ffiffiffiffiffiffiffiHffiffi
I
nte
rnationa
I
Conference
c u r r e n
t
B r e a k t h ro?.:i,
ff:i
ffi
:r#::'
r,:#
:lx
lffi::
Muhammadiyah University of Surakarta, Solo Centrat lava tndonesia )anuary 10 th,2Ats
Proceeding
International
Conference"Current Breakthrough in Pharmacy Matetials and Analyses"
/
Nurwaini ef aL, (ed) Surakarta : Muhammadiyah University'Press, 20Lsxxi, 153 pages
ISBN : 97 8- 602-7 0 429 -9 -5
Pharmacy
Chairman of Editors
Team of Pharmaceutical Technology
Setyo Nurwaini, M.Sc.
Anita Sukmawati, Ph.D., Apt, Suprapto, M.Sc., Apt.
Erindyah Retno Wikantyasning, Ph.D., Apt.
Setyo Nurwaini, M.Sc.
Dedi Hanwar, M.Si., Apt. Dr. Muhammad Da'i, M.Si., Apt. Broto Santoso, M.Sc., Apt.
Dra. Nurul Mutma'inah, M.Si., Apt.
Zaklry Cholisoh, M.Clin. Pharm., Ph.D., Apt. Arifah Sri Wahyuni, M.Sc., Apt.
Tanti Azizah Sujono, M.Sc., Apt.
Zakky Cholisoh, M.Clin. Pharm., Ph.D., Apt. Arifah Sri Wahyuni, M.Sc., Apt.
Dra. Nurul Mutma'inah, M.Si., Apt.
Ratna Yuliani, M.Biotech.St.
Azis, Saifudin, Ph.D., Apt.
Ratna Yuliani, M.Biotech.St.
Azis, Saifudin, Ph.D., Apt.
Team of Chemical Analyses
Team of Pharmacy Community and Clinic
Team of Pharmacological Study
Team of Herbal Medicine
'feam of Microbiology Pharmacy
Copyright
@20L5
'.Copyright in compilers and reserved
Design cover: Pubiication and Documentation Team
Layout: Secretaris and IT Team
Published by:
Muhammadiyah University Press
Universitas Muhammadiyah Surakarta
Il.A.Yani Pabelan Tromol Pos I Kartasura SurakartaSTL02 Telp. (+62 27 L) 7 L7 4L7 -L72, E-mail: [email protected]
Proceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 97 8-602-7 0429 -9 -5
TABLE OF CONTENTS
LIST OF PARTICIPANT NON
PRESENTERS...
...ixLIST OF ORAL
PRESENTERS...
...xvLIST OF POSTER
PRESENTERS
...xviiiB.PHARMACEUTICAL
TECHNOLOGY
... 1B-OO2
DEVELOPMENT OF PARTICULATE FORMULATION, LOADING PROCEDURE ANDNANO.SCALE CHAMCTERIZATION
OF PLGA
NANOPARTICLES INCOORPOMTING ROOT EXTMCT OF Pueraria lobata (Wild) Ohwl Mardiyantol*, NeudrtAchimz
...18-005
FORMULATION OF Jatropha Multifida Linn. LATEX AS TOPICAL DosAGE FORM AND WOUND HEALING STUDY IN SWISS WEBSTER MICE Amilar, Fetri Lestarir, AbdulRahmant
...,...g8-008
EFFECT OF USE VIRGIN COCONUTOIL
[VCO) AS EMOLTENT ON PHYSICAL PROPERTIES AND STABILITY OF VITAMIN C IN TRANSPARENT SOAP Ms. Fatimah Kasorr., AnitaSukmawatit...
...16B.O1O
.
INCREASING SOLUBILITY AND DISSOLUTION OF GLIMEPIRIDE THROUGH CO. CRYSTALLIZATION METHOD Fitrianti Darusmapr*, Sundani N Soewandhiz, RachmatMauludins...
...2s8-012
SYNTHESISOF
POLY(METHACRYLICACID)
CROSS-LINKEDBY
SILVER NANOPARTICLES FOR pH-RESPONSIVE NANoSENSoR Erindyah R. Wikantyasningl*, Bunga Saviti-ir, BrotoSantosol,Suprapto
...33C-CHEMICAL
ANAIYSES.."...
...
...!...r ...42C.OO1
ELECTROCHEMICAL DETECTION OF ZANAMIVIR USING GOLDAND
GOLD-MODIFIED BORON DOPED DIAMOND ELECTRODE
wulan Tri wahyunil,2*, Ivandini rribidasari A1, Endang
Saepudin
...q2C.OOz
OPTIMIZATION OF HPLC CONDITION FOR DETERMINATION OF VITAMIN A ANDDg IN PHARMACEUTICAL PREPARATIONS
Engrid |uni Astutit, Mochammad Yuwonoz, Muhamad
Zainuddinz
...4gProceeding International Confe rence
"current Breakthrough in pharmacy Materials and Analyses,,
ISBN : 97 8-602-7 A429 -9 -S
e
ETECTROCHEMICAT DETECTION OF ZANAMIVIR USING GOI,D AND GOLD-MODIFIED
BORON DOPED DIAMOND ETECTRODE
Wulan
Tri
Wahyunir,z.,Ivandini Tribidasari
41, Endang SaepudintrDeportment of Chemistry, Faculty of Mathematics and Natural Sciences, University
of
I ndon e si a, D epok I ndonesia
z Department of Chemistry, Faculty of l4athematics and Natural Sciences, Bogor Agricultural
U niversity, B og or Indonesia
.E-mail : [email protected]
Abstract
Zanamivir
is a
neuraminidaseinhibitor
approvedby
WHOfor
treatment andprophylaxis of influenza virus A and B. Quantitative analysis of zanamivir is important to
monitor
its
contentin
medicine and aquatic environment.This
study addressed theelectrochemical detection
of
zanamivir using
gold and
gold-modifiedboron
dopeddiamond (BDD) electrodes. The measurement was conducted using cyclic voltammetry method. Logarithmic curve was consistently observed from measurement of zanamivir in wide range of concentration on three electrodes. Linear calibration curve of zanamivir was
obtained
in
the concentration rangeof
Lx
10-s-
1x
10-amol/L
(r'
-
0.969) on goldelectrode,5x10-6-1x10'+mol/L(rz=0.990)onAu-BDDelectrode,and1xL0-6-1x1.0-+
mol/L
(r,
-
0.998) on AuNPs-BDD. Meanwhile, the detectionlimit
estimated to be L.19 x1.0-6,'1..49
x
1,0-s, and 2.29x
10-6 molfL, respectivelyfor
gold, Au-BDD, and AuNPs-BDDelectrode. The precision of zanamivir measurements on three electrodes was good with
%RSD < 2.5 o/o.
Keywords : Diamond, Electrochemical, Gold, Voltammetry, Zanam ivir.
INTRODUCTION
Zanamivir (Figure 1) is a neuraminidase inhibitor approved by WHO for treatment and
prophylaxis of influenza virus A and B. This compound prevents the release and spread of the
neurly formed virion through blocking the active site of neuraminidase. Zanamivir therapy was
reported
to
be more effectivefor
influenza B treatment compareto
oseltamivir (fcrmerneuraminidase inhibitor) [1]. The use of zanamivir was rose as-oseltamivir resistant virus was
reported [2]. Examination of zanamivir content in commercial drugs should be conducted in
order
to
ensure that the patients get the appropriate.treatment dosage. lmproper dosageslead
to
resistance effect of the viruses. Furthermore, the concentration of zanamivii' in theaquatic environment also important
to
be
monitored dueto
risingcontent
by
humanProceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 978-602-7 0429-9-5
excresions and improper disposal [3]. Undesired
high
levelsof
antivirats'in
the
aquaticenvironment which contact with the viruses are potentially cause resistance.
Several analysis methods was reported
for
zanamivir determination, such as LC-MS[4,5J and detection using mercury drop electrode [5]. These techniques are effective for
zanamivir measurement, otherwise LC-MS needs several types of chemicals and sophisticated
instrument. Meanwhile, detection using mercury drop electrode was avoided because of safety reason. Due to this reasons, a alternative method for zanamivir measurement need to
be developed.
HO
Figure L Molecular structure of Zanamivir.
Electrochemical method
for
zanamivlr measurement is prospectiveto
be devetopedsince
it
is
simple,fast,
and reagentless.ln
this work,
electrochemica! measurement of'zanamivir
using gold and gold modified boron clopecl diarnond electrode (BDD) was developed.
BDD was used as working electrode due to itslow background currents, stability, and wide
potential window in aqueous solution [7]. Meanwhile, gcld used
for
BDD modification toenhanced its performace in zanamivir sensing.
MATERIALS AND METHODS
Materials
Zanamivir (Tokyo Chemical Industry Co. Ltd.), KAuCI+.4HzO (\ffako Chemicals),
allylamine (Tokyo
chemicalIndustry co.
Ltd.),
Na:csHsoz, KzHpo+, KHzpo+, HzSo+, CEHgBOa, hydrogen, oxygen, methane, siliconrlafer,
diamond powder, 2-propanol, Goldelectrode, Ag/AgCl as reference eiectrode, platinum as counter electrode, and high
purity
vrater
with
maximum conductivityof
18
MO obtainedfrom
Simply-Labwater
system(DIRECT-Q 3 UV, Millipore).
HN
)-NH-//
HN
Proceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 97 8-602-7 0429 -9 -s
Methods
Preparation of Working Electrode
Boron doped diamond (B/C atomic ratio
of
1:L000) were deposited on Si [100) wafers in microwave plasma-assisted chemical vapor deposition (MPCVD) system (ASTeXCorp.). BDD was electrochemically polarized at 3 V (vs. SSCE)
in
O.1mol/L
HzSO+ for 20min
to
obtain oxygen terminated BDD. Au-BDD was prepared through electrochemical deposition in equal volume of 2 mM HAuCI+.4HzO and HzSO+ 0.5 mM mixture at 0.2 V (vs.SSCE) for 100 min, detail preparation are describe elsewhere [8]. Meanwhile, AuNPs-BDD
was obtained by photochemical reaction using allylamine followed by chemical reaction
with
AuNPs. The AuNPs prepared according to Turkevich and Frens method [9, 10]. XPSwere used to characterize the Au-BDD and AUNPs-BDD electrode
prior
to use as working electrode.Electrochemical Measurement of Zanamivir
Electrochemical measurement
of
zanamivir
was
performed
in a
single compartment cellwith
three electrodes. A platinumwire
was used as counter electrode and silver/silver chloride electrode (SSCE) used as reference electrode. Working electrode Au-BDD and AuNPs-BDD was pressed against the bottom of the electrochemical cell by anO-ring
with
the geometric area approximately 0.L25 cmz. Phosphate buffer (PBS) 0.1 Mwas used as
the
supporting electrolyte. Cyclic voltammetry measurementsof
various conce.ntrationsof
zanamivirin
PBS 0.LM
was performed ata
scan rateof
L00 mVs-t.Precision
of
zanamivir measurementwas
expressed as percentagerelative
standarddeviation
[%
RSD) which obtained from nine replicates analysis of three concentration level of zanamivir.RESULTS AND DISCUSSION
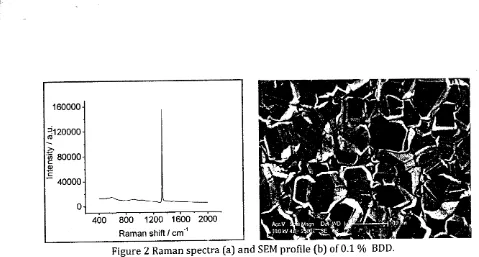
Hydrogen terminated BDD
with
0.L o/o boron has successfully prepared by MPCVDsystem. Raman spectra (Figure 2a) shows an intense peak at 1.332 cm-l which confirmed
that carbon sp3 of diamond have already deposited on Si wafer [7] and the broad peak at
500
cm'l
expectedto
be boron dopedon
diamondfilm
U7,721. Diamond crystals withapproximately 3 pm in size were covered the silica wafer (Figure 2b).
Proceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 97 8-602-7 0429 -9 -S
160000
?120000
o
'6 aoooo
C
o
c
-
40000 [image:7.595.57.536.59.316.2]400
800
1200 1600 2000 Raman shift / cm-lFigure 2 Raman spectra (a) and SEM profile (b) of 0.1 0/o BDD.
Gold was electrochemically deposited on BDD surface. Two peaks observed at83.2
eV and BT ev are attributable to Au a,fi/z a1f, Au 4F/z,respectively (Figure 3a). Au particles expected to be physically deposited at BDD surface
without
any chemical bonding sincethere was
no
significantshift of
C 1s peakat
Au-BDD electrode. Onthe
other hand,modification of the BDD surface
with
AuNPs was initiated by photochemical reaction ofBDD surface with allylamine followed by chemical reaction of amine-terminated BDD with
AuNps. Amine functional groups facilitated the chemical bonding
of
AuNPswith
BDDsurface. XpS spectra confirm that AuNPs has successfully deposited on amine-terminated
BDD. XpS peaks of Au 4frlz and 4f,p was detected at binding energies of 84 and BB eV,
respectively (F igure 3b).
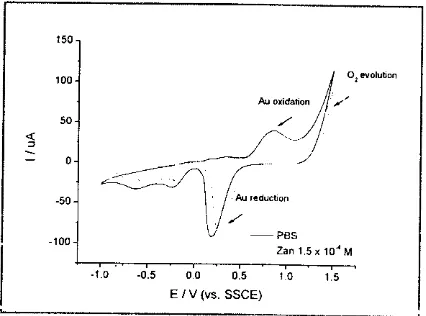
Figure
4
shows cyclic voltammograms (CV)of
0.1M
PBSpH
7
obtainedat
apotential scan rate
of
100 mVs-r in the absence andin
the presence of Zanamivir at Au electrode. There is no new oxidation or reduction peak observed in potential range of 0 V to +L.SO V. However, the CV showed that the presence of Zanamivir leads to the decreaseof oxidation and reduction peak currents
of
gold. As proposedin
our
previous report, Zanamivir was adsorp on gold electrode and reduces thedirect
contact between gold surface and waterfoxygen[8].The
condition leadsto
the
decrementof
oxidation andreduction of gold surface.This afford the chance for Zanamivir measurement using gold
and gold modified BDD.
Proceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 978-602-7 0429-9'5
(a) c1s
3CC
[image:8.595.88.539.61.241.2] [image:8.595.186.399.301.459.2]tlinding Energy / eV
Figure 3 XPS spectrum of Au-BDD (a), oxidized BDD, allylamine modified BDD, and AuNps modified BDD (b).
Zan 1.5 x l0r M
-0.5 0.0 0.5
E/V(vs.SSCE)
Figure 4 Cyclic voltammogram of 0.1 M PBS pH 7 in the absence and in the presence of
Zanamivir at Au electrode.
Logarithmic curue was consistently observed from measurement of zanamivir in wide
range
of
concentration on three electrodes (data not shown). Meanwhile,linear calibrationcurve of zanamivir was obtained in the concentration range ofL x 1-0-s - L x 1O-+mol/L on gold
electrode,5x10-6-1x10-amol/LonAu-BDDelectrode,andLxL0-e-1x1g-smol/Lon
AUNPs-BDD. The linear calibration curve could be developed based on gold oxidatiop and
reduction peak current. Otherwise, calibration curve developed from gold reduction peak current not always give excellent determination coefficient. Figrrre 5 shows the CV of 0.1 M PBS pH 7
in
the presence of Zanamivir at various concentrations and calibration curveobtained
from
measurementon
each
electrode.While
Au
electrode shows highersensitivity than Au-BDD and AuNPs-BDD electrode, higher determination coefficient can
be achieved using Au-BDD and AuNPs-BDD electrodes. The detection
limit
estimated to beProceeding International Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 97 8-602-7 0429 -9 -5
I I t'
1.19
x
LO-6,L.49x
10-s, and2.29x
10-6 molfL, respectivelyfor
gold, Au-BDD, and AuNPs-BDD electrode. Furthermore, the precision of the current responses was investigated fornine
consecutive measurements.The
high
precisionaf
Zanamivir measurement was reflectedin
the
relative standard deviations (RSDs), which are less than 2.5o/o at eachelectrodes. The summery of the analytical performance of Zanamivir at Au and Au-BDD was presented in Table 1..
(a) ,;,!-!'1 ,t: (b)
- -':ls
g-*_.14%
+ =-i 3il3x + ii5.F"? i'- =,' *r'r.
't:i n
Conc:ntrrtion cf Zrnanrivirl PII
r=---..-i L-3 : r, J,
..:
J rt ,) :iCo ncentr.3tio n of Zr n'rrn ivi r I pld
Au (a,b), Au-BDD (c, dJ, and AuNPs-BDD (e,
0
electrode.Proceeding lnternational Conference
"Current Breakthrough in Pharmacy Materials and Analyses"
ISBN : 97 8-602-7 0429 -9 -5
1| i=.:...r-l;=r
A 3z:,3: i:- ==;{
f E C, L L = .J' .:r,, o o-J
-t !-.i{ i:l :.4 = -:rl: .jr lF,'lA r,l :.: :.:
E 'V :,'s. SSC Ei
47
ll) r
f{ {:rS{ih
--t---
- i[--. __.,{-_.
{
(ej
,.
-.:
s(
=
-i:
-i. 0
-: ,l
1 - 'z{--... l: 1(8.
lr
=,'V:vE SSC:EI
{ E o L L f,-.J .:r G G,
o-
6--+..-;
4
n.ConcEntration of Zanarnivirl Pll -t
Table 1 The summary of the analytical performance of Au, Au-BDD, and AuNps-BDD
Electrode
Au-BDD AuNPs-BDD
Linearity ranee [M 1 x 10-s -1 x 10-r
5x10-o-LxL0-+
1x1.0'o-1.x1.0-sDetermination coefficient (Rz1
Oxidation peak
Reduction oeak
Limit of Detection (M) Oxidation peak
Reduction oeak
Precision of measurement n =
e (%RSD)
Oxidation peak
Reduction peak
0.990 0.990
0.946 0.998
1..25 x L0's
L.L9 x 10-o
1..53 x L0-s
1.49 x L0-s
1.89 x 10-s
2.29 x 70's
CONCLUSION
Electrochemical detection of Zanamivir
in
0.1 M phosphate buffer solution (pBSl has been successfully investigated at gold (Au) and gold-modified boron-doped diamond(Au-BDD and AuNPs-BDDJ electrodes. Measurements
of
Zanamivir basedon
reductionpeak current provide better performance than the oxidation peak. Performance of
AuNps-BDD electrode generally better than other two electrodes.
REFERENCES
1.
Kawai N, Ikematsu H,Iwaki
N, MaedaT,
Kanazawa H, KawashimaT,
Tanaka O,Yamauchi S, Kawamura K, Nagai T et al. 2OOB. A comparison of the effectiveness of
za'namivir and oseltamivir for the treatment of influenza A and B. f . Infect. 56: SI-ST.
2.
AzumaT,
NakadaN,
YamashitaN,
Tanaka H.2A1,2. Synchronous dynamics ofobserved
and
predicted valuesof
anti-influenza drugsin
environmental watersduring
a
seasonal influenza outbreak, Environ. Sci. Technol. 46: 2g73-L2gB1.3.
CunninghamVL,
Binks SP, Olson Ml.2AO9. Humanhealth
risk
assessment fromthe
presenceof
human
pharmaceuticalsin
the
aquatic
environment. Regul.Toxicol. Pharmacol. 53: 39-45.
4.
Baughman TM, Wright WL, Hutton KA. 2007. Determination of zanamivir in rate andmonkey plasma
by
positive
ion
hydrophilic interaction
chromatography IHILIC)/tandem mass spectrometry. J. chromatogr B. g52: s0s-
511.5.
Ge J, [,iu F, I{olmes EH, Ostrander GK,Li
QX.}OLZ Aqueousnormal
phase Iiquidchromatography
coupled
with
tandem
time-o?-flight quadrupole
massspectrometry
for
deterrninationof
zanamivir in humah serum. |. Chromatogr B.58
-
62,6.
Skrzypek S. 2010. Electrochemical Studies of the Neuraminidase Inhibitor Zanamivirand its Voltammetric Determination in Spiked Urinb. Electroan al.22:2339-2346.
7.
Fujishirna A, Einaga Y, Rao TN, Tryk DA. Diamond Electrochemistry. First ed. Elsevier Science. Tokyo: 2005.8.
Wahyuni WT, Ivandini TA,fiwanti
PK, Saepudin E Gunlazuardif,
Einagay..Z0l4.
Electrochemical Detection of Neuraminidase based on Zanamivir lnhibition using
Gold-Proceeding International Conference
"current Breakthrough in Pharmacy Materiars and Anaryses"
ISBN : 97 8-602-7 0429 -9 -5
Modified Boron-Doped Diamond Electrode. Research Report. lndonesia: University of lndonesia.
Turkevich
f,
Stevenson PC,Hiller
J. 1951.A
studyof the
nucleation and growthprocesses in the synthesis of colloidal gold. Discuss. Faraday Soc. 11: 55-75.
Frens
C.
1'973- Controlled nucleationfor the
regulationof
the
particle
size in monodisperse gold suspensions. Nat. phys. Sci.24'1.: zo-zz.ACKNOWTEDGMENT
Directorate
of
higher
education Republicof
Indonesiaand
Department ofChemistry Keio University, fapan.
OPTIMIZATION OF HPLC CONDITION FOR DETERMINATION OF VITAMIN A AND D3
IN PHARMACEUTICAT PREPARATIONS
Engrid f uni Astutit, Mochammad Yuwonoz, Muhamad Zainuddinz
t Pharmacy Deportment,
Il niversity of Muhammadiyah Molang
zFaculty of Pharmacy, Airlangga IJniversier Surabaya
Abstract
The
purpose
of
the present study wasto
achieve the HPLC conditionfor
theseparation
of
vitamin
A
and Dgin
pharmaceutical formulations.The
optimization of method was carriedout by
investigating the effectof
HPLC conditions, namely mobilephase composition,
flow
rate, packingof
columnand
analytical wavelengthon
peak symmetry, tailing factor, resolution and retention time reproducibility of the analytes ofinterest.
A
good
simultaneous separationof
vitamin
A
and
De
in
pharmaceutical fprmulations was obtained using a chromolith@ column Rp-1ge (100 mm x 4.6 mm) withmethanol L00 o/o as mobile phase at flow rate
of L ml/min. The detector was set
at
265.gnm and total analysis time was 12 min. The system suitability test demonstratecl good
resolution (Rs>5),
tailing
factor<
2, theoretical plate>
2000 and selectivityfactoi
>1.Relative standard deviation (RSD) of retention time was 0.1 1.0/o for vitamin A and o.A7o/a
for vitamin D3, whilst RSD of peak area 'was 0.09o/o and 0.99o/o for vitamin A and vitamin
D3, resp€ctively.
Keyword : vitamin A and D3, HpLC, Method optimization.
INTRODUCTION
Vitamins are
a
groupof
organic compoundsthrt
,..,
in
very
small amounts, essential for normal metabolism, growth and development, and regulationof cell function. Thirteen vitamins are recognized in human nutrition which may be conveniently classified
into two groups according to their solubility : as water-soluble (B-complex and C) and fat-soluble (A, D, E and K) t1l.
Proceeding International Conference
"current Breakthrough in pharmacy Materiars and Analyses"
ISBN : 97 8-602-7 0429-9 -s
9.
10.