We Learned?
Andrew L. Stoll, Perry F. Renshaw, Deborah A. Yurgelun-Todd, and
Bruce M. Cohen
New technologies are offering increasingly powerful means to obtain structural, chemical, and functional images of the brain during life, often without the use of ionizing radiation. Bipolar disorder, with its clear physi-ologic features, would appear to be a prime candidate for the application of current brain imaging; however, only a modest number of studies have been reported to date, and most studies have small sample sizes and heterogeneous subject groups. Nonetheless, there are a few consistent findings among these studies, including the following: 1)
Structural imaging studies suggest an increased number of
white matter hyperintensities in patients with bipolar disorder. These may be lesions unique to bipolar disorder and its treatment, or related to cardiovascular risk factors, which are more common in bipolar patients. Decreased cerebellar size and anomalies of cerebellar blood volume have also been reported. Increased sulcal prominence and enlargement of the lateral and third ventricles are less consistently observed findings. 2) Spectroscopic imaging suggests abnormalities of metabolism of choline-contain-ing compounds in symptomatically ill bipolar patients and, possibly, treatment-induced changes in choline- and myo-inositol– containing compounds. Each of these groups of metabolites serves as a component of membrane phospho-lipids and cellular second-messenger cycles. 3) Metabolic
and blood flow studies provide evidence for decreased
activity of the prefrontal cortex (PFC) in bipolar patients during depression. It is not clear if these changes are restricted to particular subregions of the PFC, nor if they are reversed with mania.
No single pathophysiologic mechanism yet explains these findings, although all might be due to regional alterations in cellular activity and metabolism or changes in cell membrane composition and turnover.
The development of imaging technologies has far outpaced their use in bipolar disorder. The promise of future studies is great, with more powerful magnetic resonance scanners, additional ligands for positron emission tomography and single photon emission computed tomography imaging, and
improved image generation and processing already avail-able. Biol Psychiatry 2000;48:505–517 © 2000 Society of Biological Psychiatry
Key Words: Bipolar disorder, imaging, MRI, SPECT, PET
Introduction
N
euroimaging technologies are maturing rapidly. Ear-lier techniques, such as x-ray computed tomography (CT) and electroencephalography, have been joined by magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), functional magnetic resonance (fMR), positron emission tomography (PET), and single photon emission computed tomography (SPECT). In ad-dition, these recent techniques have steadily become more sensitive, more reliable, and more affordable (Rauch and Renshaw 1995).Increasingly in the last decade, brain imaging has been applied to the study of psychiatric disorders and, most notably, to research on schizophrenia and depression, leading to new clues on cause and treatment. By compar-ison, bipolar disorder has received less attention. This is unfortunate, as bipolar disorder is relatively common, often quite disruptive to normal living, and clearly medi-ated by biological factors.
Genetic studies have the most direct evidence of a biological basis for bipolar disorder, notably the concor-dance rates for monozygotic identical twins being twofold higher than those of same sex dizygotic (fraternal) twins. In fact, depending on definition of illness, identical twin concordance rates vary from 50% to nearly 100%, indi-cating a substantial role for inherited factors. Family and adoption studies confirm these findings (National Institute of Mental Health’s Genetics Workgroup 1999).
Further evidence of a biological etiology and pathogen-esis in bipolar disorder stems from pharmacologic re-search, where specific drugs can mimic or ameliorate bipolar symptoms. For example, antidepressants, cortico-steroids, and other classes of drugs can induce or
exacer-From Psychopharmacology Research Laboratory and Brain Imaging Center, McLean Hospital, Belmont, and the Department of Psychiatry, Harvard Medical School, Boston, Massachusetts.
Address reprint requests to Bruce M. Cohen, M.D., Ph.D., Harvard University, Department of Psychiatry, McLean Hospital, 115 Mill Street, Belmont MA 02178.
Received February 15, 2000; revised July 3, 2000; accepted July 6, 2000.
bate mania. Psychostimulants, such as D-amphetamine, cocaine, and others can induce hypomaniclike states at low doses and maniclike psychosis at high and repeated doses. In contrast, mood-stabilizing and antimanic medications, such as lithium carbonate and valproate, and antipsychotic agents, such as haloperidol and clozapine, reduce or eliminate manic symptoms (Baldessarini 1996).
Clues to the neural substrate of affective symptoms in bipolar disorder come from studies of normal volunteers as well as subjects with unipolar major depression. Areas of the brain that appear to mediate both normal and abnormal mood include anterior limbic and paralimbic structures, such as the prefrontal cortex and anterior cingulate cortex; subcortical structures, such as the thala-mus, basal ganglia, and nucleus accumbens; and temporal lobe structures, notably the amygdala and hippocampus (Davidson et al 1999; Dougherty and Rauch 1997; Kennedy et al 1997; Ketter et al 1996; Soares and Mann 1997b). Studies of brain lesions, mostly stroke and tumors, show a relationship between left-sided frontal regions and depression and right-sided regions and mania, though appearance of the latter is less frequent than the former (Soares and Mann 1997a; Starkstein et al 1991). Subcor-tical lesions, especially of the caudate and thalamus, can also present with mood dysregulation, with right-sided lesions again being more commonly associated with mania (Starkstein et al 1991).
Bipolar disorder is not only typified by the affective symptoms of mania and depression. Physiologic and somatic symptoms of altered sleep, energy, and appetite suggest involvement of brain stem nuclei and the hypo-thalamus. By analogy to findings in schizophrenia, hallu-cinations and cognitive symptoms suggest involvement of the temporal cortex and temporal lobe structures as well as the parietal and prefrontal cortices, respectively.
Thus, there are numerous leads in the search for candidate neural substrates involved in the syndrome of bipolar disorder. The background evidence and the pro-found and often severe clinical presentation of patients with bipolar disorder suggest that sufficiently sensitive and specific brain imaging technologies will be able to detect the crucial brain substrate of bipolar illness.
In preparation for this article, a review of the literature was conducted using the software OVID to search the MEDLINE database. The subject heading bipolar
disor-der, combined with imaging, was searched for the years
1966 –2000. In this review we will discuss some of the newer imaging technologies (specifically, MRI, MRS, fMR, PET, and SPECT), review data from the application of these techniques to the study of bipolar disorder, and provide recommendations on the use of current and developing imaging approaches in future research.
The purpose of this article is not to review individual
articles, but to succinctly and clearly describe the most consistent findings among the neuroimaging studies in bipolar disorder (Table 1). In addition, this review focuses on the common methodological limitations and strengths of existing studies, design issues for future research, and emerging technology for this work.
Recent excellent reviews of individual study results are available for structural (Altshuler et al 1995; Jeste et al 1988; Norris et al 1997; Soares and Mann 1997a; Vide-bech 1997; Yurgelun-Todd and Renshaw 1999), chemical (Kato et al 1998; Moore and Renshaw 1997; Soares et al 1996), and functional (Dougherty and Rauch 1997; Soares and Mann 1997b) brain imaging in bipolar disorder, and for affective disorders in general. Details of published studies and techniques can be found in these and the original sources cited.
Imaging Technologies: An Overview
MR Scanning
Magnetic resonance scanning works by immersing tissues in a magnetic field. Due to their unpaired neutrons or protons, a number of atomic nuclei of biological relevance will line up with (in a lower, more stable energy state) or against (in a higher, less stable energy state) the static magnetic field. Brief radio frequency pulses are generated, which are absorbed by some of the lower energy nuclei, moving them into the higher energy, less stable state. When the radio frequency pulse is shut off, some of the nuclei that were shifted into the higher energy state revert back to the lower energy state, releasing radio frequency energy in the process. This released energy decays with two relaxation times, T1and T2.
The T1time represents the spin–lattice (or longitudinal)
relaxation time and T2 is the spin–spin (or transverse)
relaxation time. T1measures the time required for nuclei
as a whole to return to their baseline lower energy state in the magnetic field. The T2 relaxation time is the time
between turning off the radio frequency pulse and lack of directionality to the sum of the individual nuclear spins.
Table 1. Imaging Findings in Bipolar Disorder
The most consistently and frequently observed findings Increased white matter hyperintensities
Cerebellar structural abnormalities
Mild sulcal prominence and ventricular enlargement
Increased choline-containing compounds in the basal ganglia, when subjects are symptomatic
Decreased activity of the prefrontal cortex, when subjects are depressed
No specific pathophysiologic mechanism has yet been demonstrated that unifies these findings
The frequency and relaxation times of the atomic nuclei in tissues are dependent on the molecule containing each nucleus and the chemical milieu of each molecule. There-fore, the electromagnetic signal can be analyzed to yield anatomic (structural or MRI) data, chemical (MRS) data, or functional (blood flow and volume or fMRI) data.
MRI
Magnetic resonance imaging, a technique for structural or anatomic imaging of the brain, is based largely on signals from protons in water (H2O), the most prevalent chemical
in the brain. The association of water with local lipids produces most of the regional signal differences in MRI scans. Magnetic resonance imaging has replaced x-ray CT in most clinical and almost all psychiatric neuroscience research applications. This is because MRI has superior contrast and soft tissue imaging capability, and because MRI does not expose the research subject to ionizing radiation. Therefore, subjects can be imaged repeatedly. Newer contrast agents, such as gadolinium, have improved the ability to differentiate certain pathologic processes, such as tumors, from normal tissue.
MRS
The physical chemistry governing MRI also applies to MRS. With some minor modifications, a standard MRI machine, instead of producing structural imaging data, can generate concentration data for a number of psychiatri-cally relevant compounds in a given volume (voxel) of brain tissue. The most common magnetic resonant nucleus used for MRS is hydrogen (1H, or proton), since as with structural imaging, the high concentration of hydrogen in biological tissues and the great sensitivity of 1H in a
magnetic field permits the detection of compounds in lower concentrations within the brain. Commonly mea-sured compounds include those containing inositol, cho-line, creatine, or N-acetyl-aspartate. Phosphorous (31P) MRS is also frequently used to measure phosphomo-noesters (PMEs) and phosphodiesters (PDEs), including sugars and phospholipid-associated metabolic and cata-bolic components of cell membranes, as well as energy storage metabolites such as phosphocreatine (PCr) and nucleotide phosphates. Magnetic resonance spectroscopy technology has been applied to many other biologically important nuclei, including7Li, the most abundant isoto-pic component of natural lithium, including lithium given as a medication,19F, to detect the many
fluorine-contain-ing psychotropic drugs; and a low abundance form of carbon,13C, to label biological compounds. 23Na, 25Mg, and39K are also observable by MRS.
fMR
Functional magnetic resonance produces images related to cerebral blood flow or volume that, on average, correlate with the relative degree of activity of regions of the brain. Cerebral blood volume is based on the detection of the flow of intravenously administered paramagnetic tracers, such as gadolinium, through brain tissue, with measure-ments that can be made as frequently as one per second by a technique called dynamic susceptibility contrast MRI (DSCMRI; Belliveau et al 1991; Levin et al 1996; Rosen et al 1991). Alternatively, regional blood flow can be estimated by blood oxygenation level detection (Kwong et al 1992), which takes advantage of the fact that deoxyhe-moglobin is paramagnetic, whereas oxygenated hemoglo-bin is not. Brain regions that are activated receive in-creased blood flow, delivering oxygen beyond their immediate needs, leading to a reduction of deoxyhemo-globin, greater regional coherence of the magnetic field, and a slightly greater MR signal, which identifies the activated region.
PET
Positron emission tomography scanning is based on com-pounds containing unstable isotopic forms of atomic nuclei (radioisotopes) that emit positrons when they dis-integrate. When positrons encounter electrons, the two particles annihilate each other, releasing high energy gamma rays. The gamma rays radiate in opposite direc-tions and can be detected by coincidence counters. Be-cause the half-lives of these radioisotopes are brief, an on-site cyclotron or fast delivery from an off-site cyclotron is required to generate fresh isotopes on an as-needed basis. Positron emission tomography studies can be used to determine local brain activity through measurements of either regional cerebral blood flow or glucose utilization. As with fMR, PET works because blood flow and glucose metabolism are tightly linked to regional brain activity. The radioisotope-containing compounds typically used to estimate regional brain activity include11C-glucose and
18
F-deoxyglucose for metabolism and15O-water for blood flow.
Positron emission tomography can also be used to detect and quantify radioisotope-containing ligands de-signed to bind to specific receptors and other sites of interest in the brain, such as neurotransmitter transporters or enzymes.
SPECT
accelera-tor is required, as the isotopes used in SPECT have longer half-lives and, accordingly, lower energy. The lower energy of the SPECT radioisotopes and the lack of coincidence detection due to the emission of just a single photon per disintegration produce poorer spatial resolution than PET. Because of its relative simplicity and lower costs, SPECT is frequently used clinically and in clinical research to measure regional blood flow and, thereby, regional neuronal activity.
As in PET, SPECT can be divided into studies of regional blood flow (regional brain activity) and radioli-gand binding. For blood flow measurements, several techniques are commonly employed. The first, utilizing inhaled radioisotope 133xenon gas, is used to determine absolute cerebral blood flow measurements. The xenon method is generally reliable only for cortical regions. A second technique, using 99technetium-containing com-pounds, including hexamethylpropyleneaminoxime (99 Tc-m-HMPAO), can only determine relative values of re-gional blood flow, but can be used in studies not only of the cerebral cortex but also of subcortical structures. Other radioisotopes, such as 123I-N-isopropyl iodoamphetamine (IMP), can also be employed to measure local cerebral blood flow.
Radioligand-binding studies with SPECT employ com-pounds containing either 99technetium or, more com-monly,123iodine. As with PET, these SPECT radioligands can be used to determine receptor, transporter, or enzyme alterations in the brain.
Brain Imaging Findings in Bipolar Disorder
MRI
The recurrent nature of bipolar disorder has often sug-gested a search for a chemical abnormality; however, structural anomalies can also potentially cause episodic illness or dyscontrol of mood. Although there are strik-ingly few postmortem studies of bipolar disorder, in vivo anatomic studies go back at least to the early 1980s, with some x-ray CT studies finding enlarged ventricles in subjects with bipolar disorder.
Magnetic resonance imaging provides high resolution, in vivo anatomic data, including separation of white and gray matter, and is well suited to identify structural abnormalities in psychiatric disorders. At least 30 MRI studies in patients with bipolar disorder have been pub-lished since 1987. Their most consistent finding is a higher than expected presence of white matter hyperintensities (WMHs). Two recent reviews (Altshuler et al 1995; Videbech 1997) document seven reports of a statistically significant increase of WMHs in patients with bipolar disorder. In fact, of 12 studies overall, all but two show at least a trend to an overall increase in the presence of
WMHs. On average, the risk of having WMHs was elevated more than threefold in bipolar subjects.
White matter hyperintensities, best visualized with T2
-weighted images, are small areas where the signal inten-sity is high relative to the surrounding tissue. Most often seen in aging and cerebrovascular disorders, WMHs are extremely rare in healthy individuals without any personal history of psychiatric or neurologic illness who are under the age of 30 – 40 years (Steffens and Krishnan 1998). Demyelination, astrogliosis, or axonal loss as well as dilated perivascular spaces or diverticula of the lateral ventricles can also cause WMHs; however, the precise cause and relevance of WMHs in bipolar disorder are unclear, although data exist to suggest that the WMHs are present early in the course of illness (Botteron et al 1992; McDonald et al 1999), often at the first significant episode of illness (Strakowski et al 1993a), and are higher in bipolar patients at all ages (Woods et al 1995). Some studies have reported that WMHs are associated with a worse prior clinical course and a worse clinical outcome, but many have not (Altshuler et al 1995; Soares and Mann 1997a). Whether the nature of the WMHs in subjects with bipolar disorder is similar to that of WMHs seen with aging has never been studied; however, bipolar patients have more alcohol and substance abuse (Strakowski et al 1998), more frequent smoking histories (Yates and Wal-lace 1987), and greater cardiovascular risk factors (such as hypertension) than healthy comparison subjects (Aylward et al 1994; Figiel et al 1991; Steffens and Krishnan 1998; Yates and Wallace 1987), all of which could lead to cerebrovascular lesions. Most patients with bipolar disor-ders do not have WMHs, nor is there a clear association between medications, such as lithium, that can alter lipid metabolism and lead to MR signal changes and the frequency of encountering WMHs (Figiel et al 1991; Norris et al 1997).
may be related to alterations in fluid homeostasis. Periven-tricular WMH, the most common WMH, might also be related to apparent VBR increases.
Many cortical and subcortical regions have been exam-ined for a relationship to bipolar disorder. The most consistent report may be of a decrease in cerebellar size. Various studies using CT or MRI suggest cerebellar atrophy in psychosis, in general, and bipolar disorder, in particular (DelBello et al 1999; Heath et al 1979; Soares and Mann 1997a). Several studies observed statistically significant cerebellar atrophy, on average, in all or a subset of patients who were older or had multiple episodes of bipolar disorder (DelBello et al 1999; Nasrallah et al 1982a; 1982b; Yates et al 1987). All of the remaining studies of bipolar disorder show a substantial trend in the same direction (Loeber et al 1999; Weinberger et al 1982). Drug and especially alcohol abuse (Lippmann et al 1982), which can cause cerebellar atrophy, may be responsible for this finding. Lithium treatment may cause cerebellar changes as well (Roy et al 1998). If cerebellar abnormal-ities are illness rather than drug related, the pathophysio-logic changes may be mediated, in part, by dysregulated cerebellar control over limbic and cortical regions (Schmahmann and Sherman 1998). Studies of the volumes of the striatum, amygdala, other temporal lobe structures, and the prefrontal cortex have yielded less consistent results. Drevets and associates (1997, 1998) have reported reduced volume and altered activity of the subgenual prefrontal cortex, an area activated during the production of normal moods, as well as a reduction of glial cells postmortem in bipolar subjects. Hirayasu and colleagues (1999) observed decreased subgenual cingulate cortex volume in subjects with first-episode affective psychosis, most of whom had bipolar disorder; however, the abnor-mality was only seen in patients with a family history of affective illness. Also, patients with schizophrenia had the same mean subgenual cingulate cortex volume as patients with affective illness and a positive family history of illness. These findings in the prefrontal cortex are well worth following up.
MRS
By its origins, MR scanning is a technique for chemical identification, and MRS has the potential to measure the concentrations of various endogenous and exogenous compounds in the brain. Most studies in bipolar disorder have used1H or31P MRS technology.
Choline is highly visible with 1H MRS and, as a precursor and metabolite of membrane phospholipids, second-messenger compounds, and the neurotransmitter acetylcholine, is among the more interesting compounds to study. Overall, several studies suggest a modest increase
in choline concentration in the basal ganglia (Kato et al 1998; Moore and Renshaw 1997; Sharma et al 1992) and, possibly, the anterior cingulate (Soares et al 1999) in mania or depression, but not during euthymia.
Several groups using 31P MRS agree that there are elevations in PMEs, most likely in the frontal lobes in symptomatic patients with bipolar disorder (Deicken et al 1995; Kato et al 1998; Ketter et al 1996). Evidence has been reported for abnormal levels of other compounds readily observed by 1H MRS or 31P MRS, including
myo-inositol, phosphocreatine, and PDEs, but these
find-ings have not been repeatable across studies (Kato et al 1998; Moore et al 1999; Soares et al 1996).
The choline resonance by1H MRS represents combined
signals from several choline-containing compounds, in-cluding phosphocholine and glycerophosphocholine. Sim-ilarly, the PME resonance by31P MRS, often taken to be a measure of precursors for membrane lipids, contains signals from numerous metabolites, including phospho-choline, phosphoethanolanine, and phosphoinositol (Moore and Renshaw 1997). It is possible that increased choline and PME resonances represent alternative mea-sures of higher concentrations of the same molecule, phosphocholine, as its signal appears in each peak. No direct test of this possibility has yet been performed. It is also possible that both increases in choline and PME resonances are due to lithium treatment. Lithium inhibits choline transport, increases choline-containing metabolites in cells (Stoll et al 1992), and inhibits the breakdown of inositol monophosphate (IMP), leading to an intracellular increase in IMP (Berridge et al 1989). Both of these effects have been observed in nonhuman studies as well as in some in vivo studies in human subjects (Silverstone et al 1999; C.M. Demopoulos et al, unpublished data).
The ability to see changes in the choline and PME resonances in vivo is notable, as MRS can only observe compounds present in micromolar quantities. Such com-pounds, due to high concentration, serve as osmolytes, and can only show limited change without being balanced by alterations of other highly concentrated substances. A candidate for such changes would be taurine (Pasantes-Morales et al 1998), but while visible by MRS, its levels have not been studied in bipolar disorder. The substantial and consistently reported increases found for choline and PME may simply reflect changes in membrane turnover or cellular metabolism, activities that may be greatly altered regionally during the symptomatic phases of bipolar disorder.
fMR
disorder. In fact, our search found only one. It noted decreased cerebral blood volume (measured by DSCMRI) in bipolar versus schizophrenic or healthy comparison subjects in each of nine regions measured, regionally significant for the cerebellar tonsils (Loeber et al 1999). Other studies have suggested reduced cerebellar volume (see above). Functionally, the cerebellum participates in regulation of numerous areas thought to be associated with control of mood and the symptoms of affective illness, including limbic and paralimbic brain regions and the monoaminergic nuclei of the midbrain (Loeber et al 1999; Soares and Mann 1997a, 1997b).
PET
Positron emission tomography has been used predomi-nantly in metabolic (18F-fluorodeoyglucose) studies in bipolar disorder. Studies mixing subjects with unipolar and bipolar depression, with most subjects having unipolar illness, have tended to see a reduction of metabolism in the prefrontal cortex (Soares and Mann 1997b), which may correlate with severity of illness (Soares and Mann 1997b). For bipolar depression or mania, alone, no con-sensus of findings suggests altered metabolism in the brain as a whole or in the prefrontal cortex or its constituent parts (Ketter et al 1996; Soares and Mann 1997b). Single studies, without independent reports of replication, have observed increased metabolism in basal ganglia and the thalamus in bipolar depression (Baxter et al 1989) and increased metabolism in the left amygdala in mania (Al-Mousawi et al 1996). A single cerebral blood flow study with H215O suggested increased activity in the
subgenual prefrontal cortex during mania (Drevets et al 1997).
SPECT
Cerebral blood flow studies, performed with 133xenon, IMP, or 99Te tend, like metabolic studies, to suggest a reduction in prefrontal activity with depression, but most studies mixed patients with unipolar and bipolar illness. No consistent, or replicated, differences have been re-ported for bipolar patients for the whole brain, temporal lobe structures, subcortical nuclei, or lateralized activity in the brain (Dougherty and Rauch 1997; Ketter et al 1996; Soares and Mann 1997b).
Ligand Binding Studies
There are individual, unreplicated reports in patients with bipolar disorder of 1) decreased frontal lobe but not striatal dopamine (D1) binding sites (Suhara et al 1992), 2) no
difference in D2 binding sites versus control subjects
(Wong et al 1985), or 3) an increase in D2binding sites in
subjects with psychosis versus those without psychosis (Pearlson et al 1995). A recent report observed a decrease in serotonin (5-HT1A) binding sites in various brain
regions in 17 subjects with mood disorders (N 5 4 subjects with bipolar disorder; Drevets et al 1999); how-ever, postmortem studies show no consistent decrease in 5-HT1Areceptors in affective disorders. Neither do genetic
studies show an association or linkage between structural genes for dopamine or serotonin receptors and bipolar disorder. Perhaps any in vivo abnormalities are related to changes due to medications or the course of illness.
Summary of Findings
Whether for anatomic, biochemical, or functional ques-tions, the current data on bipolar disorder are from a modest number of studies, often differing in design and technical approaches and spanning different regions of the brain. Despite the preliminary nature of these data, some themes arise and some results appear replicable (Table 1). Structurally, it appears highly likely that there is an increase in WMHs in bipolar patients, even when they are young. This increase may be related to illness per se; medication, alcohol, or smoking; or cardiovascular factors. Bipolar patients may, on average, have a modest increase in ventricular size, an effect that seems to be generalized. Among brain regions, there may be anomalies of the cerebellum, which again may be related to drugs as well as illness.
Biochemically, there appear to be altered levels of compounds containing choline and PMEs in basal ganglia and the frontal cortex. These alterations could represent detection of the same molecule or closely related ones as measured by1H MRS and 31P MRS, respectively. Here, too, the alterations could be a consequence of treatment as well as underlying pathophysiology.
Glucose metabolic and blood flow studies suggest decreased frontal cortical activity in depression, whether unipolar or bipolar. Of interest, WMHs may be most common in frontal white matter or in periventricular regions in the frontal pole (Viedbech 1997). Individual studies of other regions thought to mediate mood, although not replicated, show abnormal metabolism in blood flow in various temporal lobe and subcortical nuclei.
cognition and behavioral control (prefrontal cortex), all of which are altered in bipolar disorder. Future, more tar-geted studies can address these findings through the testing of hypotheses regarding specific molecular and functional abnormalities (see below).
Limitations in Interpreting Findings
Anyone who performs clinical psychiatric research will confirm that these studies are very difficult to design, complete, and interpret. While technologic advances make such studies more powerful, they do not necessarily make them easier, as they add complexity and an element of constant change through upgrades of techniques. The study of bipolar disorder by imaging technology is further confounded by historically low funding that cannot cover the cost of dedicated subject recruitment and evaluation staff or the full expense of scans and scan analysis. For these reasons, numerous caveats must be considered in the interpretation of existing studies.
By and large, sample sizes in the studies reported were small, often under 10 subjects, leading to a high likelihood of missed findings (false negative or type II error). By comparison, measures were often many, leading to a likelihood of findings by chance alone, a false positive or type I error.
These issues are compounded by the fact that bipolar disorder is likely heterogeneous in etiology and patho-physiology and is certainly heterogeneous in course, illness phase, and treatment response. Most studies do not or, given their small size, cannot control for these factors. Treatment is highly variable, and medications can affect brain volume as well as chemistry and function. The enlarged caudate in patients treated with neuroleptics illustrates this point well (Chakos et al 1994). Past or recent medication is often not documented, but drug effects can last weeks to years after exposure (Cohen et al 1992). Alcohol and substance use are also relevant, al-though are rarely documented, and dietary supplements, from stimulants to membrane precursors, such asv-3 fatty acids, choline, and inositol, can potentially change brain structure and function to a degree detectable by current brain imaging technologies.
Matching of populations is also crucial for ensuring valid results, but is difficult to control. Few studies matched for sociodemographic conditions, IQ, ethnicity, or even age or gender, though these can have profound effects on all brain measures and cognitive/functional performance.
Similarly, on the technologic side, a variety of machines and techniques were used, as were a variety of analytic programs. This is partly an irreducible consequence of instrumentation and technical advance. Nonetheless, in
comparing and interpreting findings it is important to recall that brain images are extensively processed through multiple stages of analysis, are highly dependent on machine type and analytic assumptions, and are not direct pictures of the brain.
Structural imaging by MRI produces high resolution scans, but boundaries between regions are often uncertain or arbitrary. Measures used vary between line, area, and volume.
Chemical imaging by MRS currently provides informa-tion only on compounds at very high concentrainforma-tions in the brain. These compounds tend to have multiple functions as precursors, metabolites, and osmolytes. Seemingly indi-vidual MRS resonance peaks almost always contain sig-nals from multiple related compounds, as well as unrelated compounds with overlapping resonance. The interpreta-tion of changes in peak height or area are often oversim-plified and inadequately blinded. Not only may they not take into account the numerous underlying sources of signal, but they also rarely take into account changes in the rate of disappearance (relaxation) of signals that may reflect aspects of tissue unrelated to concentration of the compound observed. Thus, a change in peak area or height may not mean a change in concentration of substances contributing to the peak.
Functional imaging represents a set of techniques vari-ously measuring regional blood flow, blood volume, metabolism, or radioisotope binding. None of the tech-niques are equivalent measures nor are their signals a direct measure of neuronal firing, receptor level, or activ-ity. Resolution on in vivo functional brain imaging com-bines information from many different cell groups, not a single coordinated group of cells. In addition, regional and whole brain activity is highly dependent not only on illness, but also on medication and on whatever the patient is thinking or doing. Cognitive state or task is controlled in very few studies.
These limitations suggest clear guidelines for improve-ment in future studies, including larger sample sizes, more thorough assessment, better matching, and greater atten-tion to subject characteristics and analysis and interpreta-tion of findings. Nonetheless, for a small number of studies, existing data provide both clear leads and a good reason to believe more specific hypotheses will be gener-ated and tested. Advances in technology suggest the same.
Future Studies
both brain imaging techniques and design, some areas of future promise are listed here.
MR Scanners
• Most current studies are performed on 1.5-T clinical
magnets. Increasingly 3-T, 4-T, and even 7-T mag-nets with bore sizes large enough for human heads or entire bodies are becoming available. These ma-chines will improve all MR scanning, providing higher resolution structural and functional MRI and greater separation of compounds and sensitivity to compounds at lower concentrations by MRS.
MRI
• Past studies have concentrated on volumetric (size)
analysis, but abnormalities may only affect part of a brain region or nucleus. Increasingly, analytic tech-niques are available for sophisticated morphometric (shape) analysis of nuclei, which may reveal previ-ously unseen disease-related phenomena.
• No studies have compared in vivo to postmortem
findings in the same individual. Also, structural MRI and histologic analysis can both be performed on a postmortem brain. In this fashion, the nature of WMHs or other anomalies seen by MR in bipolar
disorder can be associated with a lesion at the cellular or molecular level.
MRS
• Proton decoupling (Luyten et al 1989; Murphy et al
1993), spectral editing (Mescher et al 1998; Rothman et al 1992), double quantum filtering (Keltner et al 1997; Thompson and Allen 1998) and two-dimen-sional MRS (Hurd et al 1998; Ryner et al 1995) can separate resonances from different compounds that normally appear in a single peak, thus allowing changes to be associated with individual metabolites or with metabolites that could not previously be studied.
• Diffusion-weighted imaging and diffusion tensor
im-aging take advantage of constraints imposed on molecular movement by cell membranes and axons to study cellular integrity and define white matter tracts in the brain (Rowley et al 1999; Shimony et al 1999).
• Chemical shift imaging (Nelson et al 1997;
Vikhof-Baaz et al 1999) allows spectroscopic information to be rapidly obtained from large regions of the brain, with subsequent processing leading to the analysis of chemical signals from smaller, specific localized volumes.
• 13C can be used to label naturally occurring
com-pounds (Bluml 1999; Petersen et al 1999) to follow brain metabolism of nutrients such as choline, inosi-tol, orv-3 fatty acids, thus allowing the study of cell membrane turnover and second-messenger signaling.
• Many psychotropic drugs contain one or more
fluo-rine atoms, which may make them visible by MRS with high–field strength magnets (Christensen et al 1998; Strauss et al 1998).
• Magnetic resonance signals contain far more
infor-mation than is extracted. In MRS studies, peak amplitudes are usually measured at one particular time following excitation; however, different com-pounds under the same peak, as well as the same compound in different tissues or chemical back-grounds, can show very different rates of signal disappearance (relaxation time). These delay charac-teristics can be responsible for significant signal differences and can provide a new window on tissue abnormalities in bipolar disorder or other illnesses.
Functional Imaging
• For all forms of functional imaging, fMR, PET, or
SPECT, cognitive tasks can be applied that activate specific brain circuits. Using such tasks adds
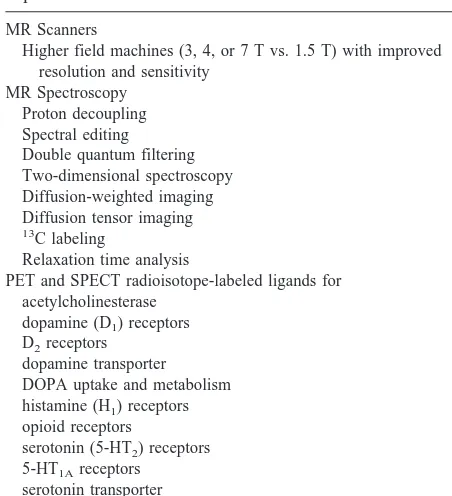
addi-Table 2. Selected New Technologies for Brain Imaging in Bipolar Disorder
MR Scanners
Higher field machines (3, 4, or 7 T vs. 1.5 T) with improved resolution and sensitivity
MR Spectroscopy Proton decoupling Spectral editing Double quantum filtering Two-dimensional spectroscopy Diffusion-weighted imaging Diffusion tensor imaging 13C labeling
Relaxation time analysis
PET and SPECT radioisotope-labeled ligands for acetylcholinesterase
dopamine (D1) receptors D2receptors
dopamine transporter DOPA uptake and metabolism histamine (H1) receptors opioid receptors
serotonin (5-HT2) receptors 5-HT1Areceptors
serotonin transporter
tryptophan uptake and metabolism
tional controls over subject state and provides data on system responsitivity, not just baseline regional activity.
• For SPECT, multiple-head cameras and dedicated
ring-detector brain cameras have increased signal resolution (Noto and Scheiner 1999).
• New scintillators are under development for more
sensitive g-ray signal detection for both PET and SPECT (Noto and Scheiner 1999).
Ligand Binding
• Radioisotope-labeled ligands are available for PET
scanning of dopamine D1and D2receptors and the
dopamine transporter (Ouchi et al 1999); brain up-take and metabolism of DOPA (Ito et al 1999); 5-HT2(Attar-Levy et al 1999) and 5-HT1Areceptors;
the serotonin transporter (McCann et al 1998; Szabo et al 1999); brain uptake and metabolism of trypto-phan (Benkelfat et al 1999); opioid receptors (Dun-can 1999; Smith et al 1999; Zubieta et al 1999); histamine (H1) receptors (Kim et al 1999); g
-ami-nobutyric acid A (GABAA) receptors (Weber et al
1999); and levels of acetylcholinesterase (Namba et al 1999). Single photon emission computed tomog-raphy ligands are available for D2receptors
(Kuen-stler et al 1999), the dopamine transporter (Booij et al 1999), and the GABAAreceptor (Morimoto 1999).
• Positron emission tomography ligands under
devel-opment include agents binding to muscarinic (Kiesewetter et al 1999) and nicotinic acetylcholine (Valette et al 1999) receptors and the N-methyl-D
-aspartate glutamate receptor (Ametamey et al 1999).
• Single photon emission computed tomography
li-gands under development include agents binding to the serotonin transporter (Dresel et al 1999) and nicotinic acetylcholine receptors (Musachio et al 1999).
Study Design
Many design issues have already been mentioned in the section on the limitations of current studies and tech-niques. Among them, attention to control and documenta-tion of past and current medicadocumenta-tion, drugs of abuse, alcohol, smoking, and dietary supplements is very useful. Larger numbers of subjects are desirable in imaging studies and can help produce subgroups with some homo-geneity by state, symptoms, clinical history, and drug response. Matching for age, gender, and ethnic, socioeco-nomic, and educational background are essential, though of varying degrees of difficulty.
Other design issues worth considering include the following:
• Magnetic resonance techniques, particularly those
involving no ionizing radiation, are ideal for longi-tudinal studies of structure, chemistry, or function through time, treatment, and different stages of illness. There is great power gained on state-related phenomena when a subject can be his or her own control subject.
• Similarly, MR techniques are well suited for use in
children, either those who have early-onset illness or those at risk for illness. Including only the more severe presentations, lifetime risk to a first-degree relative of an individual with bipolar disorder is approximately 25%. Adding less severe manifesta-tions, like cyclothymia, brings the risk close to 50%. Longitudinal studies of relatives can provide crucial information on those who will develop illness but who are currently asymptomatic or minimally symp-tomatic and are untreated.
• Bipolar disorder appears, as noted, to be a disease of
heterogeneous origin and presentation. For this rea-son, it is wise not to look just at mean differences between subject groups, but to look at variances and to study outliers on a measure. Outliers may repre-sent a subgroup of individuals who are more homo-geneous in etiology or pathophysiology. Larger sub-ject numbers aid in such an analysis.
Finally, it is important to recognize that brain imaging requires multidisciplinary teams of clinicians, scientists, and technologists. Often these collaborators do not under-stand the strengths and limitations of one another’s fields. Thus, the clinician may not appreciate the degree to which imaging data require postimage processing. The nonclini-cal technologist may have little appreciation of the diffi-culties and importance of high quality clinical recruiting, evaluation, and classification.
Ultimately, imaging studies are most powerful if a team of experts, including pharmacologists, epidemiologists, and generalists as well as clinicians and imaging special-ists, is involved. Collaborations need not be on site, but they should involve as much information sharing and longer term association as possible.
We hope this review will serve to enhance such sharing in this young and promising field.
Some of the work described here was performed with support by a grant from the Stanley Foundation Bipolar Disorders Research Center at McLean Hospital, gifts from Joan M. Collins and Jeanne and Sanford Robertson, and federal Grants Nos. MH57520 (BMC) and MH58681 (PFR).
References
al-Mousawi AH, Evans N, Ebmeier KP, Roeda D, Chaloner F, Ashcroft GW (1996): Limbic dysfunction in schizophrenia and mania. A study using 18F-labelled fluorodeoxyglucose and positron emission tomography. Br J Psychiatry 169:509 – 516.
Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R (1995): T2 hyperintensities in bipolar disorder: Magnetic resonance imaging comparison and literature meta-analysis.
Am J Psychiatry 152:1139 –1144.
Ametamey SM, Samnick S, Leenders KL, Vontobel P, Quack G, Parsons CG, et al (1999): Fluorine-18 radiolabelling, biodis-tribution studies and preliminary PET evaluation of a new memantine derivative for imaging the NMDA receptor. J
Recept Signal Transduct Res 19:129 –141.
Attar-Levy D, Martinot JL, Blin J, Dao-Castellana MH, Crouzel C, Mazoyer B, et al (1999): The cortical serotonin2 receptors studied with positron-emission tomography and [18F]-se-toperone during depressive illness and antidepressant treat-ment with clomipramine. Biol Psychiatry 45:180 –186. Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris
GJ, Geer M, et al (1994): Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J
Psychiatry 151:687– 693.
Baldessarini RJ (1996): Drugs and the treatment of psychiatric disorders: Antipsychotic and antidepressant agents. In: Hard-man JG, Limbird LE, Molinoff PB, Ruddon RW, GilHard-man AG, editors. Goodman and Gilman’s The Pharmacologic Basis of
Therapeutics, 9th ed. New York: McGraw-Hill, 431– 459.
Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al (1989): Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen
Psychiatry 46:243–250.
Belliveau JW, Kennedy D Jr, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, et al (1991): Functional mapping of the human visual cortex by magnetic resonance imaging.
Science 254:716 –719.
Benkelfat C, Young SN, Okazawa H, Leyton M, Diksic M (1999): The validity of the PET/alpha-[11C]methyl-L-trypto-phan method for measuring rates of serotonin synthesis in the human brain. Neuropsychopharmacology 21:153–157. Berridge MJ, Downes CP, Hanley MR (1989): Neural and
development actions of lithium: A unifying hypothesis. Cell 59:411– 419.
Bluml S (1999): In vivo quantitation of cerebral metabolite concentrations using natural abundance 13C MRS at 1.5T. J
Magn Reson 136:219 –225.
Booij J, Hemelaar TG, Speelman JD, deBruin K, Janssen AG, van Royen EA (1999): One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123]FPCIT SPECT. J Nucl Med 40:753–761.
Botteron KN, Figiel GS, Wetzel MW, Hudziak J, VanEerdewegh M (1992): MRI abnormalities in adolescent bipolar affective disorder. J Am Acad Child Adolesc Psychiatry 31:258 –261. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Wu H, et al (1994): Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 151:1430 –1436.
Christensen JD, Babb SM, Cohen BM, Renshaw PF (1998):
Quantitation of dexfenfluramine/d-norfenfluramine concen-tration in primate brain using 19F NMR spectroscopy. Magn
Reson Med 39:149 –154.
Cohen BM, Tsuneizumi T, Baldessarini RJ, Campbell A, Babb SM (1992): Differences between antipsychotic drugs in per-sistence of brain levels and behavioral effects.
Psychophar-macology 108:338 –344.
Davidson RJ, Abercrombie H, Nitschke JB, Putnam K (1999): Regional brain function, emotion and disorders of emotion.
Curr Opin Neurobiol 9:228 –234.
Deicken RF, Fein G, Weiner MW (1995): Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am J Psychiatry 152:915–918.
DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW (1999): MRI analysis of the cerebellum in bipolar disorder: A pilot study. Neuropsychopharmacology 21:63– 68.
Dougherty D, Rauch SL (1997): Neuroimaging and neurobiolog-ical models of depression. Harv Rev Psychiatry 5:138 –159. Dresel SH, Kung MP, Huang X, Plossl K, Hou C, Shiue CY, et al (1999): In vivo imaging or serotonin transporters with [99mTc]TRODAT-1 nonhuman primates. Eur J Nucl Med 26:342–347.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al (1999): PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 46:1375–1387.
Drevets WC, Ongur D, Price JL (1998): Neuroimaging abnor-malities in the subgenual prefrontal cortex: Implications for the pathophysiology of familial mood disorders. Mol
Psychi-atry 3:220 –226.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824 – 827. Duncan JS (1999): Positron emission tomography receptor
stud-ies in epilepsy. Rev Neurol (Paris) 155:482– 488.
Ellis PK, Reilly M, Bell KE (1995): Bilateral subdural collec-tions invisible on a CT brain scan. Ulster Med J 64:98 –100. Figiel GS, Krishnan KRR, Rao VP, Doraiswamy M, Ellinwood EH Jr, Nemeroff CB, et al (1991): Subcortical hyperintensi-ties on brain magnetic resonance imaging: a comparison of normal and bipolar subjects. J Neuropsychiatry Clin Neurosci 3:18 –22.
Heath RG, Franklin DE, Shraberg D (1979): Gross pathology of the cerebellum in patients diagnosed and treated as functional psychiatric disorder. J Nerv Ment Dis 167:585–592. Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG,
Fischer IA, et al (1999): Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry 156:1091–1093. Hurd RE, Gurr D, Sailasuta N (1998): Proton spectroscopy without water suppression: The oversampled J-resolved ex-periment. Magn Reson Med 40:343–347.
Ito K, Morrish PK, Rakshi JS, Uema T, Ashburner J, Bailey DL, et al (1999): Statistical parametric mapping with 18F-dopa PET shows bilaterally reduced striatal and nigral dopaminer-gic function in early Parkinson’s disease. J Neurol Neurosurg
Psychiatry 66:754 –758.
Kato T, Inubushi T, Kato N (1998): Magnetic resonance spec-troscopy in affective disorders. J Neuropsychiatry Clin
Neu-rosci 10:133–147.
Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S (1994): Phosphorus-31 magnetic resonance spectroscopy and ventricular enlargement in bipolar disorder.
Psychiatry Res 55:41–50.
Keltner JR, Wald LL, Frederick BD, Renshaw PF (1997): In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magn Reson Med 37:366 –371. Kennedy SH, Javanmard M, Vaccarino FJ (1997): A review of
functional neuroimaging in mood disorders: Positron emis-sion tomography and depresemis-sion. Can J Psychiatry 42:467– 475.
Ketter TA, George MS, Kimbrell TA, Benson BE, Post RM (1996): Functional brain imaging, limbic function, and affec-tive disorders. The Neuroscientist 2:55– 65.
Kiesewetter DO, Carson RE, Jagoda EM, Herscovitch P, Eckel-man WC (1999): Using single photon emission tomography (SPECT) and positron emission tomography (PET) to trace the distribution of muscarinic acetylcholine receptor (MACHR) binding radioligands. Life Sci 64:511–518. Kim SE, Szabo Z, Seki C, Ravert HT, Scheffel U, Dannals RF,
et al (1999): Effect of tracer metabolism on PET measure-ment of [11C]pyrilamine binding to histamine H1 receptors.
Ann Nucl Med 13:101–107.
Kuenstler U, Juhnhold U, Knapp WH, Gertz HJ (1999): Positive correlation between reduction of handwriting area and D2 dopamine receptor occupancy during treatment with neuro-leptic drugs. Psychiatry Res 90:31–39.
Kwong KK, Belliveau JW, Chester DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al (1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89:5675–5679. Levin JM, Ross MH, Harris G, Renshaw PF (1996): Applications
of dynamic susceptibility contrast magnetic resonance imag-ing in neuropsychiatry. Neuroimage 4:147–162.
Lippmann S, Manshadi M, Baldwin H, Drasin G, Rice J, Alrajeh S (1982): Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients.
Am J Psychiatry 139:667– 668.
Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA (1999): Differences in cerebellar blood volume in schizophrenic and bipolar disorder. Schizophr Res 37:81– 89. Luyten PR, Bruntink G, Sloff M, Vermeulen JW, van der Heijden J, den Hollander JA, Heerschap A (1989): Broadband proton decoupling in human 31
P NMR spectroscopy. NMR
Biomed 1:177–183.
McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA (1998): Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 352:1433–1437.
McDonald WM, Tupler LA, Marsteller FA, Figiel GS, DiSouza S, Nemeroff CB, et al (1999): Hyperintense lesions on magnetic resonance images in bipolar disorder. Biol
Psychi-atry 45:965–971.
Mescher M, Merkle MM, Kirsch J, Garwood M, Gruetter R (1998): Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266 –272.
Moore CM, Breeze JL, Kukes TJ, Rose SL, Dager SR, Cohen BM, et al (1999): Effects of myo-inositol ingestion on human brain myo-inositol levels: A proton magnetic resonance spectroscopic imaging study. Biol Psychiatry 45:1197–1202. Moore CM, Renshaw PF (1997): Magnetic resonance spectros-copy studies of affective disorders. In: Krishnan KRR, Do-raiswamy PM, editors. Brain Imaging in Clinical Psychiatry. New York: Marcel Decker, 185–214.
Morimoto K (1999): Benzodiazepine receptor imaging in the brain: Recent developments and clinical validity. Jpn J Nucl
Med 36:307–313.
Murphy BJ, Stoyanova R, Srinivasan R, Willard T, Vigneron D, Nelson S, et al (1993): Proton-decoupled31
P chemical shift imaging of the brain in normal volunteers. NMR Biomed 6:173–180.
Musachio JL, Villemagne VL, Scheffel UA, Dannals RF, Dogan AS, Yokoi F, et al (1999): Synthesis of an I-123 analog of A-85380 and preliminary SPECT imaging of nicotinic recep-tors in baboon. Nucl Med Biol 26:201–207.
Namba H, Iyo M, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al (1999): Human cerebral acetylcholinesterase activity measured with positron emission tomography: Procedure, normal values and effect of age. Eur J Nucl Med 26:135–143. Nasrallah HA, McCalley-Whitters M, Jacoby CG (1982a): Ce-rebral ventricular enlargement in young manic males: A controlled CT study. J Affect Disord 4:15–19.
Nasrallah HA, McCalley-Whitters M, Jacoby CG (1982b): Cor-tical atrophy in schizophrenia and mania: A comparative CT study. J Clin Psychiatry 43:439 – 441.
National Institute of Mental Health’s Genetics Workgroup (1999): Genetics and mental disorders. Biol Psychiatry 45: 559 –573.
Nelson SJ, Vigneron DB, Star-Lack J, Kurhanewicz J (1997): High spatial resolution and speed in MRSI. NMR Biomed 10:411– 422.
Norris SD, Krishnan KR, Ahearn E (1997): Structural changes in the brain of patients with bipolar affective disorder by MRI: A review of the literature. Prog Neuropsychopharmacol Biol
Psychiatry 21:1323–1337.
Noto RB, Scheiner JD (1999): Advances in nuclear medicine: Current concepts. Med Health RI 82:190 –194.
Ouchi Y, Yoshikawa E, Okada H, Futatsubashi M, Sekine Y, Iyo M, et al (1999): Alterations in binding site density of dopamine transporter in the striatum, orbitofrontal cortex, and amygdala in early Parkinson’s disease: Compartment analysis for beta-CFT binding with positron emission tomography.
Ann Neurol 45:601– 610.
Pasantes-Morales H, Quesada O, Moran J (1998): Taurine: An osmolyte in mammalian tissues. In: Schaffer S, Lombardini JB, Huxtable RJ, editors. Taurine 3: Cellular and Regulatory
Mechanisms. New York: Plenum Press, 209 –217.
Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, et al (1995): In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder.
Arch Gen Psychiatry 52:471– 477.
Petersen SJ, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, et al (1999): Determination of the rate of the glutamate/ glutamine cycle in the human brain by in vivo 13C NMR.
Rauch SL, Renshaw PF (1995): Clinical neuroimaging in psy-chiatry. Harv Rev Psychiatry 2:297–312.
Raz S, Raz N (1990): Structural brain abnormalities in the major psychoses: A quantitative review of the evidence from com-puterized imaging. Psychol Bull 108:93–108.
Rosen BR, Belliveau JW, Aronen HJ, Kennedy D, Buchbinder BR, Fischman A, et al (1991): Susceptibility contrast imaging of cerebral blood volume: Human experience. Magn Reson
Med 22:293–299.
Rothman DL, Hanstock CC, Petroff OA, Novotny EJ, Prichard JW, Shulman RG (1992): Localized 1H NMR spectra of glutamate in the human brain. Magn Reson Med 25:94 –106. Rowley HA, Grant PE, Roberts TP (1999): Diffusion MR imaging. Theory and applications. Neuroimaging Clin N Am 9:343–361.
Roy M, Stip E, Black D, Lew V, Langlois R (1998): Cerebellar degeneration following acute lithium intoxication. Rev
Neu-rol (Paris) 154:546 –548.
Ryner LN, Sorenson JA, Thomas MA (1995): 3D localized 2D NMR spectroscopy on an MRI scanner. J Magn Reson 107:126 –137.
Schmahmann JD, Sherman JC (1998): The cerebellar cognitive affective syndrome. Brain 121:561–579.
Sharma R, Venkatasubramanian PN, Barany M, Davis JM (1992): Proton magnetic resonance spectroscopy of the brain in schizo-phrenic and affective patients. Schizophr Res 8:43– 49. Shimony JR, McKinstry RC, Akbudak E, Aronovitz JA, Snyder
AZ, Lori NF, et al (1999): Quantitative diffusion-tensor anisotropy brain MR imaging: Normative human data and anatomic analysis. Radiology 212:770 –784.
Silverstone PH, Rotzinger S, Pukhovsky A, Hanstock CC (1999): Effects of lithium and amphetamine on inositol metabolism in the human brain as measured by 1
H and 31
P MRS. Biol
Psychiatry 46:1634 –1641.
Smith JS, Zubieta JK, Price JC, Flesher JE, Madar I, Lever JR, et al (1999): Quantification of delta-opiod receptors in human brain with N19-([1]C]methyl) naltrindole and positron emis-sion tomography. J Cereb Blood Flow Metab 19:956 –966. Soares JC, Boada F, Spencer S, Wells KF, Mallinger AG, Frank
E, et al (1999): NAA and choline measures in the anterior cingulate of bipolar disorder patients. Biol Psychiatry 45: 119S.
Soares JC, Krishnan KR, Keshavan MS (1996): Nuclear mag-netic resonance spectroscopy: New insights into the patho-physiology of mood disorders. Depression 4:14 –30. Soares JC, Mann JJ (1997a): The anatomy of mood disorders—
review of structural neuroimaging studies. Biol Psychiatry 41:86 –106.
Soares JC, Mann JJ (1997b): The functional neuroanatomy of mood disorders. J Psychiatr Res 31:393– 432.
Starkstein SE, Pedoroff P, Berthier ML, Robinson RG (1991): Manic-depressive and pure manic states after brain lesions.
Biol Psychiatry 29:149 –158.
Steffens DC, Krishnan KR (1998): Structural neuroimaging and mood disorders: Recent findings, implications for classifica-tion, and future directions. Biol Psychiatry 43:705–712. Stoll AL, Renshaw PF, Sachs GS, Guimaraes AR, Miller C,
Cohen BM, et al (1992): The human brain resonance of
choline-containing compounds is similar in patients receiving lithium treatment and controls. An in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 32:944 –952. Strakowski SM, Sax KW, McElroy SL, Keck PE Jr, Hawkins
JM, West SA (1998): Psychiatric and substance abuse syn-drome co-occurrence in bipolar disorder following a first psychiatric hospitalization. J Clin Psychiatry 59:465– 471. Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglas AW,
Stoll AL (1993a): Structural brain abnormalities in first-episode mania. Biol Psychiatry 33:602– 609.
Strakowski SM, Woods BT, Tohen M, Wilson DR, Douglass AW, Stoll AL (1993b): MRI subcortical signal hyperintensi-ties in mania at first hospitalization. Biol Psychiatry 33:204 – 206.
Strauss WL, Layton ME, Dager SR (1998): Brain elimination half-life of fluvoxamine measured by 19F magnetic reso-nance spectroscopy. Am J Psychiatry 155:380 –384. Suhara T, Nakayama K, Inoue O, Fukuda H, Shimizu M, Mori A,
et al (1992): D1 dopamine receptor binding in mood disorders measured by positron emission tomography.
Psychopharma-cology 106:14 –18.
Swayze VW, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WTC (1990): Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperin-tensities. Arch Gen Psychiatry 47:1054 –1059.
Szabo Z, Scheffel U, Mathews WB, Ravert HT, Szabo K, Kraut M, et al (1999): Kinetic analysis of [11C]McN5652: A serotonin transporter radioligand. J Cereb Blood Flow Metab 19:967–981.
Thompson RB, Allen PS (1998): A new multiple quantum filter design procedure for use on strongly coupled spin systems found in vivo: Its application to glutamate. Magn Reson Med 39:762–771.
Valette H, Bottlaender M, Dolle F, Guenther I, Fuseau C, Coulon C, et al (1999): Imaging central nicotinic acetylcholine receptors in baboons with [18F]fluoro-A-85380. J Nucl Med 40:1374 –1380.
Videbech P (1997): MRI findings in patients with affective disorder: A meta-analysis. Acta Psychiatr Scand 96:157–168. Vikhoff-Baaz B, Ljungberg M, Starck G, Forssell-Aronsson E, Jonsson E, Alpsten M, et al (1999): Performance of 2D 1H spectroscopic imaging of the brain: Some practical consider-ation regarding the measurement procedure. Magn Reson
Imaging 17:919 –931.
Weber OM, Verhagen A, Duc CO, Meier D, Leenders KL, Boesiger P (1999): Effects of vigabatrin intake on brain GABA activity as monitored by spectrally edited magnetic resonance spectroscopy and positron emission tomography.
Magn Reson Imaging 17:417– 425.
Weinberger DR, DeLisi LE, Perman GP, Targum S, Wyatt RJ (1982): Computed tomography in schizophreniform disorder and other acute psychiatric disorders. Arch Gen Psychiatry 39:778 –783.
Wong DF, Wagner HN Jr, Pearlson G, Dannals RF, Links JM, Ravert HT, et al (1985): Dopamine receptor binding of C-11-3-N-methylspiperone in the caudate in schizophrenia and bipolar disorder: A preliminary report. Psychopharmacol
Bull 21:595–598.
Age-related MRI abnormalities in bipolar illness: A clinical study. Biol Psychiatry 38:846 – 847.
Yates WR, Jacoby CG, Andreasen NC (1987): Cerebellar atro-phy in schizophrenia and affective disorder. Am J Psychiatry 144:465– 467.
Yates WR, Wallace R (1987): Cardiovascular risk factors in affective disorder. J Affect Disord 12:129 –134.
Yurgelun-Todd DA, Renshaw PF (1999): Applications of fMRI to research in psychiatry. In: Heiserman JE, Drayer BP,
editors. Neuroimaging Clinics of North America. Philadel-phia: Saunders, 295–309.
Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R (1997): Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophr Res 26:85– 92.