The detrimental effect of simmondsin on food intake and
body weight of rats
David A. York
a,*, Lori Singer
a, Julian Oliver
b, Thomas P. Abbott
b,
George A. Bray
aaPennington Biomedical Research Center,6400Perkins Road,Baton Rouge,LA70808 4124,USA bNew Crops Research,USDA-ARS-NCAUR,1815N.Uni6ersity Street,Peoria,IL61604,USA
Accepted 12 May 2000
Abstract
Background: Simmondsin is a cyanomethylene glycoside, derived from the desert shrub, Simmondsia chinensis. Simmondsin has been reported to produce weight loss and to decrease food intake, an effect that can be blocked by treatment with an inhibitor of cholecystokinin.Study design: Six experiments were conducted on male Sprague – Daw-ley rats.Results: In two acute experiments where simmondsin was either added to the diet or injected, there was a dose-related reduction in food intake. The CCKAantagonist, lorglumide, did not block the acute inhibitory effects of simmondsin on food intake and simmondsin did not produce conditioned taste aversion. In two chronic feeding studies, the high dose of simmondsin (0.5%) in the diet produced profound weight loss and death in rats. At autopsy, the kidney, heart and liver of the treated animals were larger than the pair-fed animals and there was a marked suppression of the bone marrow elements with severe anemia.Conclusion: Simmondsin is toxic with profound effects on the hematopoietic system. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Energy expenditure; Cholecystokinin; Lorglumide; Conditioned taste aversion; Capsaicin vagotomy
www.elsevier.com/locate/indcrop
1. Introduction
Simmondsia chinensis is a desert shrub that grows in the Sonoran desert of the Southwestern US. The jojoba meal extract from this plant is toxic to animals (Booth et al., 1974; Verbiscar et al., 1980). Elliger et al. (1974a,b) have identified a compound called simmondsin
[2-(cyano-methyl-ene)-3-hydroxy-4,5-dimethoxycyclohexyl b-D
-glu-coside] from jojoba meal. Cokelaere et al. (1992b) have taken up the question of whether the toxic effects of jojoba meal and the reduction of food intake have been separated with the purified sim-mondsin. This compound appears to be well ab-sorbed whether given intragastrically or mixed with food (Flo et al., 1997). Cokelaere et al. (1995a) have reported that this effect is blocked by devazepide, an antagonist of the cholecys-tokinin A receptor. The possibility that CCKA
receptors were involved in the mechanism of
ac-* Corresponding author. Tel.: +1-225-7632548; fax: + 1-225-7632525.
E-mail address:[email protected] (D.A. York).
tion of this compound prompted the present studies.
It has recently become evident that the majority of orexigenic and anorectic compounds also have effects on energy expenditure through their recip-rocal effects on the autonomic responses to feed-ing (Bray and York, 1998). Thus, orexigenic agents tend to inhibit the sympathetic drive to brown adipose tissue (BAT) to reduce BAT ther-mogenesis, whereas anorectic agents promote BAT thermogenesis and weight loss. We have investigated the possibility that simmondsin in-creases energy expenditure in addition to its anorectic properties. We have also undertaken studies to show that the anorexia induced by simmondsin does not result from the induction of a conditioned-taste aversion.
2. Methods and materials
2.1. Animals
Male Sprague – Dawley rats were purchased from Harlan Sprague – Dawley (Indianapolis, IN). They were maintained on a 12:12 h light:dark cycle (08:00 – 20:00 h) with tap water available through an automatic system.
2.2. Diets
The animals were maintained on a chow diet (Ralston-Purina Co., St. Louis, MO, Lot 5001). In some experiments, powdered chow was mixed with simmondsin at one of several concentrations. Food intake was measured at intervals of 0.5, 1, 2, 4, 6, and 24 h in the acute experiments and daily during the chronic experiments.
2.3. Procedures
2.3.1. Experiment 1
Male Sprague – Dawley rats, weighing 220 – 260 g, were housed singly and allowed to eat powdered chow that was provided in a food jar within the cage. On the experimental day at the beginning of the dark cycle (20:00 h) all rats were given fresh diet with either no simmondsin (N=
4), or simmondsin added at 0.1, 0.25, 0.5, 0.75, 1.0 or 2.0% in the powdered chow with four rats at each dose. Food intake was measured at 1, 2, 4, 6 and 24 h. After 24 h, the diet was replaced with normal powdered chow. Each rat received only one treatment.
2.3.2. Experiment2
After completion of Experiment 1 (7 days), the male Sprague – Dawley rats were fasted overnight to enhance their hunger and then given intraperi-toneal injections of 2 ml/kg containing either sa-line (0.9% w/v) vehicle (N=6) or 200, 400, 800 or 1600 mg simmondsin with N=6 rats at each dose. Food was returned within 5 min and food intake was measured at 0.5, 1, 2, 4, 6 and 24 h.
2.3.3. Experiment3
This experiment investigated the ability to block anorectic response to simmondsin with the CCKA receptor antagonist lorglumide. Thirty
male Sprague – Dawley rats (200 – 240 g) were housed singly and adapted to a powdered chow diet. All rats were deprived of food for 2 h (09:00 – 11:00 h) before they received either a sa-line vehicle injection (N=10), simmondsin, 600 mg/kg i.p. plus saline (N=10) or lorglumide (600
mg/kg) and simmondsin (600 mg/kg) (N= −10). The dose of lorglumide was injected 10 min before simmondsin. This dose of lorglumide has previ-ously been shown to block the anorectic response to CCK, but lorglumide in this dose had no effect on food intake by itself (data not shown). Food intake was measured at 0.5, 1, 2, 4, 6 and 8 h.
2.3.4. Experiment4
each was measured over a 30-min period. The intake of saccharine solution is expressed as a percent of total fluid intake.
2.3.5. Experiment 5
The chronic effect of simmondsin (0.5% in the chow diet) on food intake and body weight was compared in groups of ten animals fed ad-lib and ten animals that were pair-fed to the simmondsin group. Pair feeding was achieved by providing each rat with the intake of a simmondsin-fed rat on the previous day after correcting for spillage from the simmondsin-fed rats.
Energy expenditure of the rats was measured between the third and fifth weeks. Animals were placed in individual metabolic cages and the con-sumption of oxygen and production of carbon dioxide over 24 h was measured with a Columbus Instruments apparatus (Columbus, OH).
2.3.6. Experiment 6
A chronic dose-ranging study compared doses of 0.015, 0.05, 0.15 and 0.5% to a control group and a capsaicin-treated control group to a capsa-icin-treated group receiving 0.15% simmondsin. Rats were fed ground chow with or without the simmondsin for 4 weeks. Capsaicin treatment was performed in two groups of rats 2 weeks prior to the beginning of the study as previously described (Lin et al., 2000).
2.4. Pathology
At autopsy, dissected tissues from multiple or-gans from 16 rats in Experiment 5 (four survivors on the 0.5% simmondsin, four pair-fed rats and eight controls) were placed in 10% formalin for fixation and transferred to the veterinary patholo-gist. Tissues were embedded in paraffin, cut in 4-mm sections and stained with hematoxylin and eosin. Lesions, when present, were graded as nor-mal, mildly affected, moderately affected or severely affected.
2.5. Reagents
The simmondsin used in these experiments was kindly prepared by Thomas Abbott, Ph.D. of the USDA Laboratories in Peoria, IL.
Simmondsin was purified by the procedure de-scribed by Abbott et al. (1999). Analysis by five different laboratories determined the purity to be 94.3 – 100% and averaging 99.48% purity (Abbott et al., 2000). Analysis of this simmondsin sample (C) was performed by HPLC by an independent laboratory, comparing it to the same material recrystallized twice more (TC) and the residue from the recrystallization (R). A Sphersorb Nov-Pak C-18, 4-mm, 4.6×150 mm column (Waters) was eluted isocratically with acetonitrile/water/
acetic acid (250 ml acetonitrile in 4 l water and with pH adjusted with acetic acid to pH 3.5), eluting at 0.5 ml/min. Detection was with UV at 220 nm, refractive index (RI) and scanning fluorescence, 320 nm excitation, 450 nm emission. Only simmondsin (S), RT 13.3 min and demethyl-simmondsin (DMS) peaks (two isomers gave two peaks), RT 7.1 and 10.0 min, were observed. C gave a purity of 98.58% S and 0.50% DMS (one peak, RT 10.1) compared to 98.28% S, 0.91% DMS (one peak, RT 10.1) for TC, based on UV peak area of the observed components. R tested to be 83.67% S, 15.06% DMS by UV. Peak areas in RI gave 99.32% S, 0.35% DMS for TC, 99.33% S, 0.23% DMS for C and 88.27% S, 9.05% DMS for R. LC-MS was also performed on the three materials. The only three materials found in C and TC were identified as S and two DMS iso-mers. Very minor peaks were observed in R, which were not identifiable at these very low levels. Thin layer chromatography (TLC) on silica gel in 90/10 acetonitrile/water, 80/20 chloroform/
methanol and 80/20 methanol/water and detec-tion by UV, exposure to iodine or charring with sulfuric acid/phenol gave at most two spots for C and TC and three spots for R, none of which were at the origin. The mother liquor from the prepara-tion of C gave five spots, some with the same Rf
values as in C, TC and R. Preparative TLC was used to separate the individual spots for identifi-cation by NMR. Based on these analyses, the C and TC samples appear to be very similar in composition and of high purity with DMS as the principal minority impurity and no other detected impurities.
vehi-cle containing 10% ethanol, 10% Tween-80 before being administered to rats on three occasions at doses of 25, 25 and 50 mg/kg with 18 and 6 h between successive treatments. The effectiveness of the capsaicin treatment in destroying non-myelinated afferent fibers was confirmed by the
failure to exhibit corneal reflex in response to 1% NH4OH. The lorglumide was purchased from
Sigma and was dissolved in 0.9% saline before being administered to the rats.
2.6. Statistical analysis
Data are expressed as mean9S.E.M. An a of 0.05 was accepted as the level of statistical signifi-cance. ANOVA was used with Bonferroni’s cor-rection for multiple comparisons to compare repeat measure studies.
3. Results
3.1. Experiment 1, acute dose-response to oral simmondsin
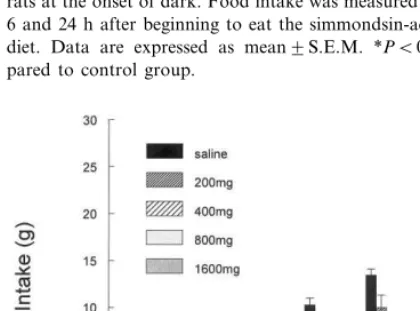
Fig. 1 shows the food intake of rats that ate a diet containing simmondsin at one of five levels beginning at the onset of the dark cycle. Begin-ning at 4 h, but more evident at 6 and 24 h, there was a significant dose-related suppression of food intake at 24 h. At 24 h, the highest dose (2.00%) had decreased food intake by \60%.
3.2. Experiment 2, acute dose-response to intraperitoneal simmondsin
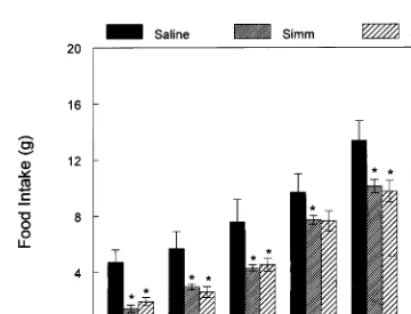
When simmondsin was administered intraperi-toneally (Fig. 2), a dose-dependent reduction in food intake was evident at 0.5 h and became striking at 1, 2, 4 and 6 h. By 24 h, it was still present, but the highest dose depressed food in-take by B50% compared to a \50% reduction at 1, 2, 4 and 6 h. The continuing effect at 24 h suggests a relatively long biological half-life for this molecule.
3.3. Experiment 3, CCK antagonist lorglumide and food intake after simmondsin
Fig. 3 shows the effect of administering 600 mg/kg of simmondsin alone or with lorglumide (600mg/kg). This dose blocks the anorectic response to CCK (data not shown). Simmondsin sup-pressed food intake by \50% at 0.5, 1 and 2 h,
Fig. 1. Effect of simmondsin on food intake. Simmondsin was added to the diet in the concentrations shown in the legend. Fresh diet with simmondsin as a first exposure was given to rats at the onset of dark. Food intake was measured at 1, 2, 4, 6 and 24 h after beginning to eat the simmondsin-adulterated diet. Data are expressed as mean9S.E.M. *PB0.05 com-pared to control group.
Fig. 3. Effect of i.p. lorglumide on intake of food after i.p. simmondsin. Overnight-fasted animals were given injections of vehicle, simmondsin 600 mg/kg or simmondsin with lorglu-mide 600mg/kg. Simmondsin reduced food intake which was not affected by simultaneous administration of lorglumide, a drug that blocks CCKA receptors. Data are expressed as
mean9S.E.M. *PB0.05 compared to control group.
and then after 4 h to see if there was any temporal effect. The suppression of food intake by sim-mondsin was unaffected by lorglumide (data not shown).
3.4. Experiment 4, conditioned taste a6ersion
Animals were conditioned to associate sim-mondsin or LiCl treatment with saccharine solu-tion. When they were subsequently given a choice of saccharine or water drinking solution (Fig. 4), the liquid intake of the vehicle-treated animals was 64912% saccharine, the liquid intake for the simmondsin pre-treated group was 4598% sac-charine, (P\0.1), whereas the lithium-treated rats showed a marked suppression of saccharine intake (791%, PB0.005 versus other intakes). Thus, simmondsin does not appear to be aversive in this context.
3.5. Experiment 5, chronic feeding study with simmondsin
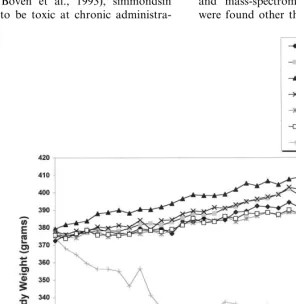
Fig. 5 shows the mean body weight of the control group, the simmondsin group (0.5% in diet) and the pair-fed group. Simmondsin pre-vented weight gain and subsequently produced weight loss in these animals. Control rats were significantly heavier than the simmondsin-treated rats by the third day of treatment. Six of the ten rats in the simmondsin group died, but none of the rats in the other group.
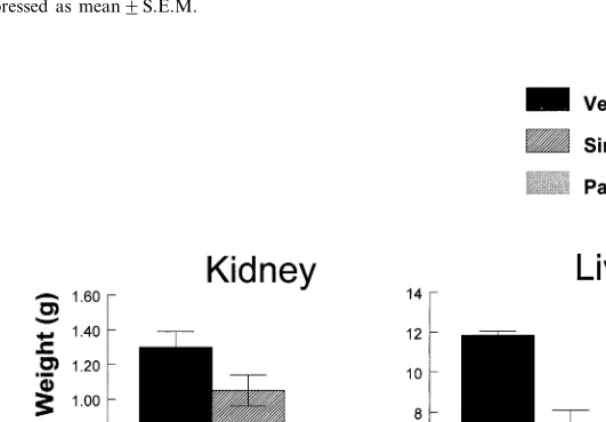
Mean food intake of the simmondsin-treated animals was reduced on Day 1 to :1590.6 g per day and fell slowly to levels of 1392 g per day until Day 40. Thereafter, food intake fell below 10 g per day as animals began to die. Rapid weight loss of individual rats began on Day 10 and between Day 35 and Day 46 for the other rats. The time of death of rats is shown by the arrows in Fig. 5. Deaths began as early as 38 days after treatment began in animals fed simmondsin. The experiment was terminated early at Day 52 because of the high death rate. At autopsy, the blood from the simmondsin-treated animals ap-peared watery and did not clot during the entire 2-h period of autopsies. Fig. 6 shows the liver and kidney weight from the three groups. Liver and
Fig. 4. Effect of simmondsin on taste aversion to saccharine. Simmondsin, 600 mg/kg, lithium chloride (81 mg/kg) and vehicle were paired with 0.15% saccharine drinking solution. After a 2-day rest, animals were again given saccharine or water to drink. The percent of saccharine intake was signifi-cantly suppressed in the animals previously treated with lithium chloride but not the animals previously treated with simmondsin. Values represent means9S.E.M. **PB0.01 compared to control group.
Fig. 5. Mean body weights of animals chronically treated with simmondsin. The animals fed the control diet gained weight progressively throughout the experiment. The animals eating the 0.5% simmondsin-adulterated diet had a decrease in body weight, as did the animals pair-fed, to those eating the simmondsin diet. The arrows indicate time of death of individual rats (two on Day 46). Data are expressed as mean9S.E.M.
Fig. 7. Metabolic rates and respiratory quotients of rats treated with simmondsin. Values represent means9S.E.M.
periartiolar sheaths and accumulation of hemosiderin-laden macrophages in the red pulp. Only the simmondsin fed animals had these le-sions. Other tissues did not contain significant changes except kidneys, which showed occasional dilated tubules filled with an amorphous, homoge-neous, eosinophilic material (protein cast).
Energy expenditure in both the simmondsin and pair-fed group was lower (Fig. 7B) (:8 kcal/100 g bw) than in the control group (12 – 14 kcal/100 g bw), but did not augment during the night in simmondsin and pair-fed rats (Fig. 7A). The respiratory quotient was higher at night (0.95 – 0.98) in all three groups than in the day (0.83 – 0.90), but did not differ between treatment groups (Fig. 7B).
3.6. Experiment 6, chronic dose-ranging study with and without capsaicin treatment
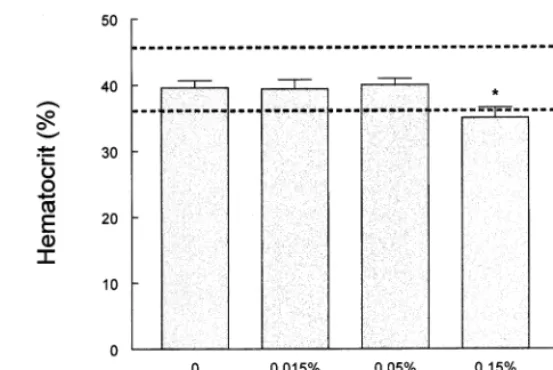
Fig. 8 shows the body weight of animals treated with simmondsin. Included in this figure are two control groups, one for the simmondsin-fed ani-mals and a capsaicin-treated control for the sim-mondsin-treated capsaicin-treated animals. Once again, the animals fed a diet with 0.5% sim-mondsin had a reduced food intake and lower body weight than the other groups. The lowest doses of simmondsin (0.015 and 0.05%) and the capsaicin control groups were slightly heavier than the control group. Simmondsin (0.15%) had no effect on body weight, but it blocked the unexpected increase that was observed in rats treated with capsaicin. However, none of these effects reached statistical significance with the ex-ception of the highest dose of simmondsin (0.5%), which significantly reduced both body weight. Likewise, there were no significant effects on food intake at any doses other than the 0.5% sim-mondsin, which significantly reduced feeding (data not shown). At autopsy, the animals treated with the highest dose of simmondsin had smaller livers and light epididymal and retroperitoneal fat pads than the ad-lib controls (data not shown). The hematocrit in the animals treated with 0.15 and 0.5% simmondsin was below the normal range for the control rats in this experiment (Fig. 9).
kidney weights of the pair-fed animals were sig-nificantly lower than in the control group. Rela-tive to the pair-fed controls, the liver and kidney of the group fed 0.5% simmondsin were signifi-cantly heavier (PB0.05), although still signifi-cantly smaller than the ad-lib controls.
4. Discussion
The experiments examined both the acute and chronic effects of simmondsin on food intake and energy expenditure of male rats. The most striking finding from the present studies was the toxicity of simmondsin when fed chronically to rats.
Simmondsin is a cyanomethylene glycoside derived from jojoba meal (Elliger et al., 1974a), an extract of the bulbous portion of the desert shrub Simmondsia chinensis. This plant grows in the Sonoran desert of the Southwest US. Jojoba meal has long known to be toxic (Booth et al., 1974). In chemical studies of separated pure sim-mondsin and its derivatives (Elliger et al., 1974a,b; Van Boven et al., 1993), simmondsin was described to be toxic at chronic
administra-tion to mice in higher doses (Verbiscar et al., 1980). This compound, simmondsin, has an anorectic effect (Cokelaere et al., 1992a) that was previously reported to be blocked by administra-tion of devazepide, a CCKA receptor antagonist
(Cokelaere et al., 1995a), suggesting that it might work through stimulation of the cholecystokinin receptors in the GI tract (Dourish et al., 1989).
One possible cause of the toxicity seen in the current experiments is that the simmondsin we used might have been contaminated with one of the other ‘toxic’ elements of jojoba meal. The simmondsin used in these experiments was pre-pared at the USDA Laboratory in Peoria, IL. When the toxicity issue arose, the lot of sim-mondsin used was reexamined by HPLC, TLC and mass-spectrometry. No other components were found other than DMS at B1%.
Fig. 9. Hematocrit in the simmondsin-treated animals. The hematocrit in the animals treated with the two highest doses of simmondsin was significantly reduced. Data are expressed as mean9S.E.M. The dashed lines are the normal range of hematocrit. *PB0.05 compared to control group.
The present studies have confirmed that sim-mondsin produces a dose-related reduction in food intake when administered acutely to rats (Flo et al., 1997). The effect was most clearly seen when the compound was injected intraperi-toneally. A single injection of simmondsin i.p., reduced food intake for the entire 24-h period.
Two experiments were conducted to test the possibility that simmondsin acted through the CCKAreceptors in the GI tract. First, lorglumide,
a CCKA receptor antagonist (de Tullio et al.,
1999) was given along with the simmondsin to male rats. The doses of lorglumide we have used in these studies had no effect on the suppression of food intake produced by simmondsin. How-ever, this same dose of lorglumide did block the effect of cholecystokinin itself. This lack of effect of lorglumide on the response to simmondsin was evident whether lorglumide was given before or with the simmondsin. Second, we tested the effect of simmondsin given in the diet to animals that had been chemically vagotomized by capsaicin (Singer et al., 1999). Simmondsin had the same effect on food intake in the presence or absence of capsaicin treatment. We were not able to confirm that simmondsin acted through CCKA receptors
(Cokelaere et al., 1995a).
Two chronic feeding studies with simmondsin demonstrated its toxicity. In the first experiment, 0.5% of simmondsin added to the diet reduced food intake by \30% and was associated with weight loss. However, after 38 days, deaths began occurring and the experiment was terminated be-fore the planned 90 days. At autopsy, the liver and kidney of the simmondsin-treated rats were larger than the weight of the corresponding or-gans in the pair-fed controls. The blood at sac-rifice did not clot and appeared ‘watery’. These results contrast with a previous report on the long-term administration of simmondsin by Coke-laere et al. (1995b). However, while that study used a similar dose of simmondsin (0.5%), it lasted for only 28 days. The first death observed in our study was not until feeding had continued for 38 days.
(1995b) were unable to detect any differences between simmondsin and pair-fed rats in their shorter chronic study, but did observe differences in breeder pullets (Cokelaere et al., 1993).
In the second chronic experiment, four doses of simmondsin were used. Once again the highest dose of 0.5% reduced food intake and body weight. The other three doses had only slight non-significant effects on body weight and food intake. At autopsy, the hematocrit of the 0.15 and 0.5% simmondsin-treated animals was below nor-mal. The highest dose also suppressed the weight of the liver and the epididymal and retroperi-toneal fat pads relative to ad-lib fed controls, and increased weight of the liver compared to pair-fed animals. These data suggest that simmondsin may have effects on hematopoiesis even at doses (0.15%) that do not affect body weight or food intake. Histopathology of bone, completed on surviving rats in the first chronic treatment study, confirmed the effects of 0.5% simmondsin on the hematopoietic cell lines in bone marrow.
Acknowledgements
Supported in part by Grants NIDDK 45278 and NIDDK 32089.
References
Abbott, T.P., Holser, R.A., Plattner, B.J., Purcell, H.C., 1999. Pilot-scale isolation of simmondsin and related jojoba con-stituents. Ind. Crops Prod. 10, 65 – 72.
Abbott, T.P., Flo, G., Frank, L., Holser, R.A., Kolodziejczyk, P., York, D., Nelsen, T., 2000. Interlaboratory comparison of simmondsin analysis. Ind. Crops Prod. 12, 209 – 213. Booth, A.N., Elliger, C.A., Waiss, A.C., 1974. Isolation of a
toxic factor from jojoba meal. Life Sci. 15, 1115 – 1120. Bray, G.A., York, D.A., 1998. The MONA LISA hypothesis
in the time of leptin. Rec. Prog. Horm. Res. 53, 95 – 117 Discussion — pp. 117 – 118.
Cokelaere, M.M., Dangreau, H.D., Arnouts, S., Kuhn, E.R., Decuypere, E.M.P., 1992a. Influence of pure simmondsin on the food intake in rats. J. Agri. Food Chem. 40, 1839 – 1842.
Cokelaere, M.M., Dangreau, H.D., Daenens, P., Bruneel, N., Arnouts, S., Decuypere, E.M.P., Kuhn, E.R., 1992b. In-vestigation of possible toxicological influences of sim-mondsin after subacute administration in the rat. J. Agri. Food Chem. 40, 2443 – 2445.
Cokelaere, M.M., Buyse, J., Daenens, P., Decuypere, E., Kuhn, E.R., van Boven, M., 1993. The influence of jojoba meal supplementation on growth and organ function in rats. J. Agri. Food Chem. 41, 1444 – 1448.
Cokelaere, M.M., Busselen, P., Flo, G., Decuypere, E., Kuhn, E.R., van Boven, M., 1995a. Devazepide reverses the anorexic effect of simmondsin in the rate. J. Endocrinol. 147, 473 – 477.
Cokelaere, M., Daenens, P., Van Boven, M., Kuhn, E.R., Decuypere, E., Darras, V., 1995b. Influence of long-term simmondsin administration on thyroid hormone levels in adult rats. Horm. Metab. Res. 27, 318 – 321.
de Tullio, P., Delarge, J., Pirotte, B., 1999. Recent advances in the chemistry of cholecystokinin receptor ligands (agonists and antagonists). Curr. Med. Chem. 6, 433 – 455. Dourish, C.T., Ruckert, A.C., Tattersal, F.D., Iversen, S.D.,
1989. Evidence that decreased feeding induced by systemic injection of cholecystokinin is mediated by CCK-A recep-tors. Eur. J. Pharmacol. 173, 233 – 234.
Elliger, C.A., Waiss, A.C., Lundin, R.E., 1974a. Cyanomethylenecyclohexyl glucosides from Simmondsia chinensis. Phytochemistry 13, 2319 – 2320.
Elliger, C.A., Waiss, A.C. Jr, Lundin, R.E., 1974b. Structure and stereochemistry of simmondsin. J. Org. Chem. 39, 2930 – 2931.
Flo, G., Daenens, P., van Boven, M., Vermaut, S., Decuypere, E., Cokelaere, M.M., 1997. Absorption and excretion of simmondsin after different administration routes in rats. J. Agri. Food Chem. 45, 185 – 188.
Lin, L., Bray, G.A., York, D.A., 2000. Enterostatin suppresses food intake in rats after near celiac arterial injections. Am. J. Physiol. 278, R1346 – R1351.
Singer, L.K., York, D.A., Berthoud, H.R., Bray, G.A., 1999. Conditioned taste aversion produced by inhibitors of fatty acid oxidation in rats. Physiol. Behav. 68, 175 – 179. Van Boven, M., Blaton, N., Cokelaere, M., Daenens, P., 1993.
Isolation, purification and stereochemistry of simmondsin. J. Agri. Food Chem. 109, 1605 – 1607.
Verbiscar, A.J., Banigan, T.F., Weber, C.W., Reid, B.L., Swingle, R.S., Trei, J.E., Nelson, E.A., Raffauf, R.F., Kosersky, D., 1980. Detoxification of jojoba meal. J. Agri. Food Chem. 28, 571 – 578.