www.elsevier.com / locate / bres
Research report
Medial prefrontal transection enhances social interaction
II: Neurochemical studies
*
Sonia Tucci , Quilianio Contreras, Ximena Paez, Luis Gonzalez, Pedro Rada,
Luis Hernandez
´
Laboratory of Behavioral Physiology, Department of Physiology, School of Medicine, Los Andes University, Merida, Venezuela
Accepted 29 August 2000
Abstract
Medial prefrontal cortex (MPFC) transection enhances social interaction in an open arena test. Social interaction enhances dopaminergic activity in the nucleus accumbens (NAC). In the present set of experiments, microdialysis probes were implanted in the NAC, and glutamate,g-aminobutyric acid (GABA) and dopamine (DA) were measured during electrical stimulation of the MPFC, after coronal transection caudal to the MPFC and after a systemic injection of amphetamine in transected rats. Electrical stimulation of the MPFC caused a transient enhancement of glutamate release in the NAC, no change in GABA levels and a long lasting increase in DA levels. Medial prefrontal transection did not change basal glutamate or GABA levels in the NAC, but increased basal DA levels. Amphetamine administration decreased GABA levels in medial prefrontal transected rats, had no effect on glutamate and increased DA levels more than in controls. The experiments suggest that glutamatergic activity in the accumbens decreases dopamine release. Medial prefrontal transection reduces glutamatergic tone and enhances dopamine release, which probably decreases GABAergic activity in the NAC. Presumably, GABA inhibition in the NAC enhances social interaction. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters and receptors
Topic: Interactions between neurotransmitters
Keywords: Microdialysis; Electric stimulation; Micellar electrokinetic chromatography; Prefrontal cortex; Nucleus accumbens; GABA–glutamate– dopamine interaction
1. Introduction the NAC [16]. In addition, the glutamatergic
cortico-accumbens neurons and the GABAergic NAC neurons A coronal transection, separating the medial prefrontal receive dopaminergic terminals that project from the cortex (MPFC) from the basal ganglia, enhances social ventral tegmental area (VTA) to the MPFC [1] and the interaction [5]. This result suggested that nerve impulses NAC [24]. Therefore, MPFC, NAC and VTA are intimate-from the MPFC modulate social interaction. Several brain ly connected and they might concert to modulate social areas receive connections from the MPFC, and those areas interaction.
might be involved in social interaction [15]. Specifically, it Pharmacological evidence suggests that the dopa-has been reported that there is an increase in dopaminergic minergic, GABAergic and glutamatergic systems control transmission in the nucleus accumbens (NAC) septi during social interaction. Systemic injections of amphetamine, a social interaction [14]. This nucleus receives glutamatergic dopaminergic agonist, and PCP, a glutamatergic antagonist, projections from the MPFC [26] and these neurons, in turn, decrease social interaction [21]. Injections of GABAergic make synaptic contact with GABAergic neurons located in agonists increase social interaction [3].Therefore, increases in social interaction due to MPFC transection might have, as their neurochemical basis, dopamine (DA), glutamate
*Corresponding author. Address for correspondence: Departamento de
and GABA changes in the prefrontal cortex, NAC and
´ ´
Fisiologıa, Apartado de correos 109, 5101-A Merida, Venezuela. Tel.:
VTA.
158-74-403111; fax:158-74-638304.
E-mail address: [email protected] (S. Tucci). In this report, we used brain microdialysis, micellar
electrokinetic chromatography (MEKC) with laser-induced guide shaft. The inlet tube of the microdialysis probe was fluorescence detection (CZE–LIFD) and high-performance connected to a syringe that was loaded with artificial liquid chromatography with electrochemical detection cerebrospinal fluid (136 mM NaCl, 3.7 mM KCl, 1.2 mM (HPLC–ED) to measure extracellular GABA, glutamate, CaCl , 1 mM MgCl and 10 mM NaHCO , at pH 7.4),2 2 3
and DA and its metabolites in the NAC of rats during which was delivered at a flow-rate of 1 ml / min. Sample electrical stimulation (ES) of the MPFC, after coronal collection started 14 h after insertion of the probe. transection caudal to the MPFC and after amphetamine
administration in both normal and transected rats.
2.4. Experiments
2. Material and methods 2.4.1. Experiment 1: microdialysis during electrical
stimulation(ES)
2.1. Subjects For glutamate and GABA analysis, samples were col-lected every 30 s (500 nl) into hematocrit tubes and stored Male albino rats of the Wistar strain and weighing in a humid chamber to minimize evaporation [19]. After between 250 and 300 g were individually housed in wire the first five samples, five animals received electrical cages with water and food ad libitum. The room tempera- stimulation for 2 min and eight additional samples were ture was 238C and the dark:light cycle was 12:12 h. collected, four during the stimulation and four after stimulation. A Grass S11 stimulator provided square pulses
2.2. Surgery of 0.35 ms duration, 145 Hz frequency and 45 V for
electrical stimulation. The pulses were passed through a Under ketamine (Ketalar, 50 mg / kg i.p.; Parke Davis) stimulus isolation unit and were delivered through an and sodium thiopental (10 mg / kg i.p.; Abbot) anesthesia, a electrical swivel joint.
guide shaft made of a 10-mm long, 21 gauge stainless steel For analysis of DA and its metabolites, samples were tubing was stereotaxically implanted above the accumbens collected every 20 min. When the chemicals in four shell (NAC-S) of 25 rats. With the level skull, the consecutive samples showed less than 10% variation, four coordinates for NAC-S were 0.7 mm lateral (L), 4.0 mm animals received electrical stimulation and then four more ventral (V) and 1.2 mm anterior (A) with respect to the samples were collected.
midsagital suture (L), the surface of the skull (V) and bregma (A) [18]. In nine rats bearing a NAC-S guide shaft,
2.4.2. Experiment 2: bilateral frontal coronal transection a nichrome wire (250mm diameter), insulated except at the
experiment
cross-sectional area of the tip and connected to an
Am-For glutamate and GABA analysis, the samples were phenol microconnector, was implanted in the ipsilateral
collected every 5 min (5ml). In all groups (four rats were prefrontal cortex at the coordinates 0.5 mm L, 4.0 mm V
operated upon and four rats received a sham operation) and 2.0 mm A. A stainless steel ground electrode was fixed
four baseline samples were taken. Then, an i.p. injection of to the skull. In eight rats bearing a NAC-S guide shaft, a
amphetamine (2 mg / kg) was administered and six addi-medial frontal coronal transection was performed,
intro-tional samples were collected. ducing and retiring a 3-mm wide blade at the coordinates
For analysis of DA and its metabolites, four samples 2.5 mm A and 7.0 mm V. Eight rats bearing a guide shaft
were collected every 20 min (20 ml). Then, all rats (four were sham-operated (craniotomy without performing the
rats were operated upon and four received a sham opera-bilateral coronal frontal transection, but disrupting the dura
tion) received an i.p. injection of amphetamine (2 mg / kg) and damaging the saggital sinus).
and five additional samples were collected. 2.3. Microdialysis
2.5. Glutamate and GABA analysis The microdialysis probe was made of a concentric
fused-silica polyimide covered capillary tubing (150 mm
2.5.1. Derivatization procedure OD375 mm ID) in 26 gauge stainless-steel tubing. A
Each sample was mixed in a 5:1:1 ratio with 20 mM cellulose hollow fiber was plugged with epoxy at one end
carbonate buffer, pH 9.4, and 2.57 mM fluorescein iso-and attached inside the 26 gauge tube with 3 mm of
thiocyanate isomer I (FITC) in acetone. A blank solution cellulose exposed. This cellulose tube had a 13,000
26
2.5.2. Capillary zone electrophoresis instrument compared by a t-test. To compare neurotransmitter levels The CZE system is a colinear instrument, model R2D2 between transected and sham rats, data were subjected to a
(Meridialysis , Merida, Venezuela), which has been de- mixed two-way ANOVA, with time and treatment as the scribed elsewhere [7,8]. Briefly, a 3 mW Argon ion laser repeated measures and independent factors, respectively. beam was tuned to 488 nm and reflected by a dichroic Concentrations at specific time points were compared by mirror centered at 510 nm. The laser beam was focused by t-tests.
means of a 0.85 NA objective on the window of the capillary. The window was located 38 cm from the anodic
end of a 48-cm long, 26mm bore fused-silica capillary that 3. Results
was filled with buffer. Fluorescence was measured by the
objective, and stray radiation was attenuated by a high pass 3.1. Experiment 1: electrical stimulation of the medial filter, centered at 520 nm and a notch filter, centered at 488 prefrontal cortex
nm. The fluorescence was focused on a R1477 multialkali
photomultiplier (PMT). The current of the PMT was Electrical stimulation of the MPFC increased glutamate converted to voltage by a voltage follower and fed to a and DA in the accumbens shell dialysates. Fig. 1A shows computer. The electropherograms were acquired and ana- the variations in glutamate and GABA concentrations in lyzed by means of a pentium II computer and ONICE the extracellular space of the NAC-S during and after
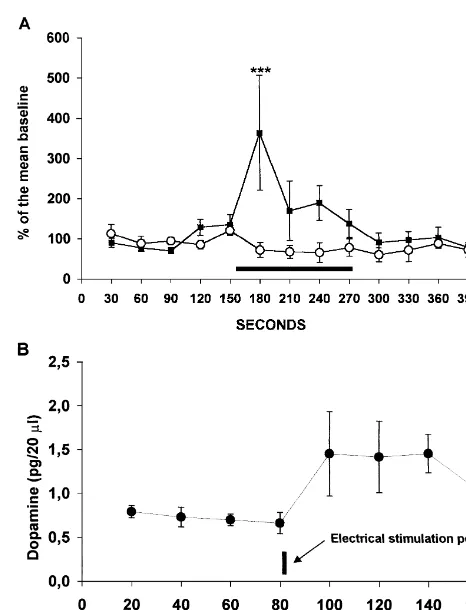
software (Dialdemo , Merida, Venezuela) electrical stimulation of the prefrontal cortex. Electrical stimulation elicited an immediate significant increase on 2.5.3. Micellar electrokinetic chromatography (MEKC) glutamate that lasted one sample and then returned to basal Micellar electrokinetic chromatography analysis con- levels (F12,4853.07; P,0.05). GABA variations were not sisted of injecting each of the three solutions, blank, statistically significant (F12,4851.74; NS). Fig. 1B shows standard and sample by the hydrodynamic method. A the variations in dopamine concentrations in the extracellu-suction of210 p.s.i. (1 p.s.i.56894.76 Pa) was applied for lar space of the NAC-S after electrical stimulation of the 1 s at the cathodic end of the capillary, while the anodic prefrontal cortex. Electrical stimulation elicited a signifi-end was immersed in the mixture reservoir. Then, the
anodic end was transferred to the buffer reservoir and a high voltage was applied using a cathode and an anode made of platinum–iridium wire. The running buffer was 80 mM sodium dodecyl sulfate (SDS), 80 mM borate, 1% acetonitrile. A high-voltage power supply supplied 26 kV between the anode and the cathode for 15 min. After each run, the capillary was rinsed with 1 M sodium hydroxide solution for 1 min, 18 mV pure water for 1 min and borate–SDS buffer for 3 min.
2.6. Analysis of DA and its metabolites
Samples were analyzed by HPLC–ED. The HPLC system consisted of a Waters 510 pump connected to a model 1725 Rheodyne valve equipped with a 20-ml loop. The chemicals were separated in an ODS, 3 mm particle, 3.2 mm bore, 10 cm long Brownlee column. They were detected in a Waters 464 electrochemical detector on a glassy carbon electrode set at 705 mV with respect to a Ag–AgCl reference electrode. 3,4-Dihydroxyphenylacetic acid (DOPAC), dopamine (DA) and homovanillic acid (HVA) were measured by comparison of the peak heights of the samples with the peak height of standards.
2.7. Statistical analysis
In both experiments, data were subjected to one-way
Fig. 1. (A) Extracellular levels of glutamate (black squares, **P,0.01,
ANOVA for repeated measures followed by Newman- post-hoc test) and GABA (open circles) before, during (black bar) and Keuls post-hoc test. In experiment 2, the mean of the basal after electrical stimulation. (B) Extracellular concentrations of dopamine
cant increase of dopamine that lasted for 1 h and then 3.2.2. Systemic amphetamine administration
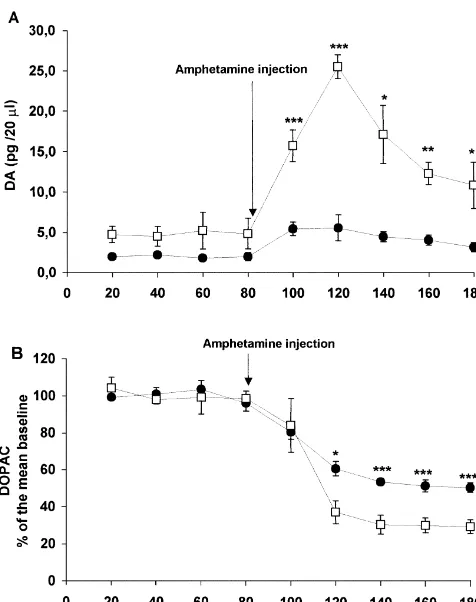
decreased in the last sample (F7,2452.51; P,0.05). Administration of amphetamine to the sham-operated DOPAC and HVA showed a slight increase after the group increased DA release in the accumbens shell. electrical stimulation that was not statistically significant Amphetamine elicited a 250% increase in DA levels that (data not shown). In summary, electrical stimulation of the lasted for four samples (80 min) and then returned to basal MPFC increased extracellular concentrations of glutamate levels (F8,2454.82; P,0.0001). In the medial prefrontal and DA. GABA, DOPAC and HVA did not change transected group, amphetamine administration elicited a significantly. 650% increase in DA levels that lasted for 100 min (F8,24518.00; P,0.0001). The difference between the 3.2. Experiment 2 sham-operated and the medial prefrontal transected group was statistically significant (time3treatment, F8,48512.49; 3.2.1. Medial prefrontal cortex coronal transection P,0.0001; treatment factor F1,65128; P,0.0001). Fig. Medial prefrontal transection increased NAC-S basal 2A shows the variations in extracellular DA concentrations levels of dopamine. Frontal transected rats had 4.36 pg / 20 after the administration of amphetamine.
ml60.3 and sham operated rats had 2.22 pg / 20 ml60.18. Amphetamine administration to the sham-operated This difference was statistically significant (t6517.15; P, group elicited an immediate 30% decrease in DOPAC 0.0001, Fig. 2A). Basal levels of DOPAC, HVA, glutamate levels that also lasted for 100 min (F8,24574.79; P,
and GABA were not different when the MPFC transected 0.0001). Amphetamine administration to the prefrontal rats were compared to sham-operated rats. transected group elicited an immediate 50% decrease in DOPAC levels that lasted for all samples collected (100 min) (F8,24536.15; P,0.0001).The difference between the sham-operated and transected groups was statistically significant (time3treatment, F8,4853.88; P,0.001; treat-ment factor F1,6510.98; P,0.005). Fig. 2B shows the variations in DOPAC concentrations in the extracellular space of the NAC-S after the administration of amphet-amine. HVA levels did not change.
In summary, lesioned rats showed higher basal levels of DA. Amphetamine administration produced a greater increase in DA levels and a greater decrease in DOPAC levels in the prefrontal transected group than in the sham-operated group.
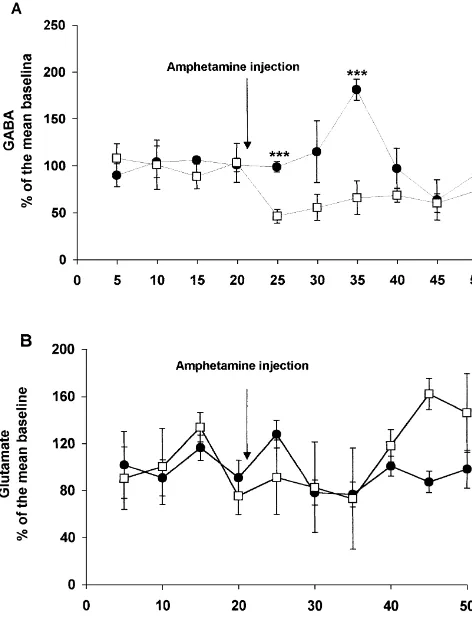
Fig. 3A shows the variations in GABA concentration in the extracellular space of the NAC-S after the administra-tion of amphetamine. In the sham-operated group, amphet-amine elicited a significant increase in GABA levels that lasted for two samples (10 min) and then returned to basal levels (F9,2753.72; P,0.005). Administration of amphet-amine to the medial prefrontal transected group decreased GABA release in the accumbens shell. This decrease lasted for three samples (15 min) and then returned to basal levels (F9,2752.29; P,0.05). The difference between the sham and transected groups was statistically significant (time3treatment, F9,5453.38; P,0.005; treatment factor
F1,6528.80; P,0.005). Glutamate concentrations in the extracellular space showed no statistically significant vari-ation after the administrvari-ation of amphetamine in both
Fig. 2. (A) Basal levels of dopamine are higher in the prefrontal groups, i.e., the medial prefrontal transected group (F 5
9,27
transected group (open squares) than in sham-operated rats (black circles).
2.24; NS) and the sham-operated group (F9,2751.44; NS),
After the systemic amphetamine injection, there was an increase in
and there was no difference between them (Fig. 3B).
extracellular dopamine concentrations that was significantly higher in medial prefrontal transected rats. (B) There is no difference in basal levels of DOPAC between the prefrontal transected group (open squares)
and the sham-operated group (black circles). After amphetamine adminis- 4. Discussion tration, there was a decrease in extracellular DOPAC in both groups,
although, in the prefrontal transected group, this decrease was greater
Electrical stimulation of the MPFC increased glutamate
than in the sham-operated group, *P,0.05, **P,0.01, ***P,0.005,
well known that extracellular glutamate is tightly reg-ulated, to prevent glutamate excitotoxicity [11].
An increase in dopamine levels in the nucleus accum-bens caused by electrical stimulation of the prefrontal cortex has also been reported [17,22]. An explanation might be that neurons from the MPFC project to the ventral tegmental area and excite dopaminergic neurons that, in turn, project into the nucleus accumbens. However, in the present set of experiments, another time discrepancy occurred. The duration of dopamine increase (almost 2 h) was longer than the stimulation time (2 min). This can only happen if excitation of the dopaminergic neurons is maintained for 2 h by a polysynaptic reverberating path-way.
The coronal transection of the MPFC did not affect basal levels of glutamate. This makes sense because most of the basal level of glutamate is of glial origin [9]. However, the same transection increased basal levels of dopamine, suggesting an inhibitory action of the prefrontal cortex on dopaminergic neurons projecting from the VTA to the nucleus accumbens. Alternatively, the lesion might injure dopaminergic neurons and cause sprouting that enhances the dopamine levels in the nucleus accumbens [13].
Systemic administration of amphetamine had no effect on extracellular levels of glutamate. Other laboratories have reported an increase in glutamate levels after
amphet-Fig. 3. (A) After the systemic administration of amphetamine in
prefron-amine injections [12,20]. There are technical differences
tal transected rats (open squares), extracellular levels of GABA showed a
between those reports and the present one. Specifically,
decrease, while in sham-operated rats (black circles), GABA levels
amphetamine was administered locally in the experiments
increased, ***P,0.005, t-test. (B) There was no significant variation in
extracellular levels of glutamate after the systemic administration of done by others. In the present experiment, amphetamine
amphetamine in both groups, i.e., prefrontal transected rats (open squares) was administered systemically. In addition, the analytical and sham-operated rats (black circles).
technique and the sample collection times were different. When HPLC is used, long sample collection times are increase in glutamate levels might be due to stimulation of required and, in the present report, micellar electrokinetic glutamatergic neurons projecting from the MPFC to the chromatography allowed shorter sample collection times. nucleus accumbens. This result confirms similar findings However, these disparate results deserve further explora-from other laboratories [26]. However, the increase in tion.
[3] R. Corbett, S. Fielding, M. Cornfeldt, R.W. Dunn, GABAmimetic
increase in accumbens dialysates in sham-operated rats is
agents display anxiolytic-like effects in the social interaction and
harder to explain. Recent evidence has shown that
amphet-elevated plus maze procedures, Psychopharmacology 104 (1991)
amine increases extracellular levels of GABA in a cal- 312–316.
cium-dependent way and through a high-affinity GABA- [4] A. Del Arco, J.L. Gonzalez-Mora, V.R. Armas, F. Mora,
Amphet-transporter mechanism [4]. One explanation could be that amine increases the extracellular concentration of glutamate in striatum of the awake rat: involvement of high affinity transporter
the increase in GABA levels is caused by amphetamine
mechanisms, Neuropharmacology 38 (1999) 943–954.
inhibition of the GABA reuptake mechanism.
[5] L. Gonzalez, M. Rujano, S. Tucci, D. Paredes, L. Hernandez, Medial
Amphetamine was more effective in enhancing extracel- frontal transection enhances social interaction. I: Behavioral studies, lular dopamine levels in lesioned rather than normal rats. Brain Res. (2000) (submitted).
In lesioned rats, both the increase in dopamine levels as [6] L. Hernandez, B.G. Stanley, B.G. Hoebel, A small, removable microdialysis probe, Life Sci. 39 (1986) 2629–2637.
well as the decrease in DOPAC levels were significantly
[7] L. Hernandez, J. Escalona, P. Verdeguer, N. Guzman, In vivo
greater than those found in intact rats. The decrease in
monitoring of brain glutamate by microdialysis coupled to capillary
GABA levels in lesioned rats might in turn increase electrophoresis and laser induced fluorescence detection, J. Liq. dopamine levels. Recent evidence suggests that dopamine Chromatrogr. 161 (1993) 2149–2160.
release in the nucleus accumbens is under GABAergic [8] L. Hernandez, S. Tucci, N. Guzman, X. Paez, In vivo monitoring of´ ´ ´ glutamate in brain microdialysis and capillary electrophoresis with
control [10]. The administration of bicuculline, through
laser induced fluorescence detection, J. Chromatogr. A. 652 (1993)
reverse microdialysis in freely moving rats, increased
393–398.
levels of extracellular dopamine in nucleus accumbens
[9] M. Herrera-Marschitz, Z.B. You, M. Goiny, J.J. Meana, R. Silveira,
dialysates [23]. If the GABA level in turn affects dopamine O.V. Godukhin, Y. Chen, S. Espinoza, E. Pettersson, C.F. Loidl, G. release, then, in the normal rat, the increase in GABA Lubec, K. Andersson, I. Nylander, L. Terenius, U. Ungerstedt, On the origin of extracellular glutamate levels monitored in the basal
levels attenuates the amphetamine-induced dopamine
re-ganglia of the rat by in vivo microdialysis, J. Neurochem. 66 (1996)
lease. In the lesioned rat, the decrease in GABA levels
1726–1735.
might remove an inhibition of the dopamine terminals, and
[10] S. Ikemoto, R.R. Kohl, W.J. McBride, GABA(A) receptor blockade
amphetamine would release more dopamine under these in the anterior ventral tegmental area increases extracellular levels of circumstances. dopamine in the nucleus accumbens of rats, J. Neurochem. 69
(1997) 137–143.
The neurochemical changes induced by MPFC
transec-[11] D. Jabaudon, K. Shimamoto, Y. Yasuda-Kamatani, M. Scanziani,
tion in the nucleus accumbens might be relevant to social
B.H. Gahwiler, U. Gerber, Inhibition of uptake unmasks rapid
interaction. The enhancement of dopaminergic activity that
extracellular turnover of glutamate of nonvesicular origin, Proc.
occurs during social interaction in normal rats might be Natl. Acad. Sci. USA 96 (1999) 8733–8738.
exaggerated in lesioned animals because the dopaminergic [12] R. Labarca, M.I. Gajardo, M. Seguel, H. Silva, S. Jerez, A. Ruiz, G.
system is already overactive. This activity of the dopa- Bustos, Effects ofD-amphetamine administration on the release of endogenous excitatory amino acids in the rat nucleus accumbens,
minergic system might enhance sensory perception and
Prog. Neuropsychopharmacol. Biol. Psychiatry 19 (1995) 467–473.
locomotor performance in lesioned rats and this might
[13] G.T. Liberatore, D.I. Finkelstein, J.Y. Wong, M.K. Horne, M.J.
contribute to augmented social interaction. However, other Porritt, G.A. Donnan, D.W. Howells, Sprouting of dopaminergic brain areas, such as the amygdala, which are connected to axons after striatal injury: confirmation by markers not dependent on
the MPFC, might also contribute to the modulation of dopamine metabolism, Exp. Neurol. 159 (1999) 565–573. [14] A. Louilot, M. Le Moal, H. Simon, Differential reactivity of
social interaction. In summary, coronal transection of the
dopaminergic neurons in the nucleus accumbens in response to
MPFC reverses the effect of amphetamine on GABA in the
different behavioral situations. An in vivo voltammetric study in free
nucleus accumbens and enhances the release of extracellu- moving rats, Brain Res. 397 (1986) 395–400.
lar dopamine induced by amphetamine. [15] D.L. Masterman, J.L. Cummings, Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors, J. Psychopharmacol. 11 (1997) 107–114.
[16] G.E. Meredith, The synaptic framework for chemical signaling in Acknowledgements
nucleus accumbens, Ann. NY Acad. Sci. 877 (1999) 140–156. [17] S. Murase, J. Grenhoff, G. Chouvet, F.G. Gonon, T.H. Svensson,
This work was supported by grants CDCHT M-653- Prefrontal cortex regulates burst firing and transmitter release in rat
9903-A and CONICIT G-97000820. mesolimbic dopamine neurons studied in vivo, Neurosci. Lett. 157 (1993) 53–56.
[18] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, Sidney, 1982.
References [19] P. Rada, S. Tucci, E. Murzi, L. Hernandez, Extracellular glutamate increases in the lateral hypothalamus and decreases in the nucleus [1] M. Cador, Y. Bjijou, S. Cailhol, L. Stinus,D-Amphetamine-induced accumbens during feeding, Brain Res. 768 (1997) 338–340.
behavioral sensitization: implication of a glutamatergic medial [20] M.S. Reid, K. Hsu Jr., S.P. Berger, Cocaine and amphetamine prefrontal cortex–ventral tegmental area innervation, Neuroscience preferentially stimulate glutamate release in the limbic system: 94 (1999) 705–721. studies on the involvement of dopamine, Synapse 27 (1997) 95– [2] S.L. Castro, Y. Wu, A.C. Michael, M.J. Zigmond, Inhibitory effect of 105.
be-haviour, and locomotor activity in rats, Neuropsychopharmacology tion by mesolimbic GABA receptors—an in vivo electrochemicalA
19 (1998) 18–25. study, Brain Res. 798 (1998) 156–165.
[22] M.T. Taber, H.C. Fibiger, Electrical stimulation of the prefrontal [25] Q.S. Yan, S.G. Yan, Blockade of GABAA receptors within the cortex increases dopamine release in the nucleus accumbens of the nucleus accumbens increases accumbal dopamine release: A mi-rat: modulation by metabotropic glutamate receptors, J. Neurosci. 15 crodialysis study, Soc. Neurosci. Part 2 (1999), Abstract 882.19. (1995) 3896–3904. [26] Z.B. You, T.M. Tzschentke, E. Brodin, R.A. Wise, Electrical [23] B.H. Westerink, H.F. Kwint, J.B. deVries, The pharmacology of stimulation of the prefrontal cortex increases cholecystokinin, mesolimbic dopamine neurons: a dual-probe microdialysis study in glutamate, and dopamine release in the nucleus accumbens: an in the ventral tegmental area and nucleus accumbens of the rat brain, J. vivo microdialysis study in freely moving rats, J. Neurosci. 18
Neurosci. 16 (1996) 2605–2611. (1998) 6492–6500.