Value-adding to Honey
by Dr Joan Dawes and Dr David Dall
May 2014
© 2014 Rural Industries Research and Development Corporation.
The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances.
While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication.
The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors.
The Commonwealth of Australia does not necessarily endorse the views in this publication.
This publication is copyright. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. However, wide dissemination is encouraged. Requests and inquiries concerning reproduction and rights should be addressed to RIRDC Communications on phone 02 6271 4100.
Researcher Contact Details
In submitting this report, the researcher has agreed to RIRDC publishing this material in its edited form.
RIRDC Contact Details
Rural Industries Research and Development Corporation Level 2, 15 National Circuit
BARTON ACT 2600
Electronically published by RIRDC in May 2014
Foreword
At present the commercial value of Australian honeys primarily relates to taste quality, but stronger health awareness by consumers has created scope for adding value to Australian honeys by exploiting properties of the honeys that convey health benefits. This project has examined three such potential attributes of commercially-prepared Australian eucalypt honeys: Glycaemic Index; prebiotic properties; and therapeutic activity.
The project found that all the Australian eucalypt honeys tested were prebiotic food, stimulating the growth of gut bacteria that contribute to human health and reducing the growth of deleterious gut bacteria. Australian honey packers and marketers have already started to explore how to take advantage of this finding.
Although the honeys were found to be low to medium Glycaemic Index foods, the Index was also found not to be a useful parameter to apply to honeys. In the competitive market for honey products, the industry will need to consider the implication of this finding. No commercially useful antibacterial or antifungal activity was detected in the samples of commercial Australian eucalypt honeys tested. This project was funded from industry revenue which is matched by funds provided by the Australian Government.
This report is an addition to RIRDC’s diverse range of over 2000 research publications and it forms part of our Honeybee R&D program, which aims to secure a productive, sustainable and more profitable Australian beekeeping industry.
Most of RIRDC’s publications are available for viewing, free downloading or purchasing online at www.rirdc.gov.au. Purchases can also be made by phoning 1300 634 313.
Craig Burns Managing Director
About the Authors
Dr Joan Dawes BA (Hons Biochem), MA, DPhil (Oxon)
Dr Dawes is a biochemist with extensive experience in management and commercialisation of scientific research, having served as Director (CEO) of the CRC for Biopharmaceutical Research, CEO of the ASX-listed company BioDiscovery Ltd and President of the Australian Biotechnology Association (now AusBiotech). She was also previously a qualified valuer of intellectual property. Dr Dawes has more than 10 years’ experience as a company director, and is the current Chair of the Governing Boards of Pestat Pty Ltd and Hopstop Australia Pty Ltd.
Dr David Dall BSc(Hons), PhD, MEnvtLaw, MIPLaw, GAICD
Dr Dall is the Managing Director of Pestat Pty Ltd. Dr Dall has scientific expertise in molecular and general microbiology and vertebrate toxicant R&D, training in environmental law and intellectual property management, and experience in regulatory affairs and product commercialisation. Dr Dall joined Pestat from an appointment as Principal Research Scientist with CSIRO Australia. Dr Dall is a member of the Australian Institute of Company Directors, and the American Association for the Advancement of Science (AAAS).
Acknowledgments
Abbreviations
ABARES Australian Bureau of Agricultural and Resource Economics and Sciences
BRS Bureau of Rural Sciences
DHA dihydroxyacetone
FAO Food and Agriculture Organization of the United Nations
FSANZ Food Standards Australia New Zealand
GC-MS gas chromatography – mass spectrometry
GI Glycaemic Index
HPLC high performance liquid chromatography
IP intellectual property
MGO methylglyoxal
MIC minimum inhibitory concentration
NMR nuclear magnetic resonance
NPSC nutrient profiling scoring criterion
PI Prebiotic Index
R&D research and development
SCFA short chain fatty acids
TGA Therapeutic Goods Administration
Contents
Foreword ... iii
About the Authors ... iv
Acknowledgments... iv
Abbreviations ... v
Executive Summary ... ix
Introduction ... 1
Objectives ... 3
Methodology ... 4
Identification and sourcing of honey samples ... 4
Composition of honey samples ... 5
Functional properties of honeys ... 6
Chapter 1. Composition of honey samples ... 7
Introduction ... 7
Floral source of honeys ... 7
Chemical content of honeys ... 7
Methodology ... 8
Floral source of honeys ... 8
Chemical analysis of honeys ... 8
Statistical analysis ... 9
Results ... 10
Floral source of honeys ... 10
Chemical analysis ... 13
Implications... 19
Recommendation ... 19
Chapter 2. Glycaemic Index of honey samples ... 20
Introduction ... 20
Methodology ... 21
Chapter 3. Prebiotic properties of honey samples ... 29
Introduction ... 29
Methodology ... 29
In vitro assessment of Prebiotic Index ... 30
In vivo measurement of Prebiotic Index ... 31
Results ... 33
In vitro assessment of Prebiotic Index ... 33
In vitro butyric acid production... 35
In vivo measurement of Prebiotic Index ... 36
In vivo butyric acid production ... 36
Implications... 36
Recommendation ... 37
Chapter 4. Antimicrobial and anti-fungal properties of honey samples ... 38
Introduction ... 38
Methodology ... 39
Test samples ... 39
Assessment of antibacterial activity ... 39
Assessment of anti-fungal activity ... 39
Hydrogen peroxide assay ... 39
Statistics ... 40
Results ... 41
Antibacterial activity of honey samples ... 41
Implications... 44
Recommendation ... 44
Chapter 5. Regulation of health and nutritional claims in Australia and New Zealand ... 45
Introduction ... 45
Methodology ... 45
Results ... 45
FSANZ Standard 1.2.7 ... 45
Health claims for honey under FSANZ Standard 1.2.7 ... 45
Recommendation ... 47
Appendix 1 ... 48
Details of researchers ... 48
Project Manager ... 48
Chapter 1. Composition of honey samples ... 48
Chapter 2. Glycaemic Index of honey samples ... 49
Chapter 3. Prebiotic properties of honey samples ... 49
Chapter 4. Antimicrobial and anti-fungal properties of honey samples ... 49
Tables
Table 0.1. Honey samples used in the project, with identifying codes and designations. ... 5
Table 1.1. Pollen content of honey samples. ... 10
Table 1.2. Electrical conductivity and designation of honey samples. ... 11
Table 1.3. Water content and pH of honey samples. ... 13
Table 1.4. Individual sugar content of honey samples. ... 14
Table 1.5. Normalised sugar content of honey samples. ... 15
Table 1.6. MGO and DHA content of honey samples. ... 18
Table 2.1. GI values of honey samples. ... 23
Table 2.2. Correlation between GI values and sugar content of honey samples. ... 24
Table 2.3. Predictive GI values of honey samples. ... 26
Table 2.4. Correlation between Predictive GI values (PGI) and sugar content of honey samples. ... 27
Table 3.1. PI values of honey samples. ... 33
Table 3.2. Correlation between PI values and sugar content of honey samples. ... 34
Table 3.3. Butyric acid production (mM) with predigested honey samples. ... 35
Table 3.4. Correlation between butyric acid production and sugar content of honey samples when adult faecal microbiota were incubated with predigested honey. ... 36
Table 4.1. Antibacterial activity of honey samples against Staphylococcus aureus. ... 41
Table 4.2. Anti-fungal activity of honey samples against Candida albicans. ... 43
Figures
Figure 1.1. Glucose versus fructose content of honey samples. ... 16Figure 2.1. Glycaemic Index in relation to glucose content of honey samples... 25
Executive Summary
What the report is about
The long-term economic viability of the Australian honey industry is particularly important to Australia, not only for the honey industry itself but also in relation to the pollination services that the honeybees provide to the horticulture industries. This viability is intrinsically linked to the prosperity of the industry and its ability not only to compete with other natural and artificial sweeteners for dietary use, but also to differentiate Australian honey from the cheaper products marketed by international competitors.
This study focussed exclusively on the potential for ‘value-adding’ to Australian eucalyptus honey products delivered through the existing commercial supply chain, and the conclusions and
recommendations of this report relate to such products. While alternative routes to honey sourcing, production and supply may offer other avenues to increased industry value and returns, they are inevitably associated with further costs and uncertainties, and were not considered in this study. At present the commercial value of Australian honeys relates only to taste quality, but stronger health awareness by consumers has created scope for adding value to Australian honeys by exploiting any properties of the honeys that convey health benefits. Anecdotal evidence has identified three such potential functional properties of Australian eucalypt honeys: Glycaemic Index, prebiotic properties and therapeutic activity. This project provides in-depth analysis of the composition of twenty samples of commercially-prepared Australian eucalypt honeys, tests whether the honey samples do in fact exhibit health-related properties, and attempts to relate honey composition to its health benefits. Australian honey packers and beekeepers could benefit directly by using some of the results of the project to derive optimal returns for honey in an increasingly competitive market. Indirect benefits will flow through to the horticulture industries as a result of the increased security of supply of pollinators.
Who is the report targeted at?
The report is targeted at the Australian honey industry, particularly honey packers.
Where are the relevant industries located in Australia?
The honey industry is represented in all States of Australia, as are the horticulture industries. The strongest honey industry representation is in NSW, which frequently contributes over 40% of Australian honey production. Western Australia and Tasmania are important to the industry because of their endemic floral sources of honey, Jarrah (Eucalyptus marginata) and Leatherwood (Eucryphia lucida).
In 2011-12 the Australian honey industry had a gross value of honey and beeswax production of $79 million which was forecast to rise to $88 million in 2012-13 and $92 million in 2013-14
(ABARES, 2013). Furthermore it has been estimated that the industry contributes directly to between $4 billion and $6 billion worth of agricultural production. In 2006-7 1,700 commercial producers with more than 50 hives each accounted for more than 90% of Australia’s honey production. Australia is recognised for the premium quality of its honey. In 2004 about 30% of honey production was exported, mostly in bulk form, to over 38 countries.
There are only a small number of large Australian packers handling this honey. The largest is
is Beechworth Honey in Corowa. There are also many other smaller honey-packing entities around Australia.
Australian honey packers and beekeepers could benefit directly by using the results of this research to derive optimal returns for honey in an increasingly competitive market. Indirect benefits will flow through to the horticulture industries as a result of the increased security of supply of pollinators. The general conclusions will benefit most of the sectors of the industry, and specific benefits can be generated for producers and suppliers of honeys from the floral sources tested: Jarrah (Eucalyptus marginata), Red Stringybark (Eucalyptus macrorrhyncha), Spotted Gum from southern New South Wales (Corymbia maculata) and Yellow Box (Eucalyptus melliodora). The beekeepers with access to these floral sources are located in Western Australia, Queensland, NSW and Victoria, and the honey packers sourcing these honeys are Capilano and the NSW packers.
Background
Anecdotal evidence and preliminary research raises the possibility that honey may confer health benefits. However, systematic studies to convincingly demonstrate such functional properties are lacking, and without them the commercial honey industry cannot make substantiated claims that would support premium pricing for honeys. Moreover, the honeys tested have not been rigorously characterised to determine whether specific physical or chemical properties contribute to functional characteristics.
Aims/objectives
The main objective of this project was to assist the Australian honey industry to maximise its revenues and enhance its public image by supply of honeys with reference to their highest-value properties. It was intended to address this objective by:
• generating high quality data examining the functional properties of these honeys as:
- low-Glycaemic Index sweeteners, - prebiotic foods and/or
- anti-fungal and antimicrobial agents;
• analysing honeys sourced from important Australian eucalypt species to link specific physical
and chemical characteristics with these health-related functional properties;
• developing proprietary tests for these functional properties, which can be used by the
Australian honey industry for identification and quality assurance testing of production batches of honey;
• using the datasets generated to support accreditation of appropriately identified honeys for
commercial supply; and
• making data and intellectual property produced by this project available to support further
research and development in other value-added contexts.
Wales (Corymbia maculata) and Yellow Box (Eucalyptus melliodora). Two other honeys, one from canola and the other a Canola/Stringybark blend, were analysed as controls. Honeys were selected for inclusion in this study after consideration of factors including general commercial availability, and prior indications of prospective but unrealised value characteristics. Honeys that already achieve premium value on the basis of characteristics such as unique flavour (eg Leatherwood honey) were not considered for inclusion.
The samples were sourced from Beechworth Honey Pty Ltd (Cowra, NSW). Jarrah honeys originated from Wescobee Limited (Bayswater, Western Australia) and were sent to Beechworth Honey for aliquoting, storage and distribution.
Groups with expertise in each field investigated were contracted to analyse the honey samples and assess their functional properties.
The floral sources of the honey samples were assigned by Beechworth Honey using the routine procedures in place at this large commercial packer, combining the information supplied on the beekeeper’s vendor declaration form with sampling and tasting to examine its colour and flavour profile.
The colour, consistency, odour and taste of each sample were also examined by Intertek Food Services GmbH (Bremen, Germany), a company with an international reputation in honey analysis. They analysed the pollen content using microscopy and measured the electrical conductivity by an in-house method.
ChemicalAnalysis Pty Ltd (Croydon, Victoria) performed chemical analysis of the honey samples, including the water content, pH, refractive index, colour and opacity. The content of glucose, fructose, sucrose and maltose + oligosaccharides was measured using High-Performance Liquid
Chromatography with Evaporative Light Scattering Detection. Methylglyoxal and dihydroxyacetone content were measured using High Performance Liquid Chromatography-Mass Spectrometry. Nuclear Magnetic Resonance spectroscopy was also carried out on one honey sample to identify
oligosaccharides.
The Glycaemic Index (GI) values of seven selected honey samples were measured in vivo. The samples included one Jarrah honey, three Red Stringybark honeys, one Spotted Gum honey and one Yellow Box honey with a spread of glucose and fructose content to optimise attempts to relate these parameters to GI values. Each sample was tested in 10 normal human subjects by the Glycemic Index Research Service, University of Sydney (SUGiRS). The methodology is regarded as the ‘gold
standard’ for measuring GI. Subjects consumed honey or a glucose control containing 50 grams of available carbohydrate, after which a 2-hour blood glucose response curve was used to calculate the GI value.
The Predictive GI was measured on 21of the honey samples used in this study. Next Instruments (Condell Park, Sydney) performed this test using the NutriScan G120 Glycemic Index Analyser, a high precision fully automated instrument that mimics the way carbohydrates are digested in the human gut.
The prebiotic potential of all 22 honey samples was assessed in vitro in the laboratory ofProfessor Patricia Conway (ProBiOz Pty Ltd), both before and after enzymic digestion and dialysis. Intestinal microcosms were derived from faecal material from two healthy human subjects. The effect of each honey sample on growth of total bacteria, the beneficial lactobacilli and bifidobacteria and the potentially harmful clostridia was determined. Short chain fatty acid metabolites produced during this process were quantified by gas chromatography.
phases each of which was four weeks in duration. Phases 1 and 3 served as wash out periods to remove the effects of previously ingested honey, and during Phases 2 and 4 subjects consumed 20 grams of honey daily. Freshly voided faecal samples were collected at the beginning of Phase 1 and at the end of each phase, and the bacterial content of each faecal sample was analysed and the Prebiotic Index calculated. In addition butyrate levels in the faecal suspensions were determined by gas chromatography.
All 22 honey samples were tested in the laboratory of Associate Professor Dee Carter (University of Sydney) for antibacterial activity against Staphylococcus aureus and anti-fungal activity against Candida albicans. Samples were assessed both as received by Beechworth Honey and as prepared for market by warming and filtration. Standard growth inhibition assays were used for antibacterial testing and a microdilution technique for anti-fungal activity. The concentration of hydrogen peroxide in honey samples was determined using a colorimetric assay.
Results/key findings
The first objective of this study has been achieved, generating high quality data examining the functional properties of commercially-prepared honeys sourced from four important Australian eucalypt species as low-Glycaemic Index sweeteners, prebiotic foods and/or anti-fungal and
antimicrobial agents. An intensive examination of the specific physical and chemical characteristics of these honeys is also reported. However, the project could not deliver a surrogate test for a health-related functional property because no characteristic of the honeys corhealth-related sufficiently well with any functional property that it could be used as the basis for developing a surrogate test for that property.
The process for accreditation of honeys with health-related functional properties has changed completely since the beginning of this project, but we have identified a potential route for claiming such properties. The data and intellectual property produced by this project are available to support further research and development in other value-added contexts.
The key findings of the project are:
• No measured physical or chemical characteristic of the honeys contributed usefully to the
assignment of floral source for Australian eucalypt honeys. It should specifically be noted that pollen analysis was not useful in this context, although both it and electrical conductivity are now extensively used in assessment and quality control of Northern hemisphere honeys. The paucity of available data and lack of expertise and experience in analysis of Australian eucalypt honeys contribute to inaccuracies in interpretation of pollen content and electrical conductivity.
• Australian eucalypt honeys are probably low to medium GI foods when consumed by the
majority of individuals, but not necessarily of lower GI value than honeys from other floral sources. The automated in vitro Predictive GItest was highly reproducible, but theresults did not correlate strongly with those from the in vivo analysis. The in vivo GI value of a honey could not be reliably predicted on the basis of its content of glucose, fructose or any other simple physical or chemical property measured in this study.
• A few of the Australian eucalypt honeys had some antibacterial activity and low levels of
anti-fungal activity, but both were entirely attributable to hydrogen peroxide, which is unstable on storage. Moreover, this is an attribute of honeys from many floral sources. There was no evidence from this study that any of the Australian eucalypt honeys tested contained stable antibacterial or anti-fungal components that could be of interest to the biotechnology or pharmaceutical industries.
• Food Standards Australia New Zealand (FSANZ) regulates health and nutritional claims in
Australia. In January 2013 it released FSANZ Standard 1.2.7, under which honey, because it is almost entirely composed of sugars and water, is effectively prevented from being
associated with health and nutritional claims. However, this barrier could be surmounted by identifying an independent expert not-for-profit organisation that would endorse such claims for Australian eucalypt honey.
Implications for relevant stakeholders
The implications for the Australian honey industry are:
• The present routine industry method for assigning floral source to Australian eucalypt honeys
is adequate and appropriate. This study did not identify any physical or chemical
characteristic, or combination thereof, that could be reliably used to differentiate between Australian eucalypt honeys sourced from different floral species.
• The Glycaemic Index, antibacterial activity and antifungal activity are not valuable properties
of Australian eucalypt honeys.
• Prebiotic potential is the health-related property of Australian eucalypt honeys that is most
likely to generate premium prices.
• No simple, cost-effective surrogate marker has been identified that could be used to analyse
batches of honey and predict their prebiotic activity.
There are also implications of this research for policy makers. Standard FSANZ 1.2.7 does not address the regulation of health-related or nutritional claims for honeys in an appropriate manner. There should be the opportunity to address this matter and change the regulation.
Recommendations
The recommendations arising from this project are addressed to the Australian honey industry, particularly the honey packers.
• The current method of assigning floral sources to Australian eucalypt honey samples remains
the best available and should not be modified to include pollen analysis or electrical conductivity, neither of which adds value to the present approach.
• Industry funds should not be expended on further analysis of the Glycaemic Index of
Australian eucalypt honeys.
• Industry funds should not be expended on further analysis of the antibacterial activity or
antifungal activity of Australian eucalypt honeys, which is unlikely to be productive.
• The industry should focus on prebiotic potential as the health-related property of Australian
• We recommend that the Australian honey industry identifies an independent expert
Introduction
The honey industry is represented in all States of Australia, as are the horticulture industries that rely on honey bees for pollination. The strongest honey industry representation is in NSW, which
frequently contributes over 40% of Australian honey production. However, it should be noted that beekeepers are highly mobile between states, typically moving their hives 500-600 kilometres to floral sources. In addition, production is highly dependent on weather events including droughts, floods and bushfires. Western Australia and Tasmania are important to the industry because of their endemic floral sources of honey, Jarrah (Eucalyptus marginata) and Leatherwood (Eucryphia lucida). In 2011-12 the Australian honey industry had a gross value of honey and beeswax production of $79 million, which was forecast to rise to $88 million in 2012-13 and $92 million in 2013-14
(ABARES, 2013). It has also been estimated that honeybees contribute directly to between $4 billion and $6 billion worth of agricultural production annually (House of Representatives Standing
Committee on Primary Industries and Resources 2008). In 2006-7 there were about 10,000 registered beekeepers with 572,000 hives, though it should be noted that registration was then not compulsory in Tasmania, the Northern Territory and the ACT. There were 1,700 commercial producers with more than 50 hives each, and these 17% of beekeepers accounted for more than 90% of Australia’s honey production (Crooks 2008). Most commercial apiarists operate between 400-800 hives but some have more than 3,000 hives. Australia is recognised for the premium quality of its honey. In 2004 about 30% of honey production was exported to over 38 countries, with key markets in the United Kingdom, Indonesia and other South East Asian countries, North America and Saudi Arabia. Most honey was exported in bulk form, but there was a significant and increasing proportion of exports shipped as retail packs (Centre for International Economics 2005).
There are only a small number of large Australian packers handling this honey. The largest is
Capilano Honey Limited, which is based in Queensland and also packs honey in Victoria and Western Australia, but receives honey from many locations in Australia. The largest NSW-based honey packer is Beechworth Honey in Corowa. There are also many other smaller honey-packing entities around Australia.
Honey has been an important part of the human diet from prehistoric times, and also has a long history of use as an active therapeutic. There is an extensive body of literature describing its physical,
chemical and functional properties, and some of this discusses honeys from native Australian floral sources. There are indications that some Australian floral species may yield honeys with potentially valuable dietary attributes such as low glycaemic indices and prebiotic properties, and therapeutic attributes such as wound healing and anti-fungal and antibacterial properties (Conway et al. 2010, Carter et al. 2010).
At present the commercial value of Australian honeys relates only to taste quality. For example, Tasmanian leatherwood honey sells at a premium because consumers find its taste superior to most other Australian honeys. A recent study commissioned by the Rural Industries Research and
Development Corporation (Kneebone 2010) indicates that a stronger health awareness by consumers has created scope for adding value to Australian honeys by exploiting any low Glycaemic Index, prebiotic potential and antibacterial and anti-fungal properties of the honeys. There is a clear
precedent in the premium commercial value of New Zealand Manuka honey based on its antibacterial activity. However, existing demonstrations of the functional properties of Australian honeys are commonly only at ‘proof-of-concept’ level.
functional properties between individual samples of the same type of honey further compounds this problem, and as a result a clear correlation between the functional attributes of Australian honey and its physical and chemical characteristics is lacking.
This study focussed exclusively on the potential for ‘value-adding’ to Australian eucalyptus honey products delivered through the existing commercial supply chain, and the conclusions and
Objectives
As noted in the Introduction above, existing research provides some indication of various functional properties of Australian honeys that may hold significant commercial potential for the industry. The main objective of this ‘Value-adding to Honey’ project was to assist the Australian honey industry to maximise its revenues and enhance its public image by supply of honeys with reference to their highest-value properties.
At the outset of the project it was envisaged that this would be addressed by means such as:
• developing datasets that link specific physical and chemical characteristics with functional
properties of honeys sourced from important Australian eucalypt species. The datasets are intended to support accreditation of appropriately identified honeys for commercial supply as:
- low-Glycaemic Index (GI) sweeteners; - prebiotic foods and/or
- anti-fungal and antimicrobial agents;
• developing proprietary assays for characteristics that are diagnostic of high-value functional
properties, which can be used for identification and quality assurance testing of production batches of honey prior to their supply for accredited purposes. It is intended that the assays will be made available by licensing to the Australian honey industry (for example, honey packers). They in turn will access the assay methods through contracted sample testing by commercial laboratories. It is envisaged that packers will pass a proportion of premiums associated with sale of high-value honeys back to producers through individual contract supply arrangements;
• submitting the datasets linking physical or chemical attributes with functional properties to
appropriate regulatory agencies to initiate a basis for accreditation of Australian honeys that conform with prescribed characteristics;
• licensing datasets linking physical or chemical attributes with anti-fungal and anti-microbial
properties to biotechnology and/or biopharmaceutical companies to underpin the further testing processes required to capture honey values as therapeutic agents, or seeking external funding for this purpose;
• making data and intellectual property (IP) produced by this project available to support further
research and development (R&D) required to enable use of honeys in other value-added contexts that are beyond the budgetary capacity of the present project. These might include advanced therapeutics such as anti-inflammatories and wound healing agents, functional foods including antioxidants and personal care products like shampoos and cosmetics. The outcomes of this project for the Australian honey industry were intended to be:
• increased industry profitability;
• enhanced industry profile and social prominence through contribution to improved
community health, and
• increased revenue for future R&D activities through exploitation of project IP.
Methodology
Identification and sourcing of honey samples
This project analysed the chemical and functional properties of 20 unifloral Australian eucalypt honey samples of known provenance, using five samples originating from each of:
• Jarrah (Eucalyptus marginata);
• Red Stringybark (Eucalyptus macrorrhyncha);
• Spotted Gum from southern New South Wales (Corymbia maculata) and
• Yellow Box (Eucalyptus melliodora).
These unifloral honeys were chosen because earlier studies indicated that Yellow Box and Stringybark honeys may be low GI foods (Holt et al. 2002; Arcot & Brand-Miller 2005), and that Jarrah and Spotted Gum honeys had antibacterial activity (Irish et al. 2011).
Two other honeys, one from canola and the other a Canola/Stringybark blend, were analysed as controls. These were chosen because canola honey was reported to have a relatively simple
carbohydrate content and a glucose:fructose ratio higher than for most eucalypt honeys (Abell et al. 1996; Holt et al. 2002), and previous reports have indicated that they are likely to have mid- to high-GI and little prebiotic activity.
The samples were sourced from Beechworth Honey Pty Ltd (Cowra, NSW). Jarrah honeys originated from Wescobee Limited (Bayswater, Western Australia) and were sent to Beechworth Honey for aliquoting, storage and distribution. Each honey sample was a 100 kilogram single batch of honey, except for the Jarrah honeys from Wescobee, which were each 84 kilogram single batches. This amount ensured that enough honey was available for all the anticipated tests, and any additional research required during the course of the project or subsequent studies. This level of acquisition was therefore both a risk management exercise and a strategic investment.
As noted, the results of this project are intended to add value to some commercial Australian honeys. Honeys for use in the project were therefore processed according to standard industry practice. On receipt of the beekeeper’s container at Beechworth Honey a subsample of 20 kilograms was removed and stored and the remaining 80 kilograms was warmed below 45oC for eight to ten hours and then filtered through a 100 micron filter. Jarrah honeys from Wescobee Limited were sampled from bulk containers in which they were delivered by beekeepers, and were not further processed before testing. Honeys were dispensed into 25 x 200 gram tubs and the remainder into 20 kilogram buckets and stored.
Table 0.1. Honey samples used in the project, with identifying codes and designations.
Sample No Source Packer’s code
1 Jarrah 1 7843WES
2 Jarrah 2 7863WES
3 Jarrah 3 8012WES
4 Jarrah 4 8105WES
5 Jarrah 5 8113WES
6 Red Stringybark 1 7264DEN
7 Red Stringybark 2 7369HOL
8 Red Stringybark 3 7460EMM
9 Red Stringybark 4 7515BBN
10 Red Stringybark 5 7526BOM
11 Spotted Gum 1 3747RUT
12 Spotted Gum 2 3854DEN
13 Spotted Gum 3 3883SNO
14 Spotted Gum 4 4442BOM
15 Spotted Gum 5 5485BOM
16 Yellow Box 1 5735SPI
17 Yellow Box 2 7130SMI
18 Yellow Box 3 7141WRI
19 Yellow Box 4 7427RUT
20 Yellow Box 5 7626DEN
21 Canola 1 8168KLI
22 Canola/Stringybark 2 8193SNO
Composition of honey samples
Methodology specific to the analysis of the composition of the honeys is described in Chapter 1. The honey samples were tested for:
• taste;
• water content;
• pH;
• refractive index;
• electrical conductivity;
• pollen content;
• content of individual monosaccharides, sucrose, maltose and oligosaccharides, and
Functional properties of honeys
Methodology specific to the analysis of the functional properties of honey is described in the relevant chapters.
The honey samples were tested for:
• Glycaemic Index;
• Prebiotic Index;
• antimicrobial activity, and
Chapter 1. Composition of honey samples
Introduction
The honey samples were analysed in detail with two specific objectives:
• to attempt to identify honey characteristics that correlate strongly with functional properties
and
• to use these results to develop assays that allow commercially viable assessment of the
functional properties of batches of honey by measuring surrogate parameters.
Floral source of honeys
Honeys are characterised in several ways. The honey source is traditionally assessed by experienced tasters using the organoleptic characteristics of taste, colour, and odour. This method is not
completely accurate; it is difficult to identify nectar sources consistently and accurately by flavour and different individuals may recognise different sources for the same, or similar, products.
Qualitative and quantitative pollen analysis, which includes identification of the botanical species present as well as their relative abundance, has also been used to examine provenance and floral source and to provide a quantitative measure of floral origin. There is no direct correlation between the pollen found in honey and the nectar from which it is produced; the pollen and nectar content in a honey depend separately on floral structure, nectar secretion and pollen production by the source plants. Originating flora for some honey sources - such as thyme - are routinely under-represented in pollen analyses to such an extent that a unifloral thyme honey can contain as little as 20% thyme pollen. By contrast unifloral manuka honey must have a manuka pollen content of at least 70%, as manuka pollen is over-represented in honey (Moar 1985). Nevertheless, pollen analysis can be a useful approach to identifying the geographic and floral source of a honey, particularly when the characteristics of a particular unifloral honey have been established. Most of the nectar sources for a honey can be recognised by pollen analysis and it is a valuable objective approach that complements traditional methods of classifying honey. Not all honey is derived from floral sources, however. Some originates from ‘honeydew’, exudations from types of insect. In Australia the main source of
honeydew honey is psyllid species such as Psylla eucalypti, which manufacture a protective shield of crystallised honeydew and are then known as lerps.
Chemical content of honeys
Honey is essentially a supersaturated solution of sugars, which also contains acids (including amino acids), vitamins, phenols, minerals and enzymes in small and varying amounts. The moisture content of Australian honeys is usually between 16 and 18%. The European Union standard for commercial honey requires a maximum moisture content of 21%, but several national standards have maxima of 18.0-18.5% and many buyers will not accept honey with a moisture content greater than 20%. Sugars comprise 95.0-99.9% of the dry weight of honey, and the specific sugar content of a honey probably defines its Glycaemic Index and prebiotic properties. The monosaccharides fructose and glucose make up about 85% of honey dry weight, with small amounts of at least 22 other more
Sucrose, maltose, trehalose and turanose are the main disaccharides in honey, which can also contain isomaltose, isomaltulose (palatinose), nigerose, kojibiose, laminaribiose and gentiobiose. A range of trisaccharides can be present, including melezitose, 3-a-isomaltosylglucose, maltotriose, l-kestose, 6-kestose and panose. Isomaltulose, panose, 1-6-kestose and 6-6-kestose are nutritionally relevant
(Bogdanov et al. 2008). These saccharides are low-glycaemic and low-insulinaemic, as their digestion by bacteria in the human intestine slowly releases the constituent glucose and fructose
monosaccharides into the bloodstream (Holub et al. 2010). Two more complex sugars,
isomaltotetraose and isomaltopentaose, have also been identified in honey samples. In most blossom honeys the great majority of sugars are reducing sugars, but many honeydew honeys have high amounts of non-reducing oligosaccharides such as melezitose, maltotriose and raffinose (Bogdanov et al. 2000).
Other compounds implicated in the functional properties of honey include DHA and MGO. MGO has been identified as the main non-peroxide antibacterial constituent of Manuka honeys (Mavric et al. 2009; Jervis-Bardy et al. 2011). MGO is produced from DHA in the honey during storage (Adams et al. 2009).
Methodology
Floral source of honeys
Routine assessment
The floral sources of the honey samples originating from Beechworth Honey, which comprised all except the Jarrah honeys, were assigned by the routine procedures in place at this large commercial packer. Thus, the source of a batch of honey was identified by the individual beekeeper on a vendor declaration form. Beechworth Honey cross-check this information with their own intelligence about the species that are flowering in each region. On receipt of the beekeeper’s container at Beechworth Honey each lot of honey is sampled and tasted to ensure that its flavour profile and colour match the characteristics of the honey identified by the beekeeper.
In this study, the colour, consistency, odour and taste of each sample were also examined by Intertek Food Services GmbH (Bremen, Germany), a company with an international reputation in honey analysis.
Pollen analysis
Pollen analysis was carried out by Intertek using microscopy to perform qualitative pollen spectrum analysis and a quantitative assessment of the relative content of the different pollens in each sample. Electrical conductivity
Electrical conductivity was measured by Intertek to detect the difference between blossom and honeydew honeys. The company’s in-house method 3110.142 was used.
Water content
Samples were analysed by Karl Fischer titration against a Hydranal standard. pH
Samples were diluted to 10% in deionised Milli-Q water for pH determination.
Refractive index
Samples were diluted with an equivalent mass of water before the refractive index was measured. The results were converted to percentage weight/weight glucose/fructose using Tables 8-59 (D-Fructose) and 8-60 (D-Glucose) in the CRC Handbook of Chemistry and Physics 87th Edition.
Individual sugar content
Samples were prepared in deionised Milli-Q water at a concentration of approximately 10 grams per litre. They were analysed using High-Performance Liquid Chromatography with Evaporative Light Scattering Detection. Calibration curves were generated for each sugar in the range 1.5-3.0 grams per litre for glucose, 2.0-5.0 grams per litre for fructose and 0.25-1.0 grams per litre for sucrose and maltose. The peaks generated by maltose and oligosaccharides overlapped; these results were therefore measured using the maltose calibration curve and reported as maltose + total
oligosaccharides. The results for individual sugars are the mean of duplicate sample preparations.
MGO and DHA content
Samples and standards were prepared at 15% weight/volume in 0.5 M sodium phosphate (pH 6.5). They were then derivatised using 1% w/v orthophenylenediamine for approximately 24 hours before analysis by HPLC-Mass Spectrometry. Samples were quantified against individual calibration curves from 1 to 100 milligrams per litre. The results are the mean of duplicate sample preparations.
Nuclear Magnetic Resonance (NMR)
2D-NMR analytical procedures followed those described by Consonni et al. (2012). A solution of the honey sample was prepared at approximately 100 mg/ml in deuterated water, and analysed by proton (with and without pre-saturation of the water peak), carbon-13 and heteronuclear single quantum coherence NMR spectroscopy.
Statistical analysis
Results
Floral source of honeys
The floral sources of the honey samples assigned by routine assessment are indicated by the sample names (see Tables).
Pollen analysis
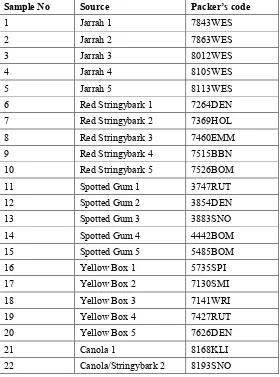
Analyses by Intertek delivered the pollen content assessments shown in Table 1.1.
Table 1.1. Pollen content of honey samples.
Sample No Packer’s code Source assigned by packer
Relative pollen type content (%)
Main (>45%) Relative (>15%) Accompanying (≥3%)
1 7843WES Jarrah 1 85% eucalypt none 12% Echium
2 7863WES Jarrah 2 58% eucalypt 32% Echium None
3 8012WES Jarrah 3 87% eucalypt none 6% Echium
4 8105WES Jarrah 4 89% eucalypt none 5% Lotus
5 8113WES Jarrah 5 82% eucalypt none 15% Echium
6 7264DEN Red Stringybark 1 78% eucalypt 19% Echium None
7 7369HOL Red Stringybark 2 72% eucalypt 24% Echium None
8 7460EMM Red Stringybark 3 48% Trifolium 41% eucalypt 5% Vicia
4% Cruciferae
9 7515BBN Red Stringybark 4 65% eucalypt 32% Echium None
10 7526BOM Red Stringybark 5 88% eucalypt none 10% Echium
11 3747RUT Spotted Gum 1 59% Echium 37% eucalypt None
12 3854DEN Spotted Gum 2 78% eucalypt 20% Echium None
13 3883SNO Spotted Gum 3 81% eucalypt none 10% Cruciferae
7% Echium
14 4442BOM Spotted Gum 4 54% eucalypt 38% Cruciferae 6% Echium
15 5485BOM Spotted Gum 5 73% eucalypt 21% Cruciferae 5% Echium
16 5735SPI Yellow Box 1 90% Echium None 7% eucalypt
17 7130SMI Yellow Box 2 50% eucalypt 44% Echium None
18 7141WRI Yellow Box 3 64% Echium 30% eucalypt 3% Cruciferae
19 7427RUT Yellow Box 4 81% eucalypt none 10% Echium
3% Trifolium
20 7626DEN Yellow Box 5 76% eucalypt 20% Echium 3% Cruciferae
Electrical conductivity
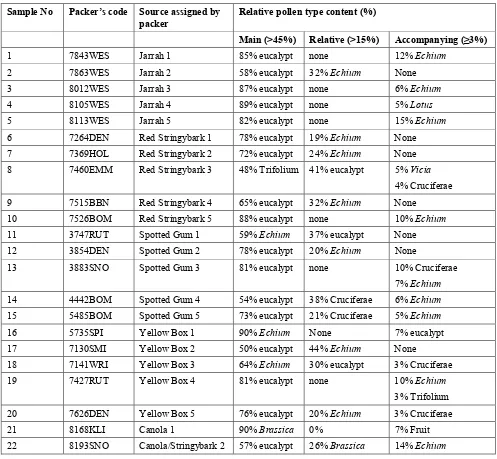
Analyses by Intertek delivered the conductivity assessments and sample designations shown in Table 1.2.
Table 1.2. Electrical conductivity and designation of honey samples.
Sample No Packer’s code Source assigned by packer
6 7264DEN Red Stringybark 1 0.521 Eucalyptus honey
7 7369HOL Red Stringybark 2 0.479 Eucalyptus honey
8 7460EMM Red Stringybark 3 0.297 Blossom honey
9 7515BBN Red Stringybark 4 0.685 Eucalyptus honey
10 7526BOM Red Stringybark 5 0.427 Eucalyptus honey
11 3747RUT Spotted Gum 1 1.029 Eucalyptus honey
12 3854DEN Spotted Gum 2 1.392 Eucalyptus honey
13 3883SNO Spotted Gum 3 1.171 Eucalyptus honey
14 4442BOM Spotted Gum 4 1.053 Eucalyptus honey with blossom honey
15 5485BOM Spotted Gum 5 1.092 Eucalyptus honey
16 5735SPI Yellow Box 1 0.359 Blossom honey
22 8193SNO Canola/Stringybark 2 0.454 Blossom honey with Eucalyptus honey
The honey samples were designated by Intertek according to the criteria outlined in the European Community Council Directive 2001/110/EC together with information in the literature. The
Intertek’s analysis indicated that:
•
Samples 1, 3, 5, 10, 13 and 19 were clearly eucalypt honeys, with >80% eucalypt pollen(Table 1.1) and no characteristics that suggest any other main source.
•
Sample 21 was clearly canola honey, with 90% canola pollen.•
Nineteen of the 22 samples contained Echium (Paterson’s Curse; Salvation Jane) pollen, butEchium is an over-represented pollen in honey (Intertek report) and as long as it did not affect the organoleptic characteristics of the honey this did not impact on sample designation, even in some cases when Echium was the main pollen species. Samples 6, 7, 9, 11 (59% Echium, 37% eucalypt pollen), 12 and 17 were designated eucalypt honey on this basis.
•
The main pollen in sample 15 was eucalypt (73%) and the presence of 21% cruciferous pollendid not affect its designation as eucalypt honey.
•
Sample 14 contained 54% eucalypt and 38% cruciferous pollen and was designated eucalypthoney with blossom honey.
•
Sample 18 contained 64% Echium and 30% eucalypt pollen and was designated blossomhoney with eucalypt honey, as was sample 22, the Canola/Stringybark control sample.
•
The main pollen in sample 8 was Trifolium (48%); it also contained 5% Vicia and 4%cruciferous as well as 41% eucalypt pollen; this sample was designated blossom honey.
•
Sample 16 contained 90% Echium and only 7% eucalypt pollen; it was also designatedblossom honey.
•
Sample 20 was designated blossom honey with Eucalyptus honey, despite containing 76%eucalypt pollen and most of the remainder being Echium pollen. In our view this is an incorrect assignment.
The electrical conductivity of honey depends on its ash and acid content. There is a linear relationship between the ash content and the electrical conductivity of a honey sample and the latter is now used in routine honey quality control procedures in Europe instead of determining the ash content.
In general for honeys produced in the northern hemisphere, blossom honeys and mixtures of blossom and honeydew honeys have conductivities of less than 0.8 and honeydew honeys have more than 0.8 milliSiemens/cm. However, eucalypt honeys provide exceptions to this relationship, with 181 samples giving a range of electrical conductivities of 0.19-1.33 milliSiemens/cm (Bogdanov et al. 2000). It can be seen from Table 1.2 that there is a very high variation between species in the conductivities of the honey samples. Three of the five Jarrah and all the Spotted Gum honey samples had electrical
These results indicated that:
• from the pollen analysis, honey samples Red Stringybark 3 (7460EMM ), Spotted Gum 4
(4442BOM), Yellow Box 1 (5735SPI) and Yellow Box 3 (7141WRI) may not be fully representative of eucalypt honey, and
• designation of honey samples Jarrah 2 (7863WES) and 4 (8105WES ) as containing
significant honeydew honey should be regarded with reservations.
Chemical analysis
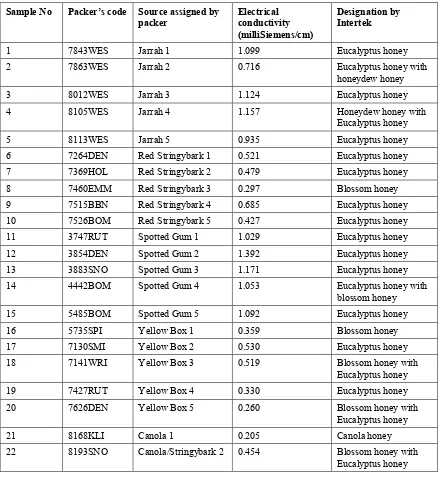
pH, water content and refractive index
The results for pH and water content of the honey samples are given in Table 1.3. The refractive index measurements were not informative; although the refractive index of honey is measured as an
indicator of the glucose:fructose ratios in the sample, the values obtained for the honeys assessed in this study did not reflect their sugar content measured by HPLC and the data are not included in this report.
Table 1.3. Water content and pH of honey samples.
These analyses indicated that:
• there was no significant difference between the water contents of the four groups of eucalypt
honeys, and
• the Jarrah honey samples were significantly less acid (P < 0.025) than those of all the other
eucalypt varieties.
Individual sugar content
Analyses by ChemicalAnalysis provided the sugar content assessments shown in Table 1.4.
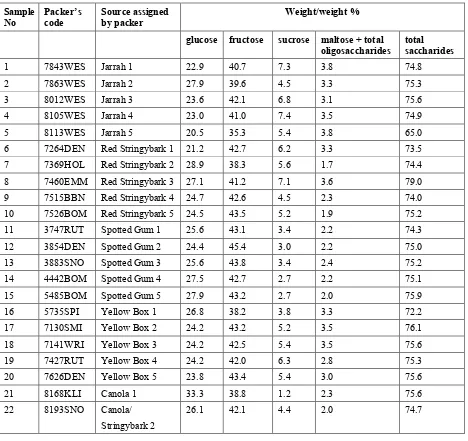
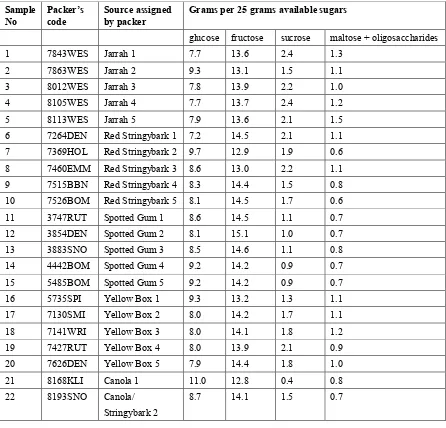
Table 1.4. Individual sugar content of honey samples.
Sample
Table 1.5. Normalised sugar content of honey samples.
Grams per 25 grams available sugars
glucose fructose sucrose maltose + oligosaccharides
1 7843WES Jarrah 1 7.7 13.6 2.4 1.3
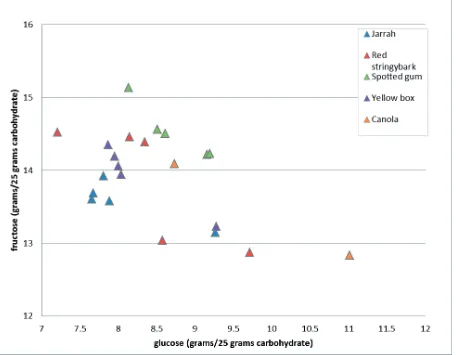
Figure 1.1. Glucose versus fructose content of honey samples.
Analyses indicated that:
• The concentrations of total saccharides in the Red Stringybark, Spotted Gum and Yellow Box
honeys were extremely similar (P > 0.8), as were those of four of the Jarrah honeys.
• The fructose content of the Spotted Gum honeys was significantly higher than that of the
Jarrah honeys (P = 0.026 when the raw data were compared, and P = 0.002 when the data had been normalised against sugar content). There were no other significant differences between fructose levels in the honeys from different eucalypt sources, and the fructose content of the Red Stringybark and Yellow Box honeys was very similar (P > 0.8).
• There were no significant differences between the glucose contents of any of the honeys from
• The amount of maltose + oligosaccharides in Spotted Gum honeys was also significantly
lower than in Jarrah honeys (P = 0.004 for the raw data and P = 0.001 for normalised data) and in Yellow Box honeys (P = 0.001 for both the raw and normalised data). When the data were normalised the content of these saccharides in Jarrah honeys was higher than in Red Stringybark honeys (P = 0.029).
• The clustered scatter-plot distribution of glucose:fructose ratios (Figure 1.1) and the clustered
scatter-plot distribution of glucose:sucrose ratios (data not shown) were consistent with the variabilities in composition noted above, but gave no indication of atypical composition for any honey sample. The outlier position of the canola-derived honey is consistent with the previously reported high relative glucose content (Abell et al. 1996).
• It should be noted that some of the individual sugar (glucose, fructose and particularly
MGO and DHA content
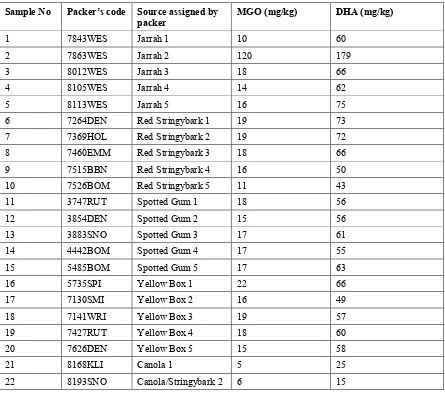
Analyses by ChemicalAnalysis provided the MGO and DHA assessments shown in Table 1.6.
Table 1.6. MGO and DHA content of honey samples.
Sample No Packer’s code Source assigned by packer
MGO (mg/kg) DHA (mg/kg)
1 7843WES Jarrah 1 10 60
2 7863WES Jarrah 2 120 179
3 8012WES Jarrah 3 18 66
4 8105WES Jarrah 4 14 62
5 8113WES Jarrah 5 16 75
6 7264DEN Red Stringybark 1 19 73
7 7369HOL Red Stringybark 2 19 72
8 7460EMM Red Stringybark 3 18 66
9 7515BBN Red Stringybark 4 16 50
10 7526BOM Red Stringybark 5 11 43
11 3747RUT Spotted Gum 1 18 56
12 3854DEN Spotted Gum 2 15 56
13 3883SNO Spotted Gum 3 17 61
14 4442BOM Spotted Gum 4 17 55
15 5485BOM Spotted Gum 5 17 63
16 5735SPI Yellow Box 1 22 66
17 7130SMI Yellow Box 2 16 49
18 7141WRI Yellow Box 3 19 57
19 7427RUT Yellow Box 4 18 60
20 7626DEN Yellow Box 5 15 58
21 8168KLI Canola 1 5 25
22 8193SNO Canola/Stringybark 2 6 15
Analyses showed that:
• There was no significant difference between either the MGO or the DHA contents of the four
groups of eucalypt honeys.
• One of the Jarrah honey samples (2) had a high content of both MGO and DHA, which have
Nuclear Magnetic Resonance
2D-NMR of Spotted Gum honey sample 3883SNO (Spotted Gum 3). The following saccharides were identified on the spectrum:
• monosaccharides: α-glucose, β-glucose;
• disaccharides: sucrose, palatinose, maltose, isomaltose, maltulose, nigerose, kojibiose,
turanose, melibiose;
• trisaccharides: maltotriose, isomaltotriose, erlose, raffinose;
• tetrasaccharides: maltotetraose.
Several of these sugars may contribute to prebiotic activity (see Chapter 3).
Implications
This study did not identify any physical or chemical characteristic, or combination thereof, that could be reliably used to differentiate between Australian eucalypt honeys sourced from different floral species.
Recommendation
Chapter 2.
Glycaemic Index of honey
samples
Introduction
The Glycaemic Index (GI) is a ranking of foods based on their overall effects on blood glucose levels. Foods with high GI values contain easily digested carbohydrates and produce a high, rapid rise and subsequent fall in blood glucose and insulin levels; those with low GI values contain carbohydrates that are digested more slowly and produce a slower, lower increase in blood glucose. The impact of the GI of a diet on a range of health outcomes is being increasingly recognised. Large-scale
epidemiological studies and meta-analyses have shown that the long-term consumption of a high GI diet can increase the risk of developing diabetes, heart disease and some cancers (FAO/WHO Report 1997; Favero et al. 1999), and more recently that the GI of the diet may be the most important dietary factor in preventing type 2 diabetes (Barclay et al. 2008). Low GI diets have been shown to reduce the risk of these diseases, improve blood glucose control and insulin sensitivity in diabetics and reduce high blood lipid levels (Jenkins et al. 1985; Brand et al. 1991; Jarvi et al. 1999; Bouché et al. 2002). They are also recommended for weight control as in addition to reducing insulinaemia, low GI foods are associated with higher satiety than high GI diets (Roberts 2000).
The International Tables of Glycemic Index and Glycemic Load Values: 2008 (Atkinson et al. 2008) are based on 205 articles published between 1981 and 2007, as well as unpublished data where the data quality could be verified. A GI value of ≤ 55 is classed as low, from 56 to 69 as moderate and ≥ 70 as high. Fructose, usually the dominant sugar in honey, has the lowest GI (19 ± 2) of any naturally occurring monosaccharide, compared with 100 for glucose and maltose, and 68 ± 5 for sucrose. The GI values of different honeys compared with glucose (100) in healthy subjects ranged from 35 ± 4 to 87 ± 8. The GI values of different Australian honeys quoted in the Table were determined in a Rural Industries Research and Development Corporation study (Arcot & Brand-Miller 2005). All five types of eucalypt honey, Yellow Box, Stringybark, Red Gum, Ironbark and Yapunya, had low GI values (< 55). The lowest was Yellow Box at 35 ± 4. Using linear correlation analysis these authors reported that fructose content was significantly associated with the average GI values of the honeys (r = - 0.76, n = 9, p<0.05) but the other individual sugars were not. An earlier publication by the same group reported that the glucose content of Australian honeys was significantly related to their mean GI, but that GI was not significantly related to the content of fructose, sucrose, maltose or organic acids (Holt et al. 2002).
The specific aims of this part of the study were to expand the preliminary studies quoted above to:
• confirm that Australian eucalypt honeys are low GI foods;
• determine whether it is possible to estimate the GI value of an Australian eucalypt honey from
its content of individual sugars, and
• assess a new in vitro Predictive GI test for its potential value in testing commercial batches of
Methodology
In vivo
GI measurement
The GI values of seven selected honey samples were measured in normal human subjects by the Glycemic Index Research Service, University of Sydney (SUGiRS). The methodology was developed at this Centre and is recognised internationally. The procedures were approved by the Human
Research Ethics Committee of the University of Sydney. Subjects
The study was conducted in ten healthy, non-smoking subjects aged 18-45 years who were within the healthy weight range, not dieting, and who did not have impaired glucose tolerance. Seven were males and three were females.
Test foods
A workshop of project stakeholders and investigators selected seven of the 22 honeys to be tested for GI using the standard in vivo methodology. Six of these honeys (samples 3, 6, 7, 9, 12 and 17) were confirmed by pollen and conductivity analyses as being ‘eucalyptus honey’ in accordance with European Council Directive 2001/110/EC, and the other was confirmed as canola honey by the same criteria (see Chapter 1). The samples included one Jarrah honey, three Red Stringybark honeys, one Spotted Gum honey and one Yellow Box honey with a spread of glucose and fructose content (see Table 1.4) to optimise attempts to relate these parameters to GI values.
Test procedure
Pure glucose dissolved in water was used as the reference food. Glucose and the honey samples were administered in portions containing 50 grams of available carbohydrate, accompanied by 250 grams of water. Each subject completed 10 individual tests. After fasting overnight for 10-12 hours, every subject consumed each of the honey samples in random order on one occasion, and the reference glucose preparation in the first, sixth and tenth test sessions. At least one day was allowed between test sessions. For each test, two fasting blood samples were first obtained. The test food was then consumed, after which additional blood samples were taken at 15, 30, 45, 60, 90 and 120 minutes. The blood samples were centrifuged and the plasma frozen until analysis.
Sample analysis
The glucose concentration of the plasma samples was assayed in duplicate using a glucose hexokinase enzymatic method (Roche Diagnostic Systems) and a Roche/Hitachi 912® automatic centrifugal spectrophotometric analyser with internal controls.
Calculation of GI values
For each test session the glucose concentrations in the two fasting plasma samples were averaged to give a baseline. The incremental area under each 2 hour glucose response curve (iAUC) was then calculated. The ratio of the iAUC for a honey sample to the averaged iAUCs for glucose for that subject, expressed as a percentage (glucose = 100%), gave the GI value for the honey. If any
Statistics
A power-based (90%) sample size calculation indicated that at least eight subjects would be required to generate statistically significant results (a difference of 1.0 standard deviation units in GI).
The researchers reported the GI value for each honey sample as the mean ± the standard error of the mean (SEM). They used analysis of variance and the Fisher PLSD test for multiple comparisons to determine whether there were significant differences between the GI values obtained.
In vitro
Predictive GI test
Test foods
All 22 honey samples used in this study were tested by Next Instruments (Condell Park, Sydney), with the exception of Red Stringybark 7264DEN, which was omitted in error from the samples sent to the testing laboratory. The laboratory instead received two samples of Yellow Box honey 7427RUT, both of which were tested.
Test procedure
The in vitro test used the NutriScan G120 Glycemic Index Analyser, a high precision fully automated instrument that mimics the way carbohydrates are digested in the human gut. It uses 50 milligrams of carbohydrate per sample, which are analysed at 37oC under gentle agitation, with physiological pH maintained throughout. Samples are initially treated with an enzyme that mimics saliva, followed by a second enzyme that breaks down fats and proteins in the sample. A further enzyme converts the sugars to glucose, and aliquots are analysed in a glucose analyser 15, 60, 120, 180, 240 and 300 minutes after initiation of the reaction. Conversion is complete at 300 minutes.
Each honey sample was assayed in duplicate at the same time as duplicate samples of the control material, glucose. One sample, canola honey 8168KLI, was assayed in duplicate on two occasions. Two samples, Red Stringybark honeys 7369HOL and 7515BBN, were first assayed in duplicate and single samples were then re-assayed on two further separate days to assess the repeatability of the assay.
Calculation of GI values
The Predictive GI was calculated using the formula:
Predictive GI = final glucose concentration (mg/ml) x final sample volume x 100/ total available carbohydrate in sample
The mean of the duplicate results was used as the final Predictive GI Value for the sample. The differences between duplicates were used to calculate the Standard Deviation of Differences (SDD).
Statistics
Results
In vivo
GI measurement
In vivo GI analyses of seven honey samples by SUGiRS resulted in the data provided in Table 2.1.
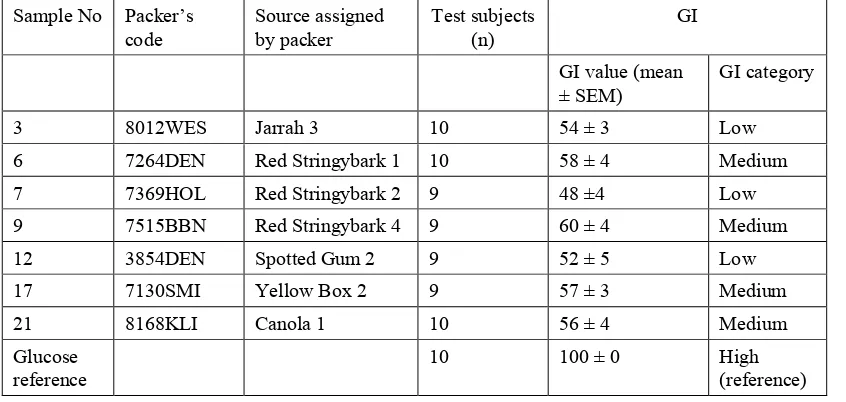
Table 2.1. GI values of honey samples.
Sample No Packer’s
The mean GI values for all the honey samples were significantly lower than that of the glucose reference and the difference was highly significant (p < 0.001) in all cases. The mean GI value for one Red Stringybark honey (7515BBN) was significantly higher than that for another (7369HOL), but there were no other significant differences amongst the mean GI values of the honey samples. Using the mean GI value, three of the eucalypt honey samples (one Jarrah, one Red Stringybark and one Spotted Gum) were rated as being ‘low’ GI and the other three samples (two Red Stringybark and one Yellow Box) as being of ‘medium’ GI, as was the canola honey. Holt et al. (2002) and Arcot and Brand-Miller (2005) reported that all the Australian eucalypt honeys they tested, including a Yellow Box honey and a Stringybark honey, had low GI values. It should be noted that although all the in vivo GI tests were performed by the same research group, the carbohydrate load used in the earlier studies was only 25 grams, half that administered to subjects in the present project. This may have affected the results.
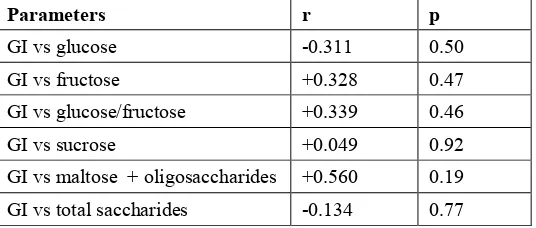
Table 2.2. Correlation between GI values and sugar content of honey samples.
Parameters r p
GI vs glucose -0.311 0.50
GI vs fructose +0.328 0.47
GI vs glucose/fructose +0.339 0.46
GI vs sucrose +0.049 0.92
GI vs maltose + oligosaccharides +0.560 0.19
GI vs total saccharides -0.134 0.77
There was no strong or significant correlation between any of the sugar contents analysed and the mean GI values for these honey samples. Similarly there was no strong or significant correlation of mean GI with the pH or content of MGO, DHA or water. The best correlation between mean GI value and any of the physical and chemical characteristics measured was with the combined maltose + oligosaccharide content. However, it was not sufficiently strong to form the basis for a valid surrogate marker of the GI value for a honey sample.
Multivariate analysis was considered as a potential means of deriving a significant correlation between GI values and two simple measurables of the honey samples, but the study did not yield sufficient data for this to be a useful approach.
Results from this study were not consistent with those of Holt et al. (2002), who previously reported a significant correlation between the GI value and glucose content of Australian honey samples.
Moreover, there was no significant correlation between the GI value and glucose content of honey samples when the mean data from this study were combined with those of Holt et al.
We observe that the results reported by Holt et al. are disproportionately affected by a single test sample (‘Commercial Blend 1’), for which the glucose content appears to have been calculated in error. If the GI result for this sample is amended their results do not show a significant correlation between GI value and glucose content. This is shown in Figure 2.1; the data from Holt et al. are labelled ‘2002’, and the re-calculated result for ‘Commercial Blend 1’ as ‘2002 amended’.
Figure 2.1. Glycaemic Index in relation to glucose content of honey samples.
Primary data from in vivo GI measurement
In vitro
Predictive GI test
In vitro measurements of the Predictive GI values for 21 honey samples by Next Instruments resulted in the data provided in Table 2.3.
Table 2.3. Predictive GI values of honey samples.
Sample
6 7264DEN Red Stringybark 1 not assessed
7 7369HOL Red Stringybark 2 51.1; 48.0; 50.5; 51.4 50.3 Low