Summary We studied assimilation, stomatal conductance and growth of Mangifera indica L. saplings during long-term exposure to a CO2-enriched atmosphere in the seasonally wet--dry tropics of northern Australia. Grafted saplings of M. indica were planted in the ground in four air-conditioned, sunlit, plastic-covered chambers and exposed to CO2 at the ambient or an elevated (700 µmol mol−1) concentration for 28 months. Light-saturating assimilation (Amax), stomatal conductance (gs), apparent quantum yield (φ), biomass and leaf area were measured periodically. After 28 months, the CO2 treatments were changed in all four chambers from ambient to the elevated concentration or vice versa, and Amax and gs were remeasured during a two-week exposure to the new regime.
Throughout the 28-month period of exposure, Amax and apparent quantum yield of leaves in the elevated CO2 treatment were enhanced, whereas stomatal conductance and stomatal density of leaves were reduced. The relative impacts of atmos-pheric CO2 enrichment on assimilation and stomatal conduc-tance were significantly larger in the dry season than in the wet season. Total tree biomass was substantially increased in re-sponse to atmospheric CO2 enrichment throughout the experi-mental period, but total canopy area did not differ between CO2 treatments at either the first or the last harvest.
During the two-week period following the change in CO2 concentration, Amax of plants grown in ambient air but meas-ured in CO2-enriched air was significantly larger than that of trees grown and measured in CO2-enriched air. There was no difference in Amax between trees grown and measured in ambi-ent air compared to trees grown in CO2-enriched air but meas-ured in ambient air. No evidence of down-regulation of assimilation in response to atmospheric CO2 enrichment was observed when rates of assimilation were compared at a com-mon intercellular CO2 concentration. Reduced stomatal con-ductance in response to atmospheric CO2 enrichment was attributed to a decline in both stomatal aperture and stomatal density.
Keywords: acclimation, apparent quantum yield, down-regu-lation, elevated CO2, gas exchange.
Introduction
The concentration of atmospheric CO2 has increased since the industrial revolution and is predicted to increase throughout the 21st century (Houghton et al. 1992). Because of the central role of CO2 in the physiology of plants, there is interest in determining the response of plants to increased atmospheric CO2 concentrations (Eamus and Jarvis 1989, Ceulemans and Mousseau 1994, Sage 1994, Eamus 1996a, 1996b).
Trees constitute a major reservoir of carbon (Gifford 1994). They also have important conservation, aesthetic, economic and social functions and can have a significant impact on atmospheric CO2 concentration and local climate (Eamus 1992). Trees within the tropics constitute a major site for the exchange of carbon between the biosphere and atmosphere. Furthermore, the tropics are characterized by high tempera-tures and light flux densities, conditions that may be expected to enhance the CO2 fertilization effect (Eamus 1996a). How-ever, there are few data on the responses of tropical trees to CO2 enrichment (Hogan et al. 1991), and in particular, of trees of the wet--dry tropics where there is distinct seasonality in atmospheric and soil water contents (Duff et al. 1997). Be-cause of the importance of source--sink relations, which are themselves highly seasonal, in determining assimilation re-sponses to atmospheric CO2 enrichment (Hilbert et al. 1991, Eamus 1996b), the relative impact of CO2 enrichment may vary seasonally. However, the impact of seasonality has rarely been considered in the context of CO2 enrichment studies for tropical trees and conflicting results have been obtained for temperate trees (Eamus 1996b). Gunderson et al. (1993) found no effect of leaf age or duration of exposure to CO2 enrichment on assimilation of yellow poplar and white oak. However, in a comparison of Castanea sativa Mill., a species with a mono-cyclic growth flush, with Fagus sylvatica L., a species with indeterminate growth (several flushes per year), Mousseau and Saugier (1992) showed that, as the growing season progressed, the impact of CO2 enrichment on assimilation declined with time for the monocyclic species.
Arp (1991) concluded that, in plants growing in pots, loss of photosynthetic capacity in response to long-term exposure to
Diurnal and seasonal changes in the impact of CO
2
enrichment on
assimilation, stomatal conductance and growth in a long-term study of
Mangifera indica
in the wet--dry tropics of Australia
JOHN GOODFELLOW, D. EAMUS and G. DUFF
School of Biological Sciences, Northern Territory University, Darwin, NT 0909, Australia
Received December 14, 1995
CO2 enrichment, often described as down-regulation, may result from restricted root volume, juvenility of tissues and frequent use of growth conditions far removed from ambient. Recent experiments with large trees growing in the ground in open top chambers (Gunderson et al. 1993), branch bag experi-ments (Teskey 1995), or free air carbon enrichment techniques (Ellsworth et al. 1995) frequently do not reveal down-regula-tion of assimiladown-regula-tion. However, these findings are not universal and down-regulation has been observed in experiments that excluded root restriction (Pettersson and McDonald 1992). Similarly, the hypothesis that atmospheric CO2 enrichment results in decreased gs has been supported in some studies (Hollinger 1987, Fetcher et al. 1988, Eamus et al. 1993) but not others (Bunce 1992, Gunderson et al. 1993, Ellsworth et al. 1995).
Because previous studies of acclimation of assimilation have involved reciprocal transfer experiments on trees growing in pots (Hollinger 1987, Fetcher et al. 1988), we carried out a reciprocal transfer experiment on trees growing in the ground by reversing the CO2 treatments at the end of a 28-month period of exposure. We report the results of a long-term (29 months) study of the assimilation and stomatal conduc-tance responses of the tropical tree species Mangifera in-dica L. cv Irwin growing in the ground in the wet--dry tropics of Australia. This species was chosen because it is economi-cally important in Australia and Asia, and because the cultivar Irwin, which fruits within one year, is amenable to manipula-tion of source--sink relamanipula-tionships. The specific hypotheses tested were that: (1) M. indica saplings can maintain an en-hanced Amax in response to atmospheric CO2 enrichment over three growing seasons; (2) time of day and seasonality influ-ence the relative response of assimilation (Amax and φ) and gs to atmospheric CO2 enrichment; (3) M. indica saplings exhibit a growth response to atmospheric CO2 enrichment and biomass accumulation that is correlated with Amax; and (4) reciprocal transfer experiments can be used to assess photo-synthetic down-regulation in trees grown in CO2-enriched air.
Materials and methods
Plant material
In June 1991, root stocks of Mangifera indica cv Kensington, (planted December 1990) were grafted with scion material from mature M. indica cv Irwin. Twenty-four grafts were planted in the ground in each of four chambers in October 1991. Trenches were dug to a depth of 1 meter before the chambers were constructed and filled with top soil to ensure uniformity of soil among chambers. All chambers (base area 10 m2 and height 2.4 m) were air-conditioned and ventilated at a rate of 50 dm3 s−1. Two chambers received CO2-enriched air (700 µmol mol−1) and two received ambient air. Chambers were spray irrigated daily with between 9 (at the start) and 127 dm3 of water (at the end) of the experiment. Chambers received 100--700 g of Osmocote slow-release fertilizer (14,6,18 N,P,K) and 4--40 g of a commercial water-soluble fertilizer (23,4,18 N,P,K) per chamber every 10--14 weeks. Irrigation and fertilizer regimes varied depending on season
and the number of trees in each chamber. At all times, irriga-tion and nutrient addiirriga-tions were maintained to minimize any limitations on growth because of water and nutrient availabil-ity. For a full description of chambers, see Berryman et al. (1993).
Measurements of assimilation and conductance
Assimilation rate (A) and gs were measured with an LI-6200 portable photosynthesis system equipped with a 1 dm3 cuvette (Li-Cor, Inc., Lincoln, NE). All measurements were made on three fully mature, upper-canopy, sunlit leaves per tree from four trees per chamber. Trees were selected from the center and both ends of each chamber to account for any possible vari-ations in microclimate within the chamber.
Diurnal measurements of A and gs were made in November 1991 (transition period between dry season and wet season when vapor pressure deficit (VPD) declines substantially, tem-perature increases but rains are infrequent and intermittent), March 1992 (wet season) and July 1992 (dry season); these periods were 3, 21 and 39 weeks, respectively, from the start of the experiment. Apparent quantum yield (φ) was determined in the morning (0630--0830 h) and in the afternoon (1630--1830 h) as the slope of the relationship between A and photo-synthetic photon flux density (PPFD).
In addition to the diurnal measurements, gs and assimilation were also measured under light-saturating conditions (PPFD > 800 µmol m−2 s−1) (Amax). Measurements were made each morning (0900--1230 h) and afternoon (1230--1600 h) on nine days between November 1991 and February 1994, during which there were three wet seasons and two dry seasons.
Stomatal density
Stomatal density of the abaxial surface of fully expanded leaves approximately two months old was measured 4 and 25 months after the beginning of the growth experiment. Three leaves per tree from four trees per chamber were examined by making a glue print (approximately 1 cm2) mid-way along each leaf. Stomatal density was calculated by observation of three view fields per print with a binocular microscope.
Growth analyses
We made three destructive harvests of four, six and eight trees per chamber at weeks 20, 60 and 80, respectively. Leaves were stripped from each tree and total leaf area was measured with a leaf area meter (Delta-T Devices, Cambridge, U.K.). Total leaf dry weight was then determined after drying to constant weight at 70 °C. The entire aboveground part of the tree (excluding leaves) was dried to constant weight at 70 °C. Root mass was estimated by excavating a 300 dm3 volume centered around the trunk and collecting all roots found.
Whole chamber reciprocal transfer experiment
ambi-ent concambi-entration. Both Amax and gs of four leaves per tree and three trees per chamber were measured 8, 13 and 15 days after transfer.
Statistical analyses of results
Statistical analyses were performed with the Systat software package (version 5.0, Wilkinson 1990). Apparent quantum yields of leaves were compared by analysis of covariance, after first testing the relationship between the dependent variable and the covariate for linearity. Data are presented as slopes ± standard error, number of data points (n) and squared multiple regression coefficient (r2). Analysis of covariance was used to determine whether a significant change in slope of the A versus PPFD relationship occurred between treatments (CO2; time of day; season) by determining the significance of the treatment
× PPFD interaction term.
Comparison of means among and between treatments was performed by a one- or two-factor nested analysis of variance. The principal factor of analysis was CO2 treatment. The secon-dary factor was time, either between morning and afternoon measurements of φ, Amax or gs, or between successive observa-tions of φ, Amax or gs. Nested terms were either trees within treatment chambers or replicate chambers within CO2 treat-ments. The probability for rejection of the null hypothesis was 0.05, unless otherwise stated.
Results
Assimilation rate increased curvilinearly with increasing PPFD in both treatments (data not shown). On all sampling dates, the elevated CO2 treatment significantly increased mean daily apparent quantum yield (φ) (mean of morning and after-noon values (P < 0.001)) (Table 1). The percentage increase in
φ in response to CO2 enrichment was largest in the dry season (July, 50%), smallest in the wet season (March, 17%) and intermediate in the transition period (November, 17%). In addition, φ was significantly smaller (P < 0.001) in the dry season than in the wet season or the transition period, irrespec-tive of treatment (Table 1).
In both treatments, φ was significantly higher in the after-noon than in the morning in both the wet and dry seasons, but
significantly lower in the afternoon compared with the morn-ing in the transition period (Table 1). Within a season, signifi-cant differences in φ between CO2 treatments occurred only in the afternoon during the transition period and the wet season with increases of 33 and 30%, respectively. In contrast, in the dry season, there were increases in φ in response to CO2 enrichment in both the morning (67%) and the afternoon (38%).
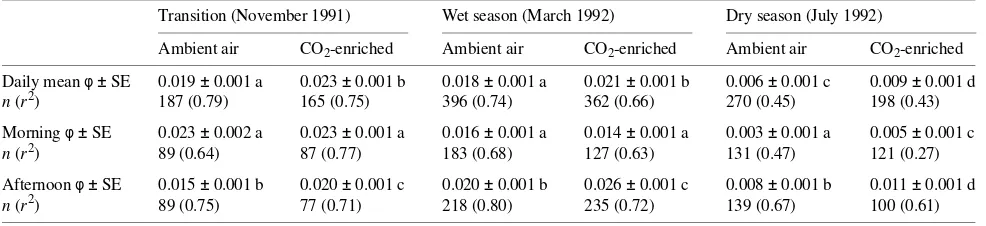
On all but one measurement day, Amax was significantly increased by atmospheric CO2 enrichment (Figure 1a). In both treatments, Amax was significantly greater in the wet season than in the dry season; however, the relative increase in Amax in response to CO2 enrichment was significantly (P < 0.01) larger in the dry season (25%) than in the wet season (16%).
The elevated CO2 treatment significantly decreased gs for all but one measurement period (Figure 1b). In both treatments, gs was larger in the wet season than in the dry season, and the relative difference in gs between CO2 treatments was also larger in the wet season than in the dry season. The elevated CO2 treatment not only reduced gs at all times of the year but also moderated the change in gs between the wet and dry seasons (Figure 1b).
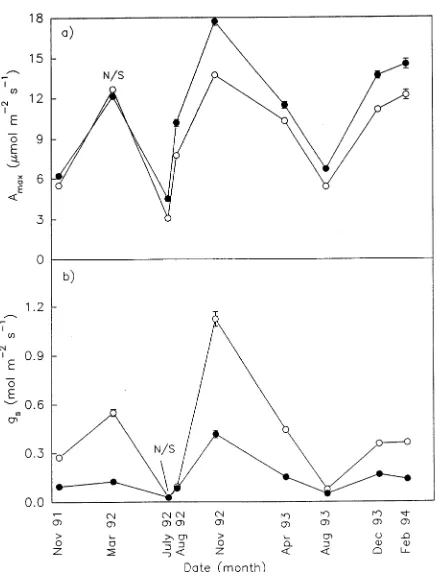
In six of seven measurements between November 1991 and December 1993, Amax was significantly less (typically 20--30%) in the afternoon than in the morning, irrespective of treatment (data not shown). For one dry season (August 1993) and one wet season (December 1993), the decline in Amax between morning and afternoon was accompanied by an increase in the the intercellular CO2 concentration/atmospheric CO2 concen-tration ratio (Ci/Ca) in the wet season but a decline in this ratio in the dry season (Figure 2a). In the dry season, the decline in gs in the afternoon was associated with a decline in Ci/Ca irrespective of treatment (Figure 2b); however, in the wet season, both gs and Ci/Ca increased in the afternoon in both treatments (Figure 2b).
In both treatments, Amax declined linearly (r2 = 0.73) with increasing leaf to air vapor pressure difference (LAVPD) (Fig-ure 3a), whereas gs declined curvilinearly over the same range of LAVPD values (Figure 3b). The elevated CO2 treatment decreased stomatal density by approximately 17%. There was no difference between stomatal density measured early in the
Table 1. Apparent quantum yield (φ mol mol−1) of Mangifera indica in CO2-enriched or ambient air during three observation periods: November 1991 (transition), March 1992 (wet season) and July 1992 (dry season). For daily mean φ, values followed by a different letter within a row are significantly different (P < 0.05). For morning and afternoon observations, means followed by a different letter within a measurement period are significantly different (P < 0.05).
Transition (November 1991) Wet season (March 1992) Dry season (July 1992)
Ambient air CO2-enriched Ambient air CO2-enriched Ambient air CO2-enriched
Daily mean φ± SE 0.019 ± 0.001 a 0.023 ± 0.001 b 0.018 ± 0.001 a 0.021 ± 0.001 b 0.006 ± 0.001 c 0.009 ± 0.001 d
n (r2) 187 (0.79) 165 (0.75) 396 (0.74) 362 (0.66) 270 (0.45) 198 (0.43)
Morning φ± SE 0.023 ± 0.002 a 0.023 ± 0.001 a 0.016 ± 0.001 a 0.014 ± 0.001 a 0.003 ± 0.001 a 0.005 ± 0.001 c
n (r2) 89 (0.64) 87 (0.77) 183 (0.68) 127 (0.63) 131 (0.47) 121 (0.27)
Afternoon φ± SE 0.015 ± 0.001 b 0.020 ± 0.001 c 0.020 ± 0.001 b 0.026 ± 0.001 c 0.008 ± 0.001 b 0.011 ± 0.001 d
growth experiment (January 1992) and late in the growth experiment (February 1994) (data not shown).
The elevated CO2 treatment increased mean daily Ci by approximately 78% (Figure 4a), and all but two measurements of Ci/Ca were significantly smaller for leaves grown at the elevated CO2 concentration than for leaves grown in ambient air (Figure 4b). In both treatments, Ci and Ci/Ca were signifi-cantly larger in the wet season than in the dry season. Mini-mum Ci/Ca ratios were observed at the time that LAVPD was maximal (Figure 4c); however, the difference in Ci/Ca between treatments was significantly larger in the wet season than in the dry season (Figure 4b).
Reciprocal transfer experiment
By Day 13 after transfer, assimilation had equilibrated to the new CO2 regime (data not shown). Figure 5a shows that Amax of leaves grown and measured in elevated CO2 was signifi-cantly larger than Amax of trees grown and measured in ambient air. On transfer of trees from ambient air to CO2-enriched air, Amax increased by 76%, which was 46% larger than the Amax of leaves grown and measured in CO2-enriched air. On transfer from CO2-enriched air to ambient air, Amax decreased by 16.5% compared to leaves grown and measured in CO2-enriched air. The Amax of leaves grown in CO2-enriched air and measured in
ambient air was similar to that of leaves grown and measured in ambient air (Figure 5a).
Stomatal conductance of leaves grown and measured in CO2-enriched air was significantly less than that of leaves grown and measured in ambient air. There was a slight but insignificant decrease in gs of leaves grown in ambient air and measured in CO2-enriched air compared to leaves grown and measured in ambient air (Figure 5b). However, gs of leaves grown in ambient air and measured in CO2-enriched air in-creased by 134% compared to leaves grown and measured in CO2-enriched air. The gs of leaves grown in CO2-enriched air and measured in ambient air increased by 32% compared to leaves grown and measured in ambient air, and by 245% compared to leaves grown and measured in CO2-enriched air (Figure 5b).
Leaves grown and measured in CO2-enriched air had a significantly higher Ci compared to leaves grown and meas-ured in ambient air (Figure 6a), resulting in a decreased Ci/Ca ratio (Figure 6b). Compared with all other measurement con-ditions, Ci was significantly larger in leaves grown in ambient Figure 1. (a) Light-saturating assimilation (Amax) and (b) stomatal
conductance (gs) determined for grafted Mangifera indica grown at ambient (s) or an elevated (d) CO2 concentration. Measurements
were made between 0900 and 1600 h over a 28-month period. Data are means ± standard error. N/S indicates a nonsignificant difference
between data points where P > 0.05. Figure 2. (a) Light-saturating assimilation (A
max) and (b) stomatal conductance (gs) as a function of Ci/Ca for grafted Mangifera indica grown at ambient (s) or an elevated (d) CO2 concentration.
air and measured in CO2-enriched air; however, the Ci/Ca ratio was not significantly different from that of leaves grown and measured in ambient air. Although Ci in leaves grown in CO2-enriched air and measured in ambient air was not signifi-cantly different from that of leaves grown and measured in ambient air, Ci/Ca ratio was significantly larger (Figures 6a and 6b).
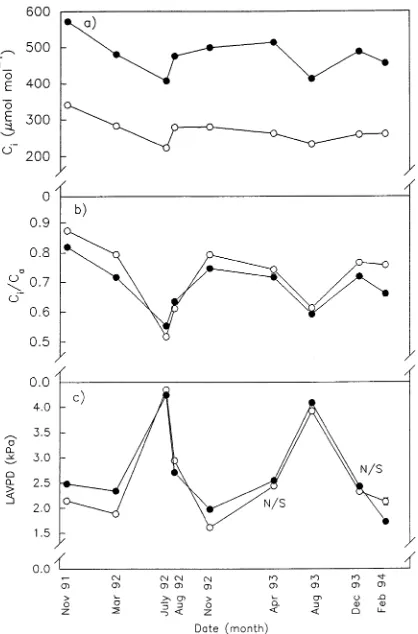
Total tree dry weight, shoot dry weight and estimated root dry weight were all significantly increased by CO2 enrichment (Figure 7) and the treatment differences appeared to increase with time. In contrast, there were no significant treatment effects on total canopy area and total canopy weight by the end of the experiment. However, throughout the growth experi-ment specific leaf area was significantly less by 14% with CO2 enrichment (data not shown).
Discussion
Apparent quantum yield----daily and seasonal variations
Apparent quantum yield (φ) increased in response to CO2 enrichment (cf. Ziska et al. 1990, Long and Drake 1991, Ziska
et al. 1991). Because assimilation is limited under low PPFD by the rate of regeneration of ribulose bisphosphate (RuBP), and decreased photorespiration results in increased availability of RuBP for carbon fixation (Stitt 1991), we postulate that the increase in φ in response to CO2 enrichment is associated with a decrease in photorespiration. Such an increase in φ may have significant implications for total daily carbon gain in tree species such as M. indica that have low light or shade habits (Eamus et al. 1993).
The response of φ to CO2 enrichment was larger in the dry season than in the transition period and wet season. The pro-portional impact of CO2 enrichment on assimilation may be enhanced when water stress is imposed (Chaves and Pereira 1992), because as gs declines during water stress, the benefit of CO2 enrichment in maintaining a high Ci becomes proportion-ally larger. Furthermore, the decline in sucrose phosphate synthase associated with drought stress can be reversed by CO2 enrichment (Vassey et al. 1990), which may also contribute to the enhancement of assimilation that occurs in response to CO2 enrichment during drought.
Both φ and gs (for all values of PPFD < 300 µmol m−2 s−1) were lower in the dry season than in the wet season, irrespec-Figure 3. (a) Light-saturating assimilation (Amax) as a function of
LAVPD, and (b) stomatal conductance (gs) as a function of LAVPD for grafted Mangifera indica grown at an elevated (d) or ambient (s)
CO2 concentration. Data are means of Amax or gs measured in the morning (0900--1230 h) and afternoon (1230--1600 h).
Figure 4. Values of (a) Ci, (b) Ci/Ca and (c) LAVPD for grafted
Mangifera indica grown at an elevated (d) or ambient (s) CO2
tive of treatment. Based on the observation that Ci decreased between wet and dry seasons for all measurements of assimi-lation when PPFD was less than 300 µmol m−2 s−1, we postu-late that the decline in gs contributed to the decline in φ.
In the transition and the wet season, differences in φ between treatments only occurred in the afternoon, whereas in the dry season there were differences between treatments for both morning and afternoon measurements. This pattern may reflect differences between seasons in the magnitude of the effect of LAVPD on gs, and hence φ. In the transition period and wet season, gs and Ci/Ca increased in the afternoon because atmos-pheric and soil water content were high and LAVPD was low because of cloud cover and reduced solar radiation load (Duff et al. 1997, Eamus and Cole 1997). However, gs was consider-ably smaller throughout the day in the dry season than in the wet season or the transition period, and was always smaller in the afternoon than in the morning. Because the proportional impact of CO2 enrichment increased as gs declined, the differ-ences in gs between seasons and between morning and after-noon may explain why the elevated CO2 treatment
significantly increased φ only in the afternoon during the wet season, but significantly increased φ in both the morning and the afternoon during the dry season.
Amax----daily and seasonal trends, and growth
On the first measurement date (November 1991), Amax in-creased in the afternoon compared to the morning, in both treatments, perhaps indicating that the trees were acclimating to the transfer from pots to the ground.
For eight of the nine measurements, Amax increased in re-sponse to CO2 enrichment, a result ascribed to decreased photorespiration and increased CO2 concentration for Rubisco (Eamus and Jarvis 1989). In both treatments, Amax was larger in the morning than in the afternoon for the majority of obser-vations (Figure 2a). In the dry season, this decline was likely the result of the large increase in LAVPD, which caused a large decline in gs and hence in the supply of CO2 to the chloroplast (Figures 4a and 4c). This hypothesis is supported by the obser-vation that Ci/Ca declined in the afternoon compared to the morning (Figures 2a and 2b) in the dry season, as would be Figure 5. (a) Light-saturating assimilation (Amax) and (b) stomatal
conductance (gs) in grafted Mangifera indica grown for 28 months at an elevated (Enr.) or ambient (Amb.) CO2 concentration and then transferred from one CO2 concentration to the other. Measurements were made before (left-hand columns) and after 8 to 15 days at the new CO2 concentration. Data are means± standard error. Bars with differ-ent letters are significantly differdiffer-ent (P < 0.05).
expected if gs limited assimilation. A similar decline in gs and Ci/Ca in response to a large increase in LAVPD has been shown in a range of tropical tree species growing in the wet--dry tropics of Australia (Berryman et al. 1993, Eamus and Cole 1997, Prior et al. 1997).
There was no treatment difference in the decline in Amax between the morning and the afternoon in the wet season. In contrast, the decline in Amax in the afternoon in the dry season was significantly larger with CO2 enrichment compared with controls. This is because trees were sink limited in the dry season with a seasonal decline in growth, yet Ci remained enhanced despite a reduction in gs, with the result that Amax remained enhanced in the dry season. An increase in the source--sink strength ratio results in end point inhibition of photosynthesis (Sage 1994). In the wet season, the decline in Amax in the afternoon was associated with increased gs and Ci/Ca (Figure 3), indicating that stomatal closure in response to increased LAVPD cannot explain the decline. A decrease in Amax despite increased gs was also reported by Eamus et al. (1993) who suggested a depletion of phosphate supply to the chloroplast could result in a lower Amax. Phosphate requirement is increased by CO2 enrichment as a result of an increase in Amax (Conroy 1992).
The significant decline in Amax in the dry season compared to the wet season in both treatments was the result of a de-creased gs caused by increased LAVPD (Figure 4). Leaf water potential did not differ between seasons indicating that soil water availability was not a limiting factor (data not shown) and so was unlikely to account for the decline in gs in the dry season. Therefore, we attribute the greater enhancement of Amax in response to CO2 enrichment in the dry season than the
wet season to the proportional increase in benefit of CO2 enrichment as stomatal limitation increased, i.e., increased Ci was maintained at a reduced gs in the dry season.
By the end of the experiment, trees grown in the CO2 enriched atmosphere had approximately 60% more biomass than trees grown in ambient air. The increase in biomass was was more than the 15% estimated increase in carbon fixed over the 28-month study (estimated from the integrated sum of Amax from Figure 1); however, this was based on rates of light-saturated assimilation. In M. indica, which has a dense closed canopy, a large and significant fraction of daily carbon gain occurs in self-shaded leaves.
Stomatal conductance was significantly decreased in leaves grown in CO2-enriched air on most measurement dates. This has been observed previously in many studies (for review see Eamus and Jarvis 1989, Sage 1994) and is the result of de-creases in both stomatal density and stomatal aperture. The percentage decline in gs between wet and dry seasons was significantly smaller for leaves grown at an elevated CO2 concentration than for leaves grown at the ambient CO2 con-centration. Carbon dioxide enrichment in the wet season (De-cember 1993) also resulted in a larger difference between morning and afternoon values of gs. A partial explanation for this may be that, in the morning, stomatal opening is at a maximum for trees grown in ambient CO2 if VPD is low. In contrast, the stomata of trees grown in elevated CO2 are not fully open (gs increases when trees grown in CO2-enriched air are transferred to ambient air), therefore, increased stomatal opening can occur in the afternoon and does so under favorable conditions.
Figure 7. (a) Tree dry weight, (b) root dry weight, (c) leaf dry weight and (d) canopy area for grafted
Mangifera indica grown in CO2 -en-riched (d) or ambient (s) air.
Acclimation of assimilation
The enhancement of assimilation in response to the elevation of CO2 concentration did not diminish over the 28-month study (cf. Gunderson et al. 1993, Idso and Kimball 1993). In con-trast, the enhancement of assimilation in response to the eleva-tion of CO2 concentraeleva-tion declined within 30 days in Castanea sativa (Mousseau and Saugier 1992), suggesting that determi-nate species with monocyclic growth flushes show a gradual decline in the response of assimilation to CO2 enrichment as the growing season progresses. Kohen et al. (1993) compared a monocyclic determinate species (C. sativa) with Fagus syl-vatica, an indeterminate species with several growth flushes, and found that F. sylvatica maintained an enhanced assimila-tion rate in response to CO2 enrichment throughout the grow-ing season. Mangifera indica is relatively indeterminate in its growth, and during the dry season, large amounts of carbon are temporarily stored in the bole of the tree (E. Chacko, CSIRO, Darwin, Australia, personal communication) to be utilized later during flowering and fruit growth. Consequently, major sinks for carbon are maintained throughout the dry season. The larger percentage increase in assimilation rate in response to CO2 enrichment in the dry season than in the wet season may be associated with sink strength.
Acclimation or down-regulation of assimilation in response to long-term exposure to an elevated atmospheric CO2 concen-tration is often inferred from a comparison of assimilation rates at a common Ca (Hollinger 1987, Ceulemans et al. 1995); however, as indicated by Sage (1994), a comparison of assimi-lation rates at a common Ci is needed to compare photosyn-thetic efficiencies. We found that comparisons at a common Ca need not result in the same Ci for trees exposed to both the ambient and an elevated CO2 concentration and therefore com-parisons of their respective assimilation rates may be mislead-ing. It was found that the assimilation rate of trees grown and measured in an elevated CO2 concentration was significantly lower than that of trees grown in ambient air and measured at an elevated CO2 concentration (Figure 5a). Such a result is frequently thought to be indicative of a loss of photosynthetic potential at an elevated CO2 concentration. However, because gs of leaves grown in ambient air but measured in CO2 -en-riched air was more than double that of leaves grown and measured in CO2-enriched air (Figure 5b), Ci will have been larger. Thus, it was to be expected that assimilation would be larger if it was CO2 limited. Thus, there may have been no loss of photosynthetic potential in the trees grown in CO2-enriched air. This view is supported by the observation that the assimi-lation rate of leaves grown in CO2-enriched air and measured in ambient air was not significantly different from that of trees grown and measured in ambient air. Furthermore, because Ci did not differ, we conclude that no loss of assimilation poten-tial occurred. A similar lack of down-regulation has been observed in mature trees (Gunderson et al. 1993, Ellsworth et al. 1995, Liu and Teskey 1995, Teskey 1995).
Stomatal conductance of leaves grown in CO2-enriched air and measured in ambient air was larger than gs of leaves grown and measured in ambient air (Figure 5b). However, Ci and Amax were similar for both sets of plants (Figures 5a and 6a).
Based on our findings and the observation that as mesophyll conductance (gm) of evergreen perennials (such as M. indica) declines, stomatal cavity CO2 concentration declines (Lloyd et al. 1992), we propose that, at an elevated CO2 concentration, stomatal density and specific leaf area decline, and that the average path length for diffusion of CO2 from the bulk air to the furthest chloroplast increases. Consequently, gm may de-cline, and hence a larger gs be required to maintain the same Ci and assimilation rate.
References
Arp, W.J. 1991. Effects of source--sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 14:869--875. Berryman, C.A., D. Eamus and G.A. Duff. 1993. The influence of CO2
enrichment on growth, nutrient content and biomass allocation of
Maranthes corymbosa. Aust. J. Bot. 41:195--209.
Bunce, J.A. 1992. Stomatal conductance, photosynthesis and respira-tion of temperate deciduous tree seedlings grown outdoors at an elevated concentration of carbon dioxide. Plant Cell Environ. 15:541--549.
Ceulemans, R. and M. Mousseau. 1994. Effects of elevated atmos-pheric CO2 on woody plants. New Phytol. 127:425--446. Ceulemans, R., X.N. Jaing and B.Y. Shao. 1995. Growth and
physiol-ogy of one year old poplar (Populus) under elevated atmospheric CO2 levels. Ann. Bot. 75:609--617.
Chaves, M.M. and J.S. Pereira. 1992. Water stress, CO2 and climate change. J. Exp. Bot. 43:1131--1139.
Cole, S.P., K.C. Woo, D. Eamus, C.E. Harwood and M.W. Haines. 1994. Field measurements of net photosynthesis and related pa-rameters in four provenances of Acacia auriculiformis. Aust. J. Bot. 42:457--470.
Conroy, J.P. 1992. Influence of elevated atmospheric CO2 concentra-tion on plant nutriconcentra-tion. Aust. J. Bot. 40:445--56.
Duff, G.A., B.A. Myers, R.J. Williams, D. Eamus, A. O’Grady and I.R. Fordyce. 1997. Seasonal patterns in soil moisture, vapour pressure deficit, tree canopy cover and pre-dawn water potential in a northern Australian savanna. Aust. J. Bot. 42:1--7.
Drake, B.G. and P.W. Leadley. 1991. Canopy photosynthesis of crops and native plant communities exposed to long-term elevated CO2. Plant Cell Environ. 14:853--860.
Eamus, D. 1992. Atmospheric CO2 and trees, from cellular to regional responses. Encycl. Earth Syst. Sci. 1:157--169.
Eamus, D. 1996a. CO2 and temperature interactions, biomass alloca-tion and stand scale modeling in the study of tree responses to CO2 enrichment. Tree Physiol. 16:43--47.
Eamus, D. 1996b. Responses of field grown trees to CO2 enrichment. Comm. For. Rev. 75:39--47.
Eamus, D. and S. Cole. 1997. Diurnal and seasonal comparisons of assimilation, phyllode conductance and water potential of three Acacia and one Eucalypt species in the wet--dry tropics of Australia. Aust. J. Bot. 42:33--40.
Eamus, D. and P.G. Jarvis. 1989. The direct effects of increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forests. Adv. Ecol. Res. 19:1--55.
Ellsworth, D.S., R. Oren, C. Huang, N. Phillips and G.R. Hendrey. 1995. Leaf and canopy responses to elevated CO2 in a pine forest under free air CO2 enrichment. Oecologia 104:1--8.
Fetcher, N., C.H. Jaeger, B.R. Strain and N. Sionit. 1988. Long-term elevation of atmospheric CO2 concentration and carbon exchange rates of saplings of Pinus taeda L. and Liquidambar styraciflua L. Tree Physiol. 4:255--262.
Gifford, R.M. 1994. The global carbon cycle: a viewpoint on the missing sink. Aust. J. Plant Physiol. 21:1--15.
Gunderson, C.A., R.J. Norby and S.D. Wullschleger. 1993. Foliar gas exchange responses of two deciduous hardwoods during 3 years of growth in elevated CO2: no loss of photosynthetic enhancement. Plant Cell Environ. 16:797--807.
Hilbert, D.W., A. Larigauderie and J.F. Reynolds. 1991. The influence of carbon dioxide and daily photon flux density on optimal leaf nitrogen concentration and root:shoot ratio. Ann. Bot. 68:365--376. Hogan, K.P., A.P. Smith and L.H. Ziska. 1991. Potential effects of elevated CO2 and changes in temperature on tropical plants. Plant Cell Environ. 14:763--778.
Hollinger, D.Y. 1987. Gas exchange and dry matter allocation re-sponse to elevation of atmospheric CO2 concentration in seedlings of three tree species. Tree Physiol. 3:193--202.
Houghton, J.T., B.A. Callender and S.K. Varney. 1992. Intergovern-mental panel for climate change. The supplementary report to the IPCC scientific assessment, Cambridge University Press, U.K., pp 1--200.
Idso, S.P. and B.A. Kimball. 1993. Effects of atmospheric CO2 enrich-ment on net photosynthesis and dark respiration rates of three Australian tree species. Plant Physiol. 141:166--171.
Kohen, A.El., L. Venet and M. Mousseau. 1993. Growth and photosyn-thesis of two deciduous forest species at elevated carbon dioxide. Funct. Ecol. 7:480--486.
Lloyd, J., J.P. Syvertsen, P.E. Kriedemann and G.D. Farquhar. 1992. Low conductance for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ. 15:873--899.
Liu, S. and R.O. Tesky. 1995. Response of foliar gas exchange to long-term elevated CO2 concentrations in mature loblolly pine trees. Tree Physiol. 15:351--359.
Long, S.P. and B.G. Drake. 1991. Effect of long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3Scirpus dyneyi. Plant Physiol. 96:221--226.
Mousseau, M. and B. Saugier. 1992. The direct effect of increased CO2 on gas exchange and growth of forest tree species. J. Exp. Bot. 43:1121--1130.
Pettersson, R. and A.J.S. McDonald. 1992. Effects of elevated carbon dioxide concentration on photosynthesis and growth of small birch plants (Betula pendula Roth.) at optimal nutrition. Plant Cell Envi-ron. 15:911--919.
Prior, L.D., D. Eamus and G.A. Duff. 1997. Seasonal and diurnal patterns of carbon assimilation, stomatal conductance and leaf water potential in Eucalyptus tetrodonta saplings in a wet--dry savanna in northern Australia. Aust. J. Bot. 42:26--32.
Sage, R.F. 1994. Acclimation of photosynthesis to increasing CO2: the gas exchange perspective. Photosynth. Res. 39:351--368.
Stitt, M. 1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 14:741--762.
Teskey, R.O. 1995. A field study of the effects of elevated CO2 on carbon assimilation, stomatal conductance and leaf and branch growth of Pinus taeda trees. Plant Cell Environ. 18:565--575. Vassey, T.C., W.P. Quick and T.D. Sharkey. 1990. Water stress, CO2,
light effects on sucrose phosphate synthase activity in Phaseolus vulgaris. Physiol. Plant. 81:37--44.
Wilkinson, L. 1990. Systat: the system for statistics. Systat Inc., Evanston, IL, USA, pp 1--200.