Diversity and distribution of nematode communities in grasslands from
Romania in relation to vegetation and soil characteristics
Iuliana Popovici
∗, Marcel Ciobanu
Institute of Biological Research, PB 229, 3400 Cluj-Napoca, RomaniaReceived 8 February 1999; received in revised form 8 October 1999; accepted 12 October 1999
Abstract
The nematode communities of 36 grassland ecosystems in Romania, belonging to different plant associations and soil types, were studied. The abundance of nematodes, the species and trophic types present, as well as their distribution in relation to plant community and soil characteristics are analyzed and discussed.
The abundance of nematodes from the 36 grasslands studied ranged between 0.41×106and 8.57×106individuals/m2, and a total of 121 genera and 145 species of nematodes were found. The highest diversity was found in grasslands developed on brown earth soil (65–67 genera and 74–76 species), with least diversity in those evolving on podzol and lithosol (33–36 genera with 25–28 identified species). Most of the dominant taxa were found in specific soil layers; some obligate plant parasitic genera (e.g.,Paratylenchus,Rotylenchus,Criconema) showed preference for deeper soil layers. The nematode diversity index (H′),
with values ranging between 2.38 and 3.47, did not differ significantly between the different types of grasslands. Plant feeding, bacterial feeding, hyphal feeding and omnivorous nematodes were the main groups in mountainous grasslands developed on different soil types. Plant feeding and bacterial feeding nematodes dominated the trophic structure and more plant feeders (62–69%) were found in communities of subalpine and alpine grasslands developed on podzol and alpine meadow soil, than in those developed on rendzina and lithosol (27–33%). The ratio of hyphal feeding to bacterial feeding nematodes (Hf/Bf) is constantly in favour of the bacterial feeding group, the values being an indicator of good soil fertility for most studied grasslands. The nematode communities of grasslands are grouped into six main clusters according to their genera affinity and distinguished by different grassland and soil types. Communities from subalpine grasslands developed on rendzina, acid brown and lithosol have the greatest similarities. An ordination of nematode communities in relation to important environmental variables is presented. Environmental variables relevant in explaining the patterns of nematode composition in grasslands, using canonical correspondence analysis (CCA), are: humus, pH, total nitrogen, exchangeable bases and soil type. No single factor could be selected. ©2000 Elsevier Science B.V. All rights reserved.
Keywords:Nematode community structure; Clusters; CCA; Grasslands; Soil variables
1. Introduction
Better information on the soil nematode fauna of natural ecosystems or ecosystems that are relatively
∗Corresponding author. Tel.:+40-64-191238;
fax:+40-64-191238.
E-mail address:[email protected] (I. Popovici).
undisturbed by human activities is providing useful insights into soil conditions in terrestrial habitats (Norton and Niblack, 1991; Hodda and Wanless, 1994b). The analysis of nematode community struc-ture is being used more frequently as a tool in ecolog-ical studies (Bongers, 1990). Pertinent multivariate analyses of nematode fauna in relation to environ-mental variables have been published by De Goede
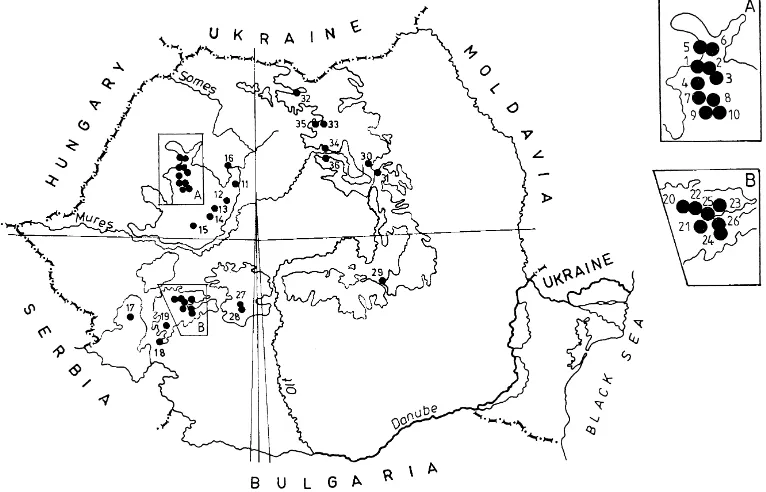
Fig. 1. Geographical distribution (using UTM grid) of the 36 grasslands studied in Romania. (A and B are enlarged areas; the numbers represent site numbers).
(1993), De Goede and Bongers (1994) and Hanel (1995).
A recent monographic compilation of the nema-tode faunal lists and soil conditions from grasslands in Europe (41 datasets) and the USA (three datasets), available also on a CD-disk, represents the most avail-able up-to-date information on these communities on a large geographical scale (De Goede and Bongers, 1998). This work includes 32 sites from Romania (Popovici, 1998). These data have not yet been anal-ysed or synthesized. In the present paper an analy-sis of the dataset from Romania including data from four additional sites (Popovici and Ciobanu, 1998) is made. The paper describes nematode abundance, di-versity and affinity for specific plant associations and soil characteristics from all 36 investigated mountain grassland ecosystems in Romania.
An extensive study on nematode communities from one protected area in Romania including data from both natural forest and grassland ecosystems has been presented previously (Popovici, 1993).
2. Material and methods
2.1. Site descriptions
The 36 grasslands (Fig. 1) were distributed at altitudes from 350 to 2270 m above sea level and represented 15 different plant associations developed on different soil types (Table 1). Half of the sites were sampled in the last decade, and 10 others be-tween 1981–1989, during an ecological project on the Carpathian fauna.
The code of sites, given in Table 1, comprises a number for site (1–36) and an abbreviation for
the mountainous massif (e.g., 1 Bih=site no.
1 from the Bihor Mountains). Plant association is given according to Coldea (1991, 1997). Soil classification is given according to Conea et al. (1980).
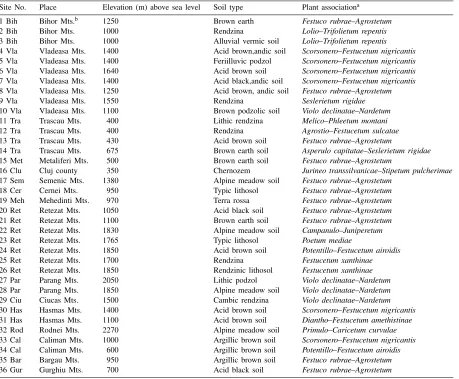
Table 1 Sites descriptions
Site No. Place Elevation (m) above sea level Soil type Plant associationa 1 Bih Bihor Mts.b 1250 Brown earth Festuco rubrae–Agrostetum
2 Bih Bihor Mts. 1000 Rendzina Lolio–Trifolietum repentis
3 Bih Bihor Mts. 1000 Alluvial vermic soil Lolio–Trifolietum repentis 4 Vla Vladeasa Mts. 1400 Acid brown,andic soil Scorsonero–Festucetum nigricantis 5 Vla Vladeasa Mts. 1400 Feriilluvic podzol Scorsonero–Festucetum nigricantis 6 Vla Vladeasa Mts. 1640 Acid brown soil Scorsonero–Festucetum nigricantis 7 Vla Vladeasa Mts. 1400 Acid black,andic soil Scorsonero–Festucetum nigricantis 8 Vla Vladeasa Mts. 1250 Acid brown, andic soil Festuco rubrae–Agrostetum
9 Vla Vladeasa Mts. 1550 Rendzina Seslerietum rigidae
10 Vla Vladeasa Mts. 1100 Brown podzolic soil Violo declinatae–Nardetum 11 Tra Trascau Mts. 400 Lithic rendzina Melico–Phleetum montani
12 Tra Trascau Mts. 400 Rendzina Agrostio–Festucetum sulcatae
13 Tra Trascau Mts. 430 Acid brown soil Festuco rubrae–Agrostetum
14 Tra Trascau Mts. 675 Brown earth soil Asperulo capitatae–Seslerietum rigidae 15 Met Metaliferi Mts. 500 Brown earth soil Festuco rubrae–Agrostetum
16 Clu Cluj county 350 Chernozem Jurineo transsilvanicae–Stipetum pulcherimae 17 Sem Semenic Mts. 1380 Alpine meadow soil Festuco rubrae–Agrostetum
18 Cer Cernei Mts. 950 Typic lithosol Festuco rubrae–Agrostetum 19 Meh Mehedinti Mts. 970 Terra rossa Festuco rubrae–Agrostetum 20 Ret Retezat Mts. 1050 Acid black soil Festuco rubrae–Agrostetum 21 Ret Retezat Mts. 1100 Brown earth soil Festuco rubrae–Agrostetum 22 Ret Retezat Mts. 1830 Alpine meadow soil Campanulo–Juniperetum
23 Ret Retezat Mts. 1765 Typic lithosol Poetum mediae
24 Ret Retezat Mts. 1850 Acid brown soil Potentillo–Festucetum airoidis
25 Ret Retezat Mts. 1700 Rendzina Festucetum xanthinae
26 Ret Retezat Mts. 1850 Rendzinic lithosol Festucetum xanthinae 27 Par Parang Mts. 2050 Lithic podzol Violo declinatae–Nardetum 28 Par Parang Mts. 1850 Alpine meadow soil Violo declinatae–Nardetum 29 Ciu Ciucas Mts. 1500 Cambic rendzina Violo declinatae–Nardetum 30 Has Hasmas Mts. 1400 Acid brown soil Scorsonero–Festucetum nigricantis 31 Has Hasmas Mts. 1100 Acid brown soil Diantho–Festucetum amethistinae 32 Rod Rodnei Mts. 2270 Alpine meadow soil Primulo–Caricetum curvulae 33 Cal Caliman Mts. 1000 Argillic brown soil Scorsonero–Festucetum nigricantis 34 Cal Caliman Mts. 600 Argillic brown soil Potentillo–Festucetum airoidis 35 Bar Bargau Mts. 950 Argillic brown soil Festuco rubrae–Agrostetum 36 Gur Gurghiu Mts. 700 Acid black soil Festuco rubrae–Agrostetum
aAccording to Coldea, 1991, 1997. bMts.=Mountains.
2.2. Sampling, extraction, identification
Five samples were taken separately from the sod layer and the A soil horizon, with an open corer (15 cm in length and 2.2 cm in diameter), down to a depth 10 or 15 cm. Each sample consisted of eight cores taken from 10 m2area and mixed for each soil layer.
The centrifugation method (De Grisse, 1969) was used for nematode extraction from each bulk sample (the mixed cores from each soil layer). Nematodes were counted before fixing in 4% formaldehyde solu-tion.
At least 150 individuals were identified for the five samples of each soil layer. The classification system is according to Andrássy (1984), Siddiqi (1986) and Bongers (1988) where appropriate and the contri-bution of trophic groups is established according to Yeates et al. (1993).
2.3. Data analysis
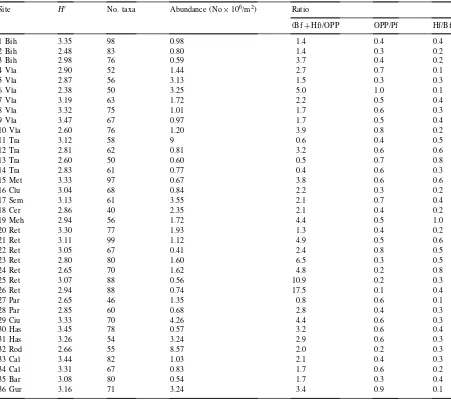
abun-Table 2
The diversity index (H′), numbers of taxa (genera
+species), abundance and trophic group ratios for nematode communities in Romanian grasslandsa
Site H′ No. taxa Abundance (No
×106/m2) Ratio
(Bf+Hf)/OPP OPP/Pf Hf/Bf
1 Bih 3.35 98 0.98 1.4 0.4 0.4
2 Bih 2.48 83 0.80 1.4 0.3 0.2
3 Bih 2.98 76 0.59 3.7 0.4 0.2
4 Vla 2.90 52 1.44 2.7 0.7 0.1
5 Vla 2.87 56 3.13 1.5 0.3 0.3
6 Vla 2.38 50 3.25 5.0 1.0 0.1
7 Vla 3.19 63 1.72 2.2 0.5 0.4
8 Vla 3.32 75 1.01 1.7 0.6 0.3
9 Vla 3.47 67 0.97 1.7 0.5 0.4
10 Vla 2.60 76 1.20 3.9 0.8 0.2
11 Tra 3.12 58 9 0.6 0.4 0.5
12 Tra 2.81 62 0.81 3.2 0.6 0.6
13 Tra 2.60 50 0.60 0.5 0.7 0.8
14 Tra 2.83 61 0.77 0.4 0.6 0.3
15 Met 3.33 97 0.67 3.8 0.6 0.6
16 Clu 3.04 68 0.84 2.2 0.3 0.2
17 Sem 3.13 61 3.55 2.1 0.7 0.4
18 Cer 2.86 40 2.35 2.1 0.4 0.2
19 Meh 2.94 56 1.72 4.4 0.5 1.0
20 Ret 3.30 77 1.93 1.3 0.4 0.2
21 Ret 3.11 99 1.12 4.9 0.5 0.6
22 Ret 3.05 67 0.41 2.4 0.8 0.5
23 Ret 2.80 80 1.60 6.5 0.3 0.5
24 Ret 2.65 70 1.62 4.8 0.2 0.8
25 Ret 3.07 88 0.56 10.9 0.2 0.3
26 Ret 2.94 88 0.74 17.5 0.1 0.4
27 Par 2.65 46 1.35 0.8 0.6 0.1
28 Par 2.85 60 0.68 2.8 0.4 0.3
29 Ciu 3.33 70 4.26 4.4 0.6 0.3
30 Has 3.45 78 0.57 3.2 0.6 0.4
31 Has 3.26 54 3.24 2.9 0.6 0.3
32 Rod 2.66 55 8.57 2.0 0.2 0.3
33 Cal 3.44 82 1.03 2.1 0.4 0.3
34 Cal 3.31 67 0.83 1.7 0.6 0.2
35 Bar 3.08 80 0.54 1.7 0.3 0.4
36 Gur 3.16 71 3.24 3.4 0.9 0.1
a(Bf+Hf)/OPP — ratio of bacterial and hyphal feeding nematodes to obligatory plant parasites, OPP/Pf — ratio of obligatory plant parasites to total plant feeding nematodes, Hf/Bf — ratio of hyphal feeding to bacterial feeding nematodes.
dance of genera, Shannon’s diversity index (H′) and
similarity of nematode communities were analyzed using the BIODIV program (Baev and Penev, 1995). The ordination of samples (32 sites), nematode genera and their relationships with environmental variables were analyzed by the canonical correspondence anal-ysis method (CCA) using the CANOCO program (Ter Braak, 1986, 1987). The genera abundance data were transformed to log(x+1). Four sites (nos. 13, 14, 17
and 22), without environmental data, were left out of the CCA analysis.
3. Results
Nematode abundance in the 36 grasslands
stud-ied ranged between 0.41×106 and 8.57×106
Table 3
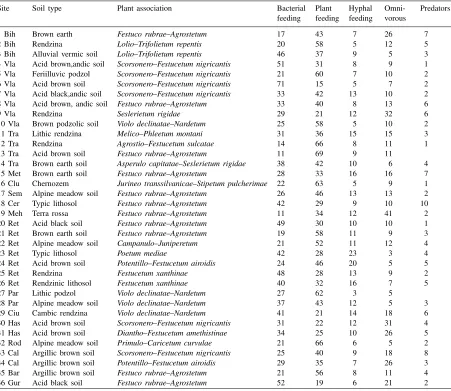
Composition of nematode feeding groups (%) in various soil types and plant associations
Site Soil type Plant association Bacterial Plant Hyphal Omni- Predators feeding feeding feeding vorous
1 Bih Brown earth Festuco rubrae–Agrostetum 17 43 7 26 7
2 Bih Rendzina Lolio–Trifolietum repentis 20 58 5 12 5
3 Bih Alluvial vermic soil Lolio–Trifolietum repentis 46 37 9 5 3
4 Vla Acid brown,andic soil Scorsonero–Festucetum nigricantis 51 31 8 9 1 5 Vla Feriilluvic podzol Scorsonero–Festucetum nigricantis 21 60 7 10 2 6 Vla Acid brown soil Scorsonero–Festucetum nigricantis 71 15 5 7 2 7 Vla Acid black,andic soil Scorsonero–Festucetum nigricantis 33 42 13 10 2 8 Vla Acid brown, andic soil Festuco rubrae–Agrostetum 33 40 8 13 6
9 Vla Rendzina Seslerietum rigidae 29 21 12 32 6
10 Vla Brown podzolic soil Violo declinatae–Nardetum 25 58 5 10 2
11 Tra Lithic rendzina Melico–Phleetum montani 31 36 15 15 3
12 Tra Rendzina Agrostio–Festucetum sulcatae 14 66 8 11 1
13 Tra Acid brown soil Festuco rubrae–Agrostetum 11 69 9 11
14 Tra Brown earth soil Asperulo capitatae–Seslerietum rigidae 38 42 10 6 4
15 Met Brown earth soil Festuco rubrae–Agrostetum 28 33 16 16 7
16 Clu Chernozem Jurineo transsilvanicae–Stipetum pulcherimae 22 63 5 9 1
17 Sem Alpine meadow soil Festuco rubrae–Agrostetum 26 46 13 13 2
18 Cer Typic lithosol Festuco rubrae–Agrostetum 42 29 9 10 10
19 Meh Terra rossa Festuco rubrae–Agrostetum 11 34 12 41 2
20 Ret Acid black soil Festuco rubrae–Agrostetum 49 30 10 10 1
21 Ret Brown earth soil Festuco rubrae–Agrostetum 19 58 11 9 3
22 Ret Alpine meadow soil Campanulo–Juniperetum 21 52 11 12 4
23 Ret Typic lithosol Poetum mediae 42 28 23 3 4
24 Ret Acid brown soil Potentillo–Festucetum airoidis 24 46 20 5 5
25 Ret Rendzina Festucetum xanthinae 48 28 13 9 2
26 Ret Rendzinic lithosol Festucetum xanthinae 40 32 16 7 5
27 Par Lithic podzol Violo declinatae–Nardetum 27 62 3 5
28 Par Alpine meadow soil Violo declinatae–Nardetum 37 43 12 5 3
29 Ciu Cambic rendzina Violo declinatae–Nardetum 41 21 14 18 6
30 Has Acid brown soil Scorsonero–Festucetum nigricantis 31 22 12 31 4 31 Has Acid brown soil Diantho–Festucetum amethistinae 34 25 10 26 5
32 Rod Alpine meadow soil Primulo–Caricetum curvulae 21 66 6 5 2
33 Cal Argillic brown soil Scorsonero–Festucetum nigricantis 25 40 9 18 8 34 Cal Argillic brown soil Potentillo–Festucetum airoidis 29 35 7 26 3
35 Bar Argillic brown soil Festuco rubrae–Agrostetum 21 56 8 11 4
36 Gur Acid black soil Festuco rubrae–Agrostetum 52 19 6 21 2
were widest in rendzina (0.56×106–4.26×106
individuals/m2) (n=7) and acid brown soils (0.57×
106–3.24×106individuals/m2) (n=7).
A total of 121 genera and 145 species were identi-fied in soils from the different Romanian grasslands (Tables 2–6 in Popovici, 1998; Table 1 in Popovici and Ciobanu, 1998). There were 33–67 genera present in each site. The lowest diversity was noted in lithosol (18 Cer, 40 taxa represented by 33 genera and 29 identified species) and the highest in brown earth soil (1 Bih, 15 Met, 21 Ret) (97–99 taxa
repre-sented by 65–67 genera and 73–74 species) (Table 2). Overall there were 30 dominant nematode genera (each representing more than 5.1% of the popula-tions) but only 2–7 were preponderant at any one site.
In alpine grasslands (27 Par, 32 Rod), Aglenchus,
FilenchusandParatylenchuswere the dominant
gen-era. In subalpine grasslands, the genera Filenchus,
Acrobeloides,Gracilacus,Paratylenchus,Plectusand
Rotylenchus were prevalent. The genera with
Filenchus, Rotylenchus, Acrobeloides, Plectus,
Ec-phyadophora, Paratylenchus, Anaplectus and
Tylen-cholaimus.
The generic diversity (H′) of the 36 sites ranged
be-tween 2.38 and 3.47, without clear differences bebe-tween the different nematode communities (Table 2). Val-ues of the Shannon’s diversity (H′) for communities
developed in rendzina, brown earth and acid brown soils (17 sites) had larger ranges (2.38–3.47) than those for communities in lithosol, podzol and alpine meadow soils (2.65–3.13) (nine sites).
The composition of the nematode communities comprised five main trophic groups: plant feeding (Pf), bacterial feeding (Bf), hyphal feeding (Hf), omnivorous (O) and predators (P). The distribution of feeding groups differed within the same type of mountainous grassland developed on varied soils (Table 3).
Plant feeding nematodes were the dominant group in 61% of the populations investigated. The bacte-rial feeding group was dominant in 28% of the sites, mainly in communities developed in rendzina, acid black soil, acid brown soil and lithosol.
In subalpine and alpine grasslands, plant feeding ne-matodes had higher representation (46–69%) in com-munities developed in acid brown, podzol and alpine meadow soil as compared to those from rendzina and lithosol where the bacterial feeding group was domi-nant (40–49%) (Table 3).
The ratios of hyphal to bacterial feeding nematodes (Hf/Bf) show a constant preponderance of the bacterial feeding group (Table 2). These values are an indicator of good soil fertility in the grasslands studied. The ratio between nematodes living on bacteria and fungi, and obligate plant parasites ((Bf+Hf)/OPP) was between
0.4 and 6.5, with two exceptions having very high values (sites 25 Ret and 26 Ret, subalpine grasslands developed on rendzina) (Table 2). The plant feeding group was mainly composed of non-obligatory plant parasites (e.g., subgroups 1e, 1f, of Yeates et al., 1993), which explains the low values of the ratio OPP/Pf (Table 2).
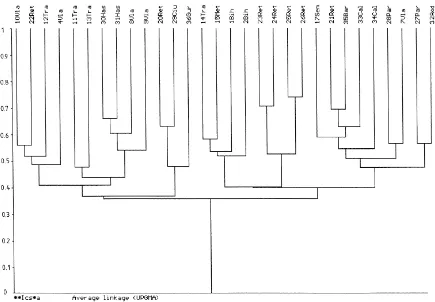
The nematode communities of 30 grassland sites were grouped into several clusters of affinity, based on the Czekanowski–Soerensen similarity coefficient of the genera (Ics) (Pesenko, 1982 in Baev and Penev, 1995) (Fig. 2). Six of the communities studied (from site nos. 3, 5, 6, 16, 18 and 19), had very low
affin-ity values, and were successively excluded from the dendrogram.
At a similarity of 37–40% six main clusters are distinguished, grouping different types of grasslands and soils. Each of these clusters is subdivided into groups having higher values of the nematode gen-era affinity. The highest similarity (70–72%) was shown by communities of subalpine grasslands on rendzina (sites 25 and 26), followed by those de-veloped on acid brown soil and lithosol (sites 23 and 24). At a similarity between 60 and 70% the nematode communities of different types of grass-lands on brown soil (sites 21, 35 and 8, 30, 31) or on acid black and rendzina (sites 20, 29) are grouped.
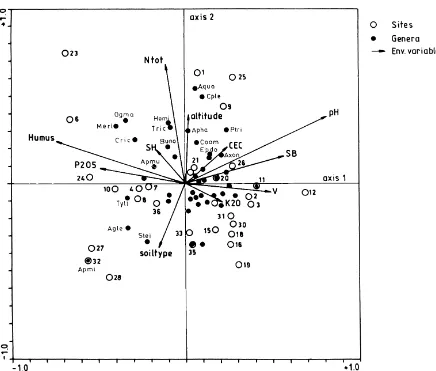
Generally, the nematode communities developed in oligotrophic soils such as argillic brown, brown pod-zolic, podzolic and alpine meadow soil (sites nos. 10, 17, 22, 27, 28, 32, 35) are grouped apart from those communities developed in eu-mesotrophic soils (brown earth, rendzina) (sites nos. 1, 2, 14, 15, 25, 26). CCA was used to assess the relative importance of environmental variables in explaining the patterns of nematode occurrence in the grasslands. On the CCA diagram (Fig. 3), Axis 1 is defined by the soil vari-ables (with long arrows): humus, pH, SB, P2O5 and
V%; Axis 2 is defined by N(tot) and soil type. The eigen values are : X1=0.155; X2=0.102; genera/
environmental correlations are X1=0.927 and
X2=0.947.
Most of the nematode genera are widespread, and are positioned in the center of the diagram.
Grasslands, and nematode genera such asAquatides,
Ceratoplectus, Paratrichodorus, Coomansus,
Ax-onchium,EpidorylaimusandChiloplacusin rendzina
and brown earth soils (sites nos. 1, 9, 21, 25, 26, located on the upper right-side of the diagram), are positively correlated with soil pH and exchange-able bases (SB) (Fig. 3). Positive correlations with humus and total nitrogen [N(tot)] can also be no-ticed for communities in grasslands evolving on acid brown soil and lithosol (sites nos. 6, 23, 24, located on the upper left-side of the diagram). The genera
Ogma, Hemicriconemoides, Merlinius, Trichodorus,
Criconema and Aporcelaimus are positioned in this
area. Soil type seems to be better correlated with
nematodes such as Aglenchus, Ecphyadophora,
Fig. 2. Dendrogram showing the clusters of soil nematode communities from grasslands according to the Ics similarity coefficient at generic level (sites codes as in Table 1).
developed on podzol and alpine meadow soil (lower left-side) which, at the same time, show negative correlations with other soil variables (Fig. 3). For a large group of nematode genera from grasslands evolving on a wide range of soil types, from rendzina to argillic soil (lower right-side of Fig. 3), no clear correlations with environmental variables could be detected.
4. Discussion
Nematode abundance in some European grasslands ranges between 0.5×106and 12×106individuals/m2
(Gerber, 1985; Wasilewska, 1994; Hodda and Wan-less, 1994b; Hanel, 1995; De Goede and Bongers, 1998; Ilieva, 1998). Nematode diversity and abun-dance in grasslands were positively correlated (Hanel, 1995) and dependent upon variations in environmen-tal conditions (Wasilewska, 1994).
The composition of nematode communities in grasslands is characterized by high specific and generic richness (Wasilewska, 1979, 1994; Gerber, 1985; Popovici, 1993, 1998; Hodda and Wanless, 1994a; De Goede and Bongers, 1994, 1998; Hanel, 1996, 1998; Ilieva, 1998; Popovici and Ciobanu, 1998). The index of generic diversity (H′) described
here confirms the results given for grasslands by
Wasilewska (1979, 1994) (H′=3.7–4.9) and Hanel
(1993) (H′=2.4–3.6).
Clear patterns in the trophic structure could be dis-tinguished. The proportion of trophic groups, domi-nated by plant feeding and bacterial feeding nema-todes, was similar to that recorded for chalk grassland (Hodda and Wanless, 1994b) and permanent pastures (Wasilewska, 1979, 1994 and Valocka and Sabova, 1997).
Our data on the ratio of (Bf+Hf)/OPP show
Fig. 3. Canonical correspondence analysis diagram (CCA) for 32 soil nematode communities of grasslands from Romania [the numbers represent site numbers (Table 1). Abbreviations on arrows: Humus, humus content (%); N(tot), total nitrogen (%); pH, in water; SB, total exchangeable bases (meq/100 g soil); SH, total hydrolytic activity (meq/100 g soil); CEC, cation exchange capacity (%); V, base saturation (%); Abbreviations for genera: Agle, Aglenchus; Apha, Aphanolaimus; Apmi, Aporcelaimium; Apmu, Aporcelaimus; Aqua, Aquatides; Axon,Axonchium; Buno,Bunonema; Cric,Criconema; Coom,Coomanus; Cple,Ceratoplectus; Epdo,Epidorylaimus; Hemi, Hemicriconemoides; Merl,Merlinius; Ogma,Ogma; Ptri,Paratrichodorus; Stei,Steinernema; Tric,Trichodorus; Tyll,Tylencholaimellus].
permanent meadows. The total number of plant feed-ing nematodes is dominated by non-obligatory plant parasites as shown by the ratio of OPP/Pf. All these re-sults reflect the different level and distribution of food sources between various types of grasslands.
Clusters with different similarity of nematode com-munities are presented by Hanel (1995, 1996). Generic composition of the nematode fauna showed the most stable community in meadows (Hanel, 1995).
5. Conclusions
A high abundance and diversity characterize the ne-matode communities of 36 Romanian grasslands. The highest values were recorded for communities devel-oped in rendzina and brown earth soil.
Plant feeding, bacterial feeding, hyphal feeding and omnivorous groups prevail in the nematode trophic structure.
Six main clusters of nematode communities are dis-tinguished at about 40% similarity, based on the affin-ity of genera. The highest affinaffin-ity was observed for communities in subalpine grasslands on rendzina, acid brown and lithosol.
Relevant environmental variables, such as soil pH, total nitrogen, humus content, exchangeable bases and soil type could explain the variations in the composi-tion of nematode communities in grasslands, but no single factor could be selected as being of overriding importance.
References
Andrássy, I., 1984. Klasse Nematoda. Bestimmungsbücher zur Bodenfauna Europas. Lief. 9, Akademie-Verlag Berlin, 509 pp. Baev,V.P., Penev, L.D., 1995. BIODIV. Program for calculating biological diversity parameters, similarity, niche overlap and cluster analysis, Version 5.1. Pensoft, Sofia, Moscow, 55 pp. Bongers, T., 1988. De Nematoden van Netherland. KNNV
Bibliotheekuitgave 46, Pirola, Schrool, Netherlands, 408 pp. Bongers, T., 1990. The Maturity Index: an ecological measure
of environmental disturbance based on nematode species composition. Oecologia 83, 14–19.
Coldea, Gh., 1991. Prodrome des associations végétales des Carpates du Sud-Est (Carpates Roumaines). Documents phytosociologiques, Camerino 13, 539 pp.
Coldea, Gh., 1997. Les associations végétales de Roumanie I. Les associations herbacées naturelles. Presses Universitaires de Cluj, 261 pp.
Conea, A., Florea, N., Puiu, St., 1980. Sistemul român de clasificare a solurilor. ASAS, ICPA, Bucuresti, 178 pp. De Goede, R.G.M., 1993. Terrestrial nematodes in a changing
environment. Ph. D. Thesis, CIP — gegevens Koninklijke Bibliotheek, Haag, 138 pp.
De Goede, R.G.M., Bongers, T., 1994. Nematode community structure in relation to soil and vegetation characteristics. Appl. Soil Ecol. 1, 29–44.
De Goede, R.G.M., Bongers, T., 1998. Nematode fauna of grassland and dwarf-shrub vegetation in The Netherlands. In: De Goede, R.G.M., Bongers, T. (Eds.), Nematode Communities of Northern Temperate Grassland Ecosystems. Focus, Giessen, pp. 79–88.
De Grisse, A.T., 1969. Redescription ou modifications de quelques techniques utilisées dans l’étude de nématodes phytoparasitaires. Meded. Fak. Landbouww. Gent 34, 351–370.
Gerber, K., 1985. Faunistische und ökologische Studie über die Nematoden einiger subalpiner Böden bei Bagdastein (Zentralalpen, Österreich). In: Franz, H. (Ed.), Veröffentlichungen des Österreichischen MaB-Programms, Band 9, Universitätsverlag Wagner, Innsbruck, pp. 113–131.
Hanel, L., 1993. Diversity of soil nematodes (Nematoda) in various types of ecosystems. Ekologia (Bratislava) 12, 259–272. Hanel, L., 1995. Secondary successional stages of soil nematodes
in cambisols of South Bohemia. Nematologica 41, 197–218. Hanel, L., 1996. Composition and seasonal changes of soil
nematode community in a South Bohemian meadow. Acta Soc. Zool. Bohem. 60, 103–114.
Hanel, L., 1998. Soil nematodes of grassland-meadow ecosystems in the Czech Republic, Central Europe. In: De Goede, R.G.M., Bongers, T. (Eds.), Nematode Communities of Northern Temperate Grassland Ecosystems. Focus, Giessen, pp. 95–122. Hodda, M., Wanless, F.R., 1994a. Nematodes from an English chalk grassland: species distributions. Nematologica 40, 116– 132.
Hodda, M., Wanless, F.R., 1994b. Nematodes from an English chalk grassland: population ecology. Pedobiologia 38, 530–545. Ilieva, Z.I., 1998. Nematode fauna of a grassland in the biospherical reserve Parangalitsa in Bulgaria. In: De Goede, R.G.M., Bongers, T. (Eds.), Nematode Communities of Northern Temperate Grassland Ecosystems. Focus, Giessen, pp. 131–138.
Norton, D.C., Niblack, T.L., 1991. Biology and ecology of nematodes. In: Nickle, W.R. (Ed.), Manual of Agricultural Nematology. Marcel Dekker, New York, pp. 85–102. Popovici, I., 1993. Structura ¸si dinamica comunitˇa¸tilor de
nematode (Nematoda). In: Popovici, I. (Ed.), Parcul Na¸tional Retezat-Studii ecologice. West Side, Bra¸sov, pp. 200–214. Popovici, I., 1998. Structure of nematode communities in mountain
grasslands from Romania. In: De Goede, R.G.M., Bongers, T. (Eds.), Nematode Communities of Northern Temperate Grassland Ecosystems. Focus, Giessen, pp. 221–240. Popovici, I., Ciobanu, M., 1998. Diversitatea ¸si distribu¸tia
nematofaunei ˆın ecosisteme din Mun¸tii Bârgˇaului ¸si Cˇaliman. Studii ¸si Cercetˇari, ¸Stiin¸tele naturii, Bistri¸ta 4, 225–240. Siddiqi, M.T., 1986. Tylenchida, parasites of plants and insects.
Farnham Royal Slough, UK, Commonwealth Agric. Bureaux, 645 pp.
Ter Braak, C.J.F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67, 1167–1179.
Ter Braak, C.J.F., 1987. CANOCO — a FORTRAN program for canonical community ordination by [partial] [detrended][canonical] correspondence analysis, principal components analysis and redundancy analysis (Version 2.1). ITI-TNO, Wageningen, 95 pp.
Wasilewska, L., 1979. The structure and function of soil nematode communities in natural ecosystems and agrocenosis. Pol. Ecol. Stud. 5, 97–145.
Wasilewska, L., 1994. The effect of age of meadows on succession and diversity in soil nematode communities. Pedobiologia 38, 1–11.
Yeates, G.W., 1984. Variation in soil nematode diversity under pasture with soil and year. Soil Biol. Biochem. 16, 95–102. Yeates, G.W., Bongers, T., De Goede, R.G.M., Freckman, D.W.,