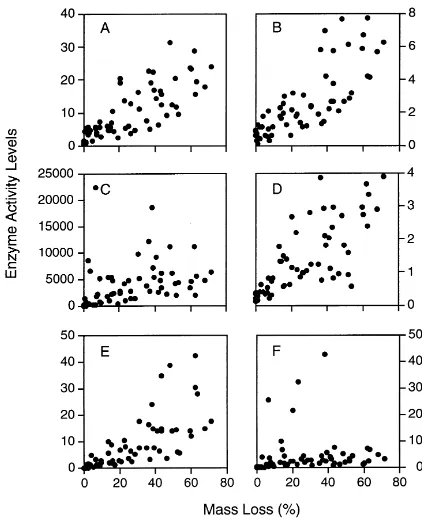
Few other field data are available for comparison, but Sinsabaugh et al. (1992) reported patterns of en-zyme activities associated with the decay of birch wood on a variety of sites in northern New York (Fig. 5). Interestingly, levels of enzyme activities were more closely related to stage of decay than time, and generally were unaffected by site factors. Several en-zymes showed increased activity with progressive de-cay (glucosidase, exocellulase, xylosidase,
phenoxi-Fig. 5. Activity levels of enzymes (units vary) associated with birch wood decay in northern New York, USA (Sinsabaugh et al., 1992). A. glucosidase (mmol gAFDM−1h−1), (B) xylosidase (mmol gAFDM−1h−1), (C) endocellulase (unit gAFDM−1h−1), (D) exocellulase (mmol gAFDM−1h−1), (E) phenol oxidase (mmol gAFDM−1h−1), (F) peroxidase (mmol gAFDM−1h−1). Data presented are pooled from eight sites, including two stream sites, two riparian zones, two hemlock stands, two deciduous stands.
dase) while others showed no relationship to decom-position (endocellulase, peroxidase). This lack of cor-respondence between endocellulase and stage of decay is consistent both with simulations and results of stud-ies by Kshattriya et al. (1992) and Joshi et al. (1993). In contrast to the field studies in India, Sinsabaugh et al. (1992) found that glucosidase activities increased with time (stage of decay), probably because suitable substrate was unavailable in woody material before the action of other enzymes (e.g., cellulases, xylosidase) released these compounds as products of cellulose and hemicellulose degradation.
correlations between levels of enzyme activity and a number of environmental factors and chemical char-acteristics of litter, but do not provide these data in more detail. Dilly and Munch (1996) only report the activities of glucosidase, and Sinsabaugh et al. (1992) did not conduct chemical analyses of decaying wood. Thus, published information is not adequate to support a more quantitative evaluation of our model results.
3.2. Laboratory study
The laboratory study of dogwood, maple and oak leaf decay (Linkins et al., 1990) provided more de-tailed measures of cellulase activities than the field studies (see above). Also, the chemical characteris-tics of these litter types are known well enough to approximate in our simulation model. The constant moisture and temperature conditions of the laboratory study suggest little limitation to decay imposed by a changing climate. Thus, we were reasonably confident that close correspondence between simulated and ob-served patterns of mass loss would yield patterns of litter chemistry dynamics that could be used to predict cellulose turnover (acid-soluble materials).
Melillo et al. (1982) and others have shown that rates of litter decay are negatively correlated
with lignin-cellulose index (LCI=lignin/[lignin+
cellulose]) and that LCI increases during decomposi-tion. We found that relationships between observed and simulated patterns of mass loss and LCI during litter decay were highly correlated (Table 4). More detailed comparisons of litter constituents were not possible because carbon fractions of residues were not evaluated during the laboratory experiment. However, patterns of exo- and endocellulase activities assayed during the experiment were compared to simulated patterns of turnover for the acid-soluble fraction of
Table 4
Correlations (r2) between observed and simulated mass (N=11) and lignin-cellulose index (LCI; N=9) values of litter residues during decomposition in laboratory incubations
Litter type N Mass LCI
Dogwood 14 0.8674 0.7109
Chestnut oak 15 0.7815 0.8875
Maple 13 0.9112 0.6747
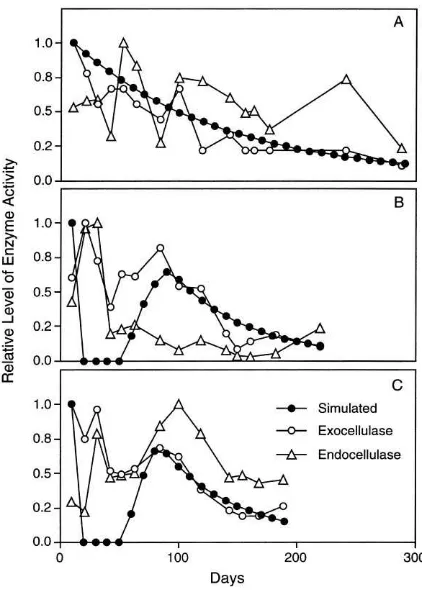
Fig. 6. Patterns of simulated turnover rates for acid-soluble frac-tions of litter and observafrac-tions of exo- and endocellulase activities during a laboratory study (Linkins et al., 1990). (A) chestnut oak, (B) flowering dogwood, (C) red maple.
litter, as a surrogate for cellulase activity (Fig. 6). Because units of activity for exo- and endocellulase activity are not comparable to each other or to units of turnover estimated by simulations, all activities were expressed as relative fractions of maximum activity levels. For example, the highest activity of endocellu-lase on maple litter was observed on Day 100 of the experiment (Fig. 6c). Thus, activities of endocellulase at all other days were expressed as a proportion of this value.
reduction in activity, a second peak of activity and then a gradual decline. This pattern also was pro-duced by simulations, although timing of the initial peak in turnover rates was earlier than observed, and the simulated decline following the initial peak was more severe than observed (Fig. 6b and c). One of the reasons why these discrepancies existed between simulations and observations is that the model shows instantaneous responses to litter availability, without constraints imposed by limits in microbial growth rates. Thus, the simulated peaks in early turnover may have been higher than was possible for micro-biota to achieve during early colonization. A similar lag in initial decay of buried litter was observed by Moorhead and Reynolds (1991).
Two reasons why simulations predicted such rapid shifts in enzyme activities result from model struc-ture and underlying assumptions. First, the model is based on the assumption that microbiota degrade extractives in preference to acid-solubles, and de-grade acid-solubles in preference to acid-insolubles, given intrinsic rates of decay and availability of ni-trogen (Moorhead and Reynolds, 1991). Hence, the precipitous decline in cellulose turnover produced by the model occurred when turnover of extrac-tives was sufficient to immobilize all available ni-trogen and none was left to support decay of the acid-soluble pool. In reality, decomposer communi-ties are likely to be more diverse in their response to nutrient-limited conditions. A second reason why the model produced such rapid shifts in predicted levels of enzyme activities is that there are no pools of enzymes explicitly included in model structure – enzyme activities were inferred from turnover rates of carbon fractions of litter. In fact, enzymes persist in the environment and continue to catalyze chemical reactions for a period of time after being produced by microbiota. For this reason, activity levels are likely to show more gradual changes over time than were predicted.
4. Conclusions
The ability to determine activities of enzymes that hydrolyze specific chemical compounds permits us to link microbial behavior to decomposition processes more clearly than has been possible with other
mea-sures of microbial attributes. As an aggregate process, decomposition is accomplished by many organisms acting in concert, but profiles of enzyme activities provide a relatively unambiguous view of micro-bial behavior that is free of assumptions regarding biomass, taxonomy or overall metabolic attributes of a community.
Recent studies have shown that patterns of litter decay are correlated with activities of key classes of enzymes, and that decomposition can be modeled as a function of these enzyme activities (Sinsabaugh et al., 1991; Sinsabaugh and Moorhead, 1994). Herein, we have shown that more traditional modeling ap-proaches can be used to predict patterns of enzyme activities as a result of estimated turnover for partic-ular chemical constituents. Moreover, simulated and observed patterns of enzyme activities were qualita-tively similar. It remains to obtain experimental data that are sufficient to conduct a more rigorous quanti-tative test of this approach, and to explore the possible integration of enzyme-based (MARCIE; Sinsabaugh and Moorhead, 1994) and more traditional (GENDEC) models.
Many experimental and modeling studies have been performed to gain insights to decomposition pro-cesses, with attention directed to more detailed chem-ical characterizations of litter (Minderman, 1968), to expanding constituent-based, decay models (Bosatta and Ågren, 1991), and to incorporating detailed as-pects of microbial physiology (McGill et al., 1981). However, the choice of mechanisms can limit, as readily as expand, the usefulness of a model. Because of their intimate relationship with decomposition processes, enzyme activities offer new opportunities for the development and testing of decomposition models.
Acknowledgements
References
Aber, J.D., McClaugherty, C.A., Melillo, J.M., 1984. Litter Decomposition in Wisconsin Forests – Mass Loss, Organic-Chemical Constituents and Nitrogen. School of Natural Resources, University of Wisconsin, Madison.
Berg, B., Karenlampi, L., Veum, A.K., 1975. Comparisons of decomposition rates measured by means of cellulose. In: Wielgolaski, F.E. (Ed.), Fennoscandian Tundra Ecosystems Part 1. Plants and Microorganisms. Springer-Verlag, New York, pp. 261–267.
Bosatta, E., Ågren, G.I., 1991. Dynamics of carbon and nitrogen in the organic matter of the soil: a generic theory. Am. Nat. 138, 227–245.
Bunnell, F.L., Tait, D.E.N., Flanagan, P.W., Van Cleve, K., 1977. Microbial respiration and substrate weight loss-I. A general model of the influences of abiotic variables. Soil Biol. Biochem. 9, 33–40.
Dilly, O., Munch, J.C., 1996. Microbial biomass content basal respiration and enzyme activities during the course of decomposition of leaf litter in a black alder (Alnus glutinosa (L.) Gaertn.) forest. Soil Biol. Biochem. 28, 1073–1081. Frankland, J.C., 1966. Succession of fungi on decaying petioles
of Pteridium aquilinum. J. Ecol. 54, 41–63.
Frankland, J.C., 1969. Fungal decomposition of bracken petioles. J. Ecol. 57, 25–36.
Frankland, J.C., 1976. Decomposition of bracken litter. Bot. J. Linn. Soc. 73, 133–143.
Joshi, S.R., Sharma, G.D., Mishra, R.R., 1993. Microbial enzyme activities related to litter decomposition near a highway in a sub-tropical forest of northeast India. Soil Biol. Biochem. 25, 1763–1770.
Kshattriya, S., Sharma, G.D., Mishra, R.R., 1992. Enzyme activities related to litter decomposition in forests of different age and altitude in northeast India. Soil Biol. Biochem. 24, 265–270.
Linkins, A.E., Melillo, J.M., Sinsabaugh, R.L., 1984. Factors affecting cellulase activity in terrestrial and aquatic ecosystems. In: Klug, M.H., Reddy, C.A. (Eds.), Current Perspectives In Microbial Ecology. American Society of Microbiology, pp. 572–579.
Linkins, A.E., Sinsabaugh, R.L., McClaugherty, C.A., Melillo, J.M., 1990. Cellulase activity on decomposing leaf litter in microcosms. Plant Soil 123, 17–25.
Long-Term Intersite Decomposition Experiment Team (LIDET). 1995. Meeting the challenge of long-term, broad-scale ecological experiments. Publ. No. 19, Long-Term Ecological Research Off., Seattle, Washington.
McGill, W.B., Hunt, H.W., Woodmansee, R.G., Reuss, J.O., 1981. PHOENIX. A model of the dynamics of carbon and nitrogen in grassland soils. Ecol. Bull. (Stockholm) 33, 49– 115.
Melillo, J.M., Aber, J.D., Muratore, J.F., 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63, 621–626.
Minderman, G., 1968. Addition, decomposition and accumulation of organic matter in forests. J. Ecol. 56, 355–362.
Moorhead, D.L., Reynolds, J.F., 1991. A general model of litter decomposition in the northern Chihuahuan Desert. Ecol. Model. 59, 197–219.
Moorhead, D.L., Reynolds, J.F., 1993. Effects of climate change on decomposition in arctic tussock tundra: a modeling synthesis. Arctic Alpine Res. 25, 403–412.
Moorhead, D.L., Reynolds, J.F., 1996. Modeling decomposition in arctic ecosystems. In: Reynolds, J.F., Tenhunen, J.D.(Ed.), Landscape function: Implications for ecosystem response to disturbance, A Case Study in Arctic Tundra. Springer-Verlag, Ecological Studies Series, vol. 120, pp. 347–367 (Chapter 16)
Moorhead, D.L., Sinsabaugh, R.L., Linkins, A.E., Reynolds, J.F., 1996. Decomposition processes: modelling approaches and applications. Sci. Total Environ. 183, 137–149.
Parnas, H., 1975. Model for decomposition of organic material by microorganisms. Soil Biol. Biochem. 7, 161–169.
Parton, W.J., Schimel, D.S., Cole, C.V., Ojima, D.S., 1987. Analysis of factors controlling soil organic matter levels in Great Plains Grasslands. Soil Sci. Soc. Am. J. 51, 1173–1179. Ryan, M.G., Melillo, J.M., Ricca, A., 1990. A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res. 20, 166–0171.
Sinsabaugh, R.L., Moorhead, D.L., 1994. Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 26, 1305–1311.
Sinsabaugh, R.L., Antibus, R.K., Linkins, A.E., 1991. An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agri. Ecosys. Environ. 34, 43–54.
Sinsabaugh, R.L., Antibus, R.K., Linkins, A.E., McClaugherty, C.A., Rayburn, L., Repert, D., Weiland, T., 1992. Wood decomposition over a first-order watershed: mass loss as a function of lignocellulase activity. Soil Biol. Biochem. 24, 743– 749.