Summary Responses of cambium to warming were re-corded three times (December 14--27, 1990, January 18--Feb-ruary 3 and Feb18--Feb-ruary 27--March 13, 1991) on 14-year-old Cryptomeria japonica D. Don and four times (December 12--26, 1990, January 18--February 2, February 26--March 12 and March 28--April 13, 1991) on 27-year-old Larix leptolepis Gord., during a period of winter cambial dormancy. Stem surfaces at breast height, mid-tree height and the crown base were warmed to 25--30 °C for 2 weeks. After heat treatment, cambia in the treated regions and in untreated regions 1 m above each treated area were examined by optical and trans-mission electron microscopy (TEM). In C. japonica, heat treatment often resulted in cambial reactivation in the treated regions, and this response to heat gradually increased as the dormant season passed from winter to spring. Conversely, in L. leptolepis, no cell division was observed in the cambial region of warmed stems until natural resumption of cambial activity, which occurred after bud break.
Keywords: Cryptomeria japonica, heat treatment, Larix lep-tolepis, winter cambial dormancy.
Introduction
In the Northern Temperate Zone of Japan, vascular cambia in conifers usually have annual periods of activity and dormancy. Cambial activity is terminated in autumn when cambial zone cells become dormant, and is resumed in spring when the cells divide and differentiate into xylem and phloem.
The resumption of cambial activity in spring is brought about both by internal chemical factors and external conditions (Savidge and Wareing 1981). We have previously shown that, in the evergreen conifer, Cryptomeria japonica D. Don, cam-bial reactivation occurs simultaneously throughout the stem before bud break, whereas in the deciduous conifer, Larix leptolepis Gord., cambial reactivation occurs a few weeks after bud flushing in stem portions of the crown, and then progresses basipetally down the stem (Oribe et al. 1993). We hypothesized from these results that factors involved in bud and leaf produc-tion regulate cambial reactivaproduc-tion in deciduous conifers, but
not in evergreen species. In an earlier study, Savidge and Wareing (1981) also suggested that temperature was a limiting factor for cambial reactivation in Pinus contorta Dougl. ex Loud., an evergreen conifer.
The objective of this study was to determine the cause of differences in cambial reactivation between evergreen and deciduous conifers. Specifically, cambia in stem portions of C. japonica and L. leptolepis were warmed for two-week peri-ods between winter and spring, and the response of cambia to this heat treatment was recorded.
Materials and methods
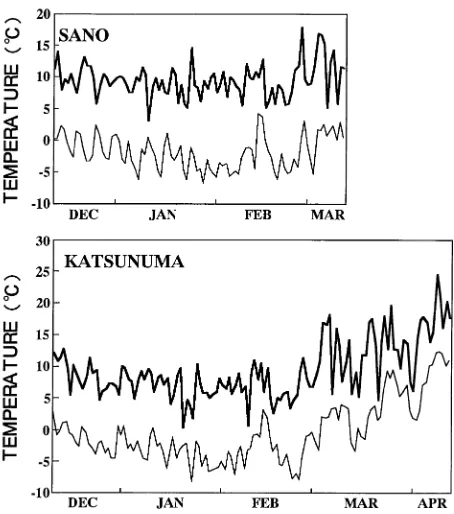
Plant materials included three 14-year-old sugi (Cryptomeria japonica) trees in the Karasawa Experimental Forest of Tokyo University of Agriculture and Technology, and four 27-year-old karamatsu (Larix leptolepis) trees in a plantation in Yamanashi Prefecture (Table 1). Between December 12, 1990 and April 13, 1991, heat treatments were carried out three times on C. japonica (December 14--27, 1990, January 18--February 3 and 18--February 27--March 13, 1991) and four times on L. leptolepis (December 12--26, 1990, January 18--Febru-ary 2, Febru18--Febru-ary 26--March 12, and March 28--April 13, 1991). One tree of C. japonica or L. leptolepis was selected on each occasion (Table 1). Maximum and minimum air temperatures during the experiments were obtained from the Sano meteoro-logical observatory located 5 km from Karasawa, and at Kat-sunuma located 10 km from the L. leptolepis plantation (Figure 1).
Three pliable, silicone-rubber electric heat tapes, 15 cm in width, were wrapped around the main stem circumference at breast height, mid-tree height and the crown base of each tree (Table 1). An alternating current of 30 V was passed through the tapes to warm the stem surfaces. The temperature between the outer bark and the heat tape was recorded by a thermometer at each location, and was adjusted to 25--30 °C. This ture range was selected to cover the range of summer tempera-tures that prevail during maximum tracheid production in conifers.
Effect of heat on cambial reactivation during winter dormancy in
evergreen and deciduous conifers
YUICHIRO ORIBE and TAKAFUMI KUBO
1Wood Technology Division, Forestry and Forest Products Research Institute, P.O. Box 16, Tsukuba City, Norin Kenkyu Danchi-nai, Ibaraki 305, Japan
2
Laboratory of Plant Morphology and Bio-Materials Physics, Department of Environmental and Natural Resource Science, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho Fuchu City, Tokyo 183, Japan
Received July 14, 1995
Immediately after each two-week heat treatment, stem disks were obtained for microscopic observation from both warmed portions and untreated portions, 1 m above the treated areas. In addition, on July 7, 1990, a disk from the breast height stem portion of a C. japonica tree was obtained to examine morpho-logical characteristics of growing cambium.
One-cm blocks containing cambium and some xylem and phloem tissues, were taken from the disks and fixed in 4% glutaraldehyde in 0.1 mol l−1 cacodylate buffer pH 7.2 for 15 min at room temperature in a vacuum. After removal of floating blocks, the remaining sinking material was fixed in glutaraldehyde for 90 min at room temperature. Specimens
were washed in 0.1 mol l−1 cacodylate buffer and trimmed to 3 mm in length before postfixation in 1% osmium tetroxide in 0.1 mol l−1 cacodylate buffer for 1 h at room temperature. Specimens were then washed in 0.1 mol l−1 cacodylate buffer, immersed in a graded ethanol--propylene oxide series followed by a propylene oxide--Araldite resin series, and embedded in Araldite resin. Glass and diamond knives on an UL-TRACUT N microtome (Nissei Sangyo. Co., Ltd., Tokyo, Ja-pan) were used to obtain transverse sections of cambia. Semi-thin sections for optical microscopy were double stained with safranin and gentian violet. Ultra-thin sections, stained with 2% uranyl acetate and lead citrate (Raynolds 1963), were observed with a JEM-100C electron microscope (JEOL, Ak-ishima City, Tokyo, Japan) at 80 kV.
Results
Cambial reactivation in C. japonica
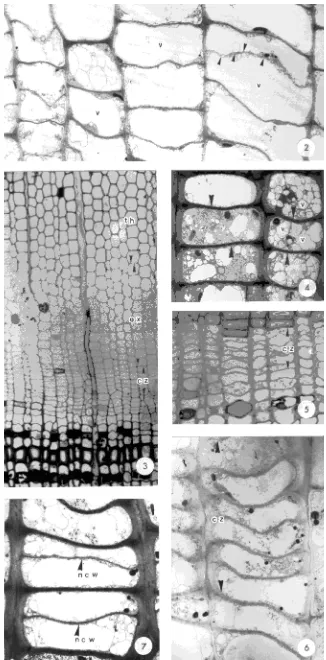
During active growth of cambial zone cells in C. japonica, cells with a cell plate, a large central vacuole and thin tangen-tial walls were observed in the cambial region (Figure 2). During the same period, cambial derivatives were radially expanding and undergoing secondary wall thickening (Fig-ure 3).
No dividing cells were observed in untreated samples col-lected on December 27, February 3 or March 13, indicating that cambia were dormant (Figure 4). Dormant cambial cells had relatively thick tangential walls and numerous subdivided vacuoles (Figure 4).
Cambial reactivation was often induced by heat treatment of the stem during the dormant period. On December 27, heat treatment of the lower portion of the stem had no effect on cambial reactivation (Figures 5 and 6), but heat treatment of the middle and upper portions of the stem induced division of previously dormant cells (Figure 7). Transverse sections of stems treated from January 18 to February 3 showed newly divided and radially expanding cells in the cambia (Figures 8--11); secondary wall thickening was observed only in cam-bial cells from the warmed, mid-tree height stem portion
(Fig-Table 1. Heat period and mensurational characteristics of the sample trees (n = 7).
Height at warmed portion (m)
Heat period Height (m) Percent crown (%) DBH1 (cm) Upper Middle Lower
Cryptomeria japonica
Dec 14--Dec 27 13.8 48.4 13.8 8.2 5.1 1.9
Jan 18--Feb 3 13.0 44.4 14.0 7.4 4.2 1.6
Feb 27--Mar 13 14.3 58.6 14.1 nm2 nm 1.2
Larix leptolepis
Dec 12--Dec 26 18.4 54.3 13.3 7.5 4.5 1.4
Jan 18--Feb 2 19.5 59.7 13.7 8.2 5.3 2.2
Feb 26--Mar 12 17.9 57.4 12.6 7.8 4.7 2.1
Mar 28--Apr 13 15.6 56.6 13.0 7.8 4.7 1.8
1 DBH = Diameter at breast height (measured at 1.2m). 2
nm = Not measured.
Figures 2 and 3. Transverse sections of active cambium in C. japonica col-lected July 7, 1990. (2) A TEM mi-crograph of cambial cells with cell plates (arrowheads) and large central vacuoles (v), ×2240. (3) Optical mi-crograph showing cell division and dif-ferentiation, ×264. Note: cz = cambial zone, ex = expanding cells, and th = wall thickening cells.
Figure 4. A TEM micrograph of nu-merous small vacuoles (v) and thick tangential cell walls (arrowheads) of dormant cambium in an untreated up-per portion of stem of C. japonica on February 3, 1991, ×2240.
Figures 5 and 6. Transverse sections of cambium in the lower stem portion of
C. japonica warmed December 14--27, 1990. (5) Optical micrograph, ×528. (6) A TEM micrograph, ×2240. Note: cz = cambial zone.
Figure 7. A TEM micrograph of cam-bium with new cell walls (ncw) in an upper stem portion of C. japonica
Figures 8 and 9. Transverse sections of cambium in the lower stem portion of C. ja-ponica warmed January 18--February 3, 1991. (8) Optical micrograph, ×429. (9) A TEM micrograph of new cell walls (ncw), ×1820. Note: cz = cam-bial zone, and ex = expanding cells.
Figure 10. Optical micrograph of cells undergoing division, ra-dial expansion and secondary wall thickening in the middle stem portion of C. japonica, warmed January 18--February 3, 1991, ×429. Note: cz = cam-bial zone, ex = expanding cells, and th = wall thickening cells.
Figure 11. Optical micrograph of cell divisions and radial ex-pansion in the upper stem portion of C. japonica, warmed January 18--February 3, 1991, ×429. Note: cz = cam-bial zone, and ex = expanding cells.
Figure 12. Optical micrograph of cells undergoing division, ra-dial expansion and secondary wall thickening in the lower stem portion of C. japonica, warmed February 27--March 13, 1991, ×429. Note: cz = cambial zone, ex = expanding cells, and th = wall thickening cells.
Figures 13 and 14. Two TEM micrographs of (13) untreated and (14) warmed cambia, col-lected on December 26, 1990 from the middle stem portion of
L. leptolepis, ×1560. Note: cambial zone cells have numer-ous small vacuoles (v) and thick tangential cell walls (ar-rowheads) and show no cell di-vision.
Figures 15 and 16. Two TEM micrographs of (15) untreated and (16) warmed cambia, collected March 12, 1991 from the middle stem portion of
L. leptolepis, ×1560. Note: cambial zone cells have numerous small vacuoles (v) and thick tangential cell walls (arrowheads) and show no cell division.
ure 10). By the third heat treatment, which was applied before natural release of cambial dormancy in spring, cambial deriva-tives producing secondary walls were also observed in the heat-treated lower portion of the stem (Figure 12).
Cambial dormancy in L. leptolepis
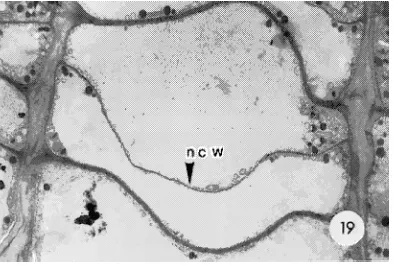
In L. leptolepis, no dividing cells were observed in untreated cambia during the experimental period from December 12 to March 12 (Figures 13 and 15), indicating that cambial cells were dormant. Transmission electron microscope (TEM) micro-graphs indicated that these cambial cells had relatively thick tangential walls and numerous subdivided vacuoles; similar cell characteristics were also observed in dormant cambia of C. japonica. By April 13, when buds had burst and cambial dormancy had been released, newly formed cell plates were frequently observed throughout the untreated regions of the stem (Figures 17 and 19). Moreover, radially expanding cells were observed in the upper untreated part of the stem.
The effect of heat treatments on dormant cambium differed in L. leptolepis from that in evergreen C. japonica. Heating stems of L. leptolepis trees failed to reactivate dormant cambial cells. Thick cell walls and numerous small vacuoles were present in warmed cambia between December and mid-March (Figures 14 and 16). By the fourth heat treatment from March 28 to April 13, cambial growth was more conspicuous in heat-treated portions than in untreated portions; however, cambial reactivation had already occurred naturally by April 13 (cf. Figures 17 and 18).
Discussion
Actively growing cambial cells were characterized by thin tangential walls and large central vacuoles. In contrast, dor-mant cambial cells of both C. japonica and L. leptolepis were characterized by thick tangential walls and numerous subdi-vided vacuoles. Similar cell structures, considered morpho-logical characteristics of dormant cambium, have previously been observed in Pinus spp., C. japonica and Salix spp., (Itoh 1971, Murmanis 1971, Lisbeth 1986).
During the dormant period, cambial growth often resumed in heat-treated portions of C. japonica stems (Table 2); similar findings have been reported for Pinus contorta (Savidge and Wareing 1981). These results suggest that, in evergreen coni-fers, cambial dormancy is imposed by low air temperatures, and that cambial reactivation is directly triggered by a rise in temperature. This conclusion implies that evergreen conifer cambia may reactivate in the absence of new shoot and leaf growth.
However, the extent of cambial activity in heat-treated stem regions of C. japonica varied on each occasion. The response to heat treatment, as measured by cambial reactivation and xylem formation, tended to increase as the period of cambial dormancy progressed. Thus, there was no cambial response to heat treatment of the lower portion of the stem in December (cf. values on December 27, February 3 and March 13 in
Table 2. Extent of cambial activity in both warmed and untreated portions of evergreen and deciduous conifer stems.
Collection date Upper portion Middle portion Lower portion
Untreated Warmed Untreated Warmed Untreated Warmed
Cryptomeria japonica
CD, RE = Cell division and radial expansion.
4
CD, RE, SWT = Cell division, radial expansion and secondary wall thickening.
5
nm = Not measured.
Table 2). These data indicate that, in addition to temperature, other factors affect cambial dormancy.
The role of plant growth regulators in cambial activity and dormancy has been demonstrated. It is well known that indol-3-ylacetic acid (IAA) is required by the vascular cambium to promote cambial cell division, radial enlargement and secon-dary wall thickening of cambial derivatives (Savidge and Wareing 1981, Little and Savidge 1987). Absence of cambial reactivation in the lower heat-treated portion of the stem in December might be a result of IAA deficiency in cambium during the early stage of dormancy. Seasonal variation in endogenous auxin concentrations may account for observed differences in cambial responses to heat treatment in C. japon-ica; however, this explanation is not supported by other obser-vations. In Abies balsamea (L.) Mill (Sundberg et al. 1987), not only was cessation of tracheid production poorly correlated with decline in IAA concentrations, but exogenous IAA, which induces similar anatomical and histochemical responses in dormant cambium as endogenous IAA (Riding and Little 1984), did not prevent cessation of cambial growth during the early stage of cambial dormancy. Furthermore, in A. balsamea (Sundberg et al. 1987), Picea sitchensis (Bong.) Carrière (Lit-tle and Wareing 1981), and P. contorta (Savidge and Wareing 1984), cambial cells retain relatively high endogenous IAA concentrations throughout dormancy.
Differences in the extent of cambial activity in heat-treated portions of the evergreen conifer stem during winter months may also be associated with a changeover between phases of dormancy. In A. balsamea, cambia that are one-year-old or more have a rest phase of dormancy followed by a quiescent phase (Little and Bonga 1974, Riding and Little 1984, 1986). During the rest phase, cambial dormancy is maintained by internal agents (Little and Bonga 1974), whereas during the quiescence phase, dormancy is imposed by external factors, such as chilling. Under favorable growing conditions, applica-tion of exogenous IAA to A. balsamea can break cambial quiescence, but it cannot break the rest phase of dormancy (Little and Bonga 1974, Riding and Little 1984, Sundberg et al. 1987). In C. japonica, cambial response to heat treatment may reflect a dormancy stage of cambium. Thus, in December, cambium in the lower portion of the stem may have been in the rest phase of dormancy.
In L. leptolepis, lack of cambial response to heat treatments before occurrence of natural cambial reactivation suggests that low air temperature is not a limiting factor for cambial reacti-vation in this species (Table 2). Previously, we found that, in L. leptolepis, cambial reactivation occurred after budbreak in stem portions located in the living crown, and progressed basipetally down the stem (Oribe et al. 1993). Based on these findings together with the fact that IAA is synthesized in young expanding leaves and transported basipetally down the stem (Savidge and Wareing 1981, Little and Savidge 1987), we postulate that cambial reactivation in L. leptolepis is regulated by IAA in the cambial region. Although it is possible that cambium remained dormant in heat-treated portions of the L. leptolepis stem because cambium is IAA deficient until bud flushing occurs, this conclusion is not supported by the study of Savidge and Wareing (1982), showing high concentrations
of IAA in dormant cambia from the main stem of L. decidua Mill.
Differences in cambial response to heat treatment between C. japonica and L. leptolepis may result from temperature differences between the bark surface and heat tape arising from variation in bark thickness and species-specific anatomy. To avoid cambial injury, cambial temperatures in heat-treated portions of stems were not directly recorded during the experi-mental period. Instead, the temperature between cambium and xylem under the heat tape was measured with copper-constan-tan thermocouples at breast height in both a C. japonica tree and a L. leptolepis tree in February. In both species, cambial temperatures gradually increased and reached stationary val-ues within the selected temperature range in 4--6 h, implying that cambia of both conifers were warmed at similar rates.
We conclude, therefore, that a rise in air temperature directly triggers breaking of cambial dormancy in the evergreen coni-fer, C. japonica, but not in the deciduous conifer, L. leptolepis. Cambial dormancy in L. leptolepis is broken by several factors associated with bud flushing.
Acknowledgments
We thank Mr. S. Matsuzaki, Karasawa Experimental Forest of Tokyo University of Agriculture and Technology for his technical assistance.
References
Itoh, T. 1971. Ultrastructure of dormant and active cambium. Wood Res. 51:33--45.
Lisbeth, S.F. 1986. Seasonal variation in the ultrastructure of the cambium in young stems of willow (Salix viminalis) in relation to phenology. Physiol. Plant. 67:529--537.
Little, C.H.A. and J.M. Bonga. 1974. Rest in the cambium of Abies balsamea. Can. J. Bot. 52:1723--1730.
Little, C.H.A. and P.F. Wareing. 1981. Control of cambial activity and dormancy in Picea sitchensis by indol-3-ylacetic and abscisic acid. Can. J. Bot. 59:1480--1493.
Little, C.H.A. and R.A. Savidge. 1987. The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul. 6:137--169.
Murmanis, L. 1971. Structural changes in the vascular cambium of
Pinus strobus L. during an annual cycle. Ann. Bot. 35:133--141. Oribe, Y., T. Kubo and M. Fushitani. 1993. Variations of cambial
reactivation within stems in deciduous and evergreen conifers. Bull. Exp. For. Tokyo Univ. Agric. Technol. 31:41--49 (In Japanese). Raynolds, E.S. 1963. The use of lead citrate at high pH as an
electron-opaque stain in electron microscopy. Cell Biol. 19:208--213. Riding, R.T. and C.H.A. Little. 1984. Anatomy and histochemistry of
Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can. J. Bot. 62:2570--2579.
Riding, R.T. and C.H.A. Little. 1986. Histochemistry of the dormant vascular cambium of Abies balsamea: changes associated with tree age and crown position. Can. J. Bot. 64:2082--2087.
Savidge, R.A. and P.F. Wareing. 1981. Plant growth regulators and the differentiation of vascular elements. In Xylem Cell Development. Ed. J.R. Barnett. Castle House Publications Ltd., London, pp 192-235. Savidge, R.A. and P.F. Wareing. 1982. Apparent auxin production and
Savidge, R.A. and P.F. Wareing. 1984. Seasonal cambial activity and xylem development in Pinus contorta in relation to endogenous indol-3-ylacetic and (S)-abscisic acid levels. Can. J. For. Res. 14:676--682.