A rapid and sensitive assay method for measuring amine oxidase
based on hydrogen peroxide – titanium complex formation
Sudipa Nag, Kalpana Saha, Monojit A. Choudhuri *
Department of Botany,Uni6ersity of Burdwan,Burdwan 713 104, India
Received 23 September 1999; received in revised form 8 January 2000; accepted 12 April 2000
Abstract
Hydrogenperoxide (H2O2) is an end product of diamine and polyamine oxidation by their respective oxidase enzymes. A new
sensitive assay method is based on a H2O2– titanium (Ti) complex formation as an indicator of H2O2production due to polyamine
oxidation. The orange – yellow coloured H2O2– Ti complex was measured at 410 nm in a Shimadzu spectrophotometer. The assay
conditions for maximum diamine oxidase (DAO) and polyamine oxidase (PAO) as standardized here using the hypocotyl tissues ofVigna catjangEndl. cv Pusa Barsati consisted of pH 7.4 (40 mM potassium phosphate buffer), 3 mM substrate (putrescine or spermine), 37°C incubation temperature and 30 min incubation time in the presence of catechol (10−2M) used as an inhibitor
of both peroxidase and catalase activity. The method described here was significantly more sensitive than the starch – iodide method [T.A. Smith, Biochem. Biophys. Res. Commun. 41 (1970) 1452 – 1456], which could be improved further if measured under the same assay conditions as described for the H2O2– Ti method. Sensitivity of the present method was tested by assaying
DAO/PAO activity in auxin treated hypocotyls of Vigna and comparing it with the starch – iodide method in two other plant samples. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Diamine oxidase; Indole-3-butyric acid; Polyamine; Polyamine oxidase;Vigna catjangEndl.;Vigna radiataL. cv 105;Brassica alba Hook cv B9
www.elsevier.com/locate/plantsci
1. Introduction
Diamine oxidase (DAO) and polyamine oxidase
(PAO) cause oxidation of diamines and
polyamines, respectively, where hydrogenperoxide (H2O2) is an end product [1,2]. Activity of these
enzymes are reportedly measured either by esti-mating the residual substrate [3] or by estiesti-mating the end product H2O2 or pyrrolline derivatives in
an indirect way [4 – 7]. One such indirect method involves the development of a starch – iodide
com-plex due to the oxidation of I− to I0 by the
liberated H2O2 [5]. Another method consists of
measurement of the rate of peroxidative oxidation
of guaiacol by H2O2 in presence of peroxidase
enzyme [6], or of the evolved O2 from the
break-down of H2O2 in presence of catalase enzyme
using an oxygen electrode [7]. A direct measure-ment of H2O2produced during DAO/PAO activity
seems to be a more sensitive method for measuring their activities in plant systems. Titanium ions strongly react with H2O2 forming a brilliant
or-ange – yellow coloured complex, which is used for measuring H2O2 in plant tissues [8]. Thus, in the
present communication, determination of DAO/
PAO activity in terms of H2O2 measurement was
standardized and established to be more sensitive than some other methods.
A valid enzyme assay is substantiated by (1) the rate of product formation which must be linear with respect to the reaction time of the assay; and (2) the quantity of product formed during a fixed reaction time that should increase proportionately Abbre6iations: H2O2, hydrogenperoxide; DAO, diamine oxidase;
PAO, polyamine oxidase; Ti, titanium; CAT, catalase; POX, peroxi-dase; IBA, indole-3-butyric acid.
* Corresponding author. Tel./fax: +91-342-56260. E-mail address:[email protected] (M.A. Choudhuri).
Table 1
Effect of inhibitor catechol (10−2 M) on authentic catalase
(CAT) and peroxidase (POX) activity (n=9)
Treatment Enzyme activity
15R, USA). The clear supernatant fraction con-taining 170 mg protein ml−1, was used as enzyme
source during assays. To test the role of catechol as an inhibitor of CAT and POX enzymes, the activity of standard CAT (Sigma, USA) was per-formed as described by Snell and Snell [10] with some modifications [11] and POX (Sigma, USA) activity was measured according to the method of Kar and Mishra [12] in the presence and absence of catechol (10−2 M).
2.3. Standardization of conditions for maximum enzyme acti6ity using putrescine as substrate
2.3.1. Assay pH
To standardize the optimum pH of assay buffer, the reaction was started by the addition of 0.5 ml of reaction mixture containing 2 mM substrate (putrescine) and 40 mM potassium phosphate buffer separately at pH 7.0, 7.2, 7.4 and 7.5 with 0.3 ml of enzyme extract at 30°C for 30 min. The pH of the actual reaction was determined after mixing enzyme extraction with reaction mixture. After incubation, the reaction was terminated by
adding 0.1 ml of 15% (W/V) titanium sulphate
(TiSO4) in 23% H2SO4. The incubation mixture
was then centrifuged at 10 000 rpm for 10 min and absorbance of the orange – yellow coloured solu-tion was taken at 410 nm in a Shimadzu UV-vis spectrophotometer. In controls, TiSO4 was added
prior to the addition of enzyme solution.
2.3.2. Substrate concentration
To standardize the optimum substrate concen-tration, the reaction was initiated by adding 0.5 ml of reaction mixture containing 0.5, 1, 2, 3, 4, 5 and 6 mM putrescine and previously standardized as-with the amount of enzyme supplied to the
reaction.
2. Materials and methods
2.1. Plant materials
Vigna catjang Endl. cv Pusa Barsati seeds were grown in sand in a controlled growth room with a 16 h photoperiod at 222 mmol m−2 s−1 intensity
(400 – 700 nm) for 7 days. Hypocotyls of 7-day-old seedlings were taken for assaying the diamine
oxi-dase (DAO) and polyamine oxidase (PAO)
activity.
2.2. Extraction and estimation of DAO/PAO
Enzymes were extracted following the method of Rinaldi et al. [7]. Hypocotyl tissue (1 g) was homogenized in a prechilled mortar using 1.5 ml of 50 mM potassium phosphate buffer (pH 7.0) containing catechol (10−2M), used as an inhibitor
of catalase (CAT) and peroxidase (POX) [9]. The homogenate was centrifuged at 10 000 rpm for 15 min at 4°C in a Beckman centrifuge (Model
GS-Table 2
Determination of actual pH of reaction mixture for maximum diamine oxidase (DAO)a
pH of the actual reaction mixture H2O2 production (DA/30 min) 9S.E.
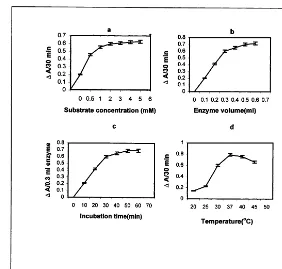
Fig. 1. (a) Determination of substrate concentration for maximum diamine oxidase (DAO) activity at incubation temperature 30°C, time 30 min and at standardized pH 7.4. (b) Determination of enzyme volume at standardized substrate concentration (3 mM putrescine), and pH 7.4 and at incubation temperature 30°C and time 30 min for maximum DAO activity. (c) Determination of incubation time at standardized substrate concentration (3 mM putrescine), pH 7.4 enzyme volume 0.3 ml and at incubation temperature 30°C for maximum DAO activity. (d) Determination of incubation temperature for maximum DAO activity at standardized substrate concentration (3 mM putrescine), pH 7.4, enzyme volume 0.3 ml and incubation time 30 min. DAO activity in terms ofDA/30 min. Bars indicate standard error (n=9).
say buffer (40 mM potassium phosphate buffer at pH 7.4) with 0.3 ml enzyme extract at 30°C for 30 min. The reaction was terminated by adding 0.1 ml of 15% TiSO4in 23% H2SO4. After
centrifuga-tion of the mixture, absorbance of the H2O2–
tita-nium complex was measured at 410 nm.
2.3.3. Enzyme concentration
To standardize the optimum enzyme concentra-tion, 0.5, 0.75, 1.00, 1.50 and 2.0 g tissue was separately extracted using 1.5 ml of 50 mM potas-sium phosphate buffer (pH 7.0) with catechol (10−2 M) and then the reaction was initiated by
adding 0.3 ml of enzyme extract separately from each extraction with 0.5 ml reaction mixture con-taining 3 mM putrescine (standardized) and 40 mM potassium phosphate buffer (pH 7.4) (stan-dardized) at 30°C for 30 min. The reaction was terminated by adding 0.1 ml of 15% TiSO4in 23%
H2SO4. After centrifugation of the mixture,
ab-sorbance of the H2O2– titanium complex was
mea-sured at 410 nm.
To standardize the optimum enzyme volume 1.0 g tissue (standardized) was extracted using 1.5 ml of 50 mM potassium phosphate buffer (pH 7.0) with catechol (10−2M) and then the reaction was
initiated by adding 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 ml of enzyme extract separately with 0.5 ml reaction mixture containing 3 mM putrescine (standard-ized) and 40 mM potassium phosphate buffer at pH 7.4 (standardized) at 30°C for 30 min. The reaction was terminated by adding 0.1 ml of 15%
Table 3
Estimation of protein contant and hydrogen peroxide produc-tion for maximum diamine oxidase (DAO) activity (n=9)
Content of H2O2Production
Tissue (g tissue/1.5
protein (DA/30 min) 9S.E. ml buffer)
(mg/ml)
0.50 80 0.2290.002
0.75 116.3 0.4490.001
0.690.003 170.3
1.00
237.3
1.50 0.6990.001
0.7290.003 333.3
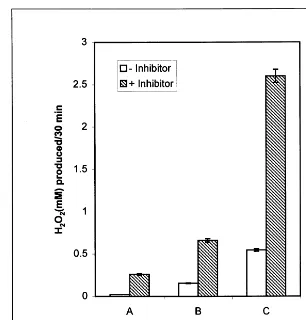
Fig. 2. (A) Diamine oxidase (DAO) activity (mM H2O2 produced/30 min) in presence and absence of catechol. Starch – iodide
method. (B) Starch – iodide method done under assay conditions of H2O2– Ti method. (C) H2O2– Ti method. Bars indidicate
standard error (n=9).
TiSO4 in 23% H2SO4. After centrifugation of the
mixture, absorbance of the H2O2– titanium
com-plex was measured at 410 nm.
2.3.4. Incubation time
By keeping all other conditions as standardized above fixed, the incubation time for maximum enzyme activity was standardized by varying the time from 10 to 60 min at intervals of 10 min at 30°C temperature and the optimum incubation time was determined. The enzyme activity was calculated as DAXTv/tX6, where A is the
sorbance of sample after incubation minus ab-sorbance at zero time control, Tv is the total
volume of the filtrate, t is the time (min) of
incubation with substrate, and 6 is the volume of
filtrate actually taken for incubation [13].
2.3.5. Temperature
To standardize the optimum temperature for incubation, the reaction mixture, consisting of 0.3 ml enzyme extract (standardized), 0.5 ml reaction
mixture containing 3 mM putrescine (standard-ized) and 40 mM potassium phosphate buffer at pH 7.4 (standardized) was separately incubated at 20, 25, 30, 37, 40, and 45°C for 30 min
(standard-ized). After incubation, 0.1 ml of 15% TiSO4
reagent was added and after centrifugation at 10 000 rpm for 10 min, absorbance of the H2O2–
titanium complex was read at 410 nm.
Table 4
Effect of IBA (25 mM) treatment (48–72 h) on diamine oxidase (DAO) and polyamine oxidase (PAO)a
PAO 9S.E. Treatment DAO 9S.E.
Control 0.7990.001 0.77090.002 IBA (48 h) 0.8990.003 0.8190.003 IBA (72 h) 1.3690.004 1.2990.003
Table 5
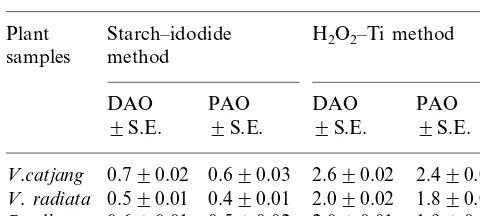
Comparison of the H2O2–Ti method and the traditional
starch–iodide method on other plant samplesa
Starch–idodide
aDAO and PAO activities in terms of mM H
2O2produced
/30 min.
2.6. DAO/PAO acti6ities in IBA treated seedlings
Changes in DAO and PAO activities were mea-sured using H2O2– Ti method in the hypocotyl
region after treating the cuttings of Vigna with IBA (indole-3-butyric acid, 25 mM) for 48 and 72 h. Where PAO activity was measured, putrescine was replaced by spermine.
2.7. Comparison of the H2O2–Ti and
starch–iodide method on a range of plant samples
To justify the significance of the H2O2– Ti
method and to make the method valid, DAO and PAO activities were measured in two other plants viz, V.radiata L. cv 105 andB. albaHook. cv B9. All the experiments were repeated three times with at least three replicates each time with repro-ducible results.
3. Results and discussion
3.1. Effect of an orthodiphenol inhibitor catechol
(10−2 M
)
It is evident from Table 1 that the standard CAT and POX showed approximately 0.0 and 0.87% activity respectively in presence of the
in-hibitor (catechol, 10−2 M). Homogenates of
hypocotyl tissues were, therefore, treated with cat-echol, an orthodiphenol compound, to inhibit the POX and CAT activity which were responsible for breakdown of enzymatically generated H2O2.
3.2. Optimum conditions for DAO acti6ity
The optimum activity of DAO was standardized under H2O2– Ti method at varying pH (7.0, 7.2,
7.4 and 7.5) (Table 2). The optimum concentration of the substrate putrescine (0.5, 1, 2, 3, 4, 5 and 6 mM) was then standardized (Fig. 1a). Concentra-tion of the enzyme (0.5, 0.75, 1.0, 1.50 and 2.0 g tissue/1.5 ml phosphate buffer, pH 7.0) was deter-mined (Table 3). Next the enzyme volume (0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 ml) was standardized and shown in Fig. 1b. This was followed by determina-tion of incubadetermina-tion time (10, 20, 30, 40, 50 and 60 min) which was depicted in Fig. 1c, and tempera-ture (20, 25, 30, 37, 40 and 45°C) was shown in Fig. 1d. It was observed that the activity of DAO 2.4. Estimation of protein content
Amount of protein was determined by the method of Bradford [14] with bovine serum albu-min as the standard.
2.5. Comparison with starch–idodide method of Smith [5]
To examine the sensitivity of the present method based on H2O2– titanium complex formation due
to diamine oxidase activity, it was compared with the starch – iodide method at the assay conditions reported by Smith [5]. DAO activity was measured employing the starch – iodide method under two assay conditions. In one, the conditions as de-scribed by Smith [5] were strictly followed, while in the other, the conditions as described above for H2O2– titanium method was followed. Thus in the
starch – iodide method, the assay mixture con-tained 0.3 ml of crude enzyme (previously ex-tracted with 100 mM potassium phosphate buffer, pH 6.5) and 0.5 ml reaction mixture consisting of 1.3% soluble starch, 20 mM potassium iodide and 10 mM putrescine in 1 mM potassium phosphate buffer (pH 5.8) for a period of 30 min at 37°C [5]. The enzyme activity was also measured following the starch – iodide formation method under the assay conditions reported here in presence and absence of catechol (10−2 M). After incubation,
the absorbance was measured at 550 nm. Enzyme activities in the above mentioned methods were expressed in terms of mM H2O2produced/30 min
(extraction buffer pH 7.0) was optimum under the assay conditions of pH 7.4, 3 mM putrescine, 1.0 g of tissue in 1.5 ml phosphate buffer equivalent to 170 mg protein ml−1, 0.3 ml enzyme volume, 30
min incubation time and 37°C temperature. It is evident from Fig. 1a that 3 mM putrescine is probably saturating since further increases in
substrate concentration (\3 mM) led to very
small changes in activity of the enzyme.
It is evident from Fig. 1b that the quantity of the product formed during the fixed reaction time (30 min) increased proportionately with the amount of enzyme supplied to the reaction up to 0.3 ml enzyme volume and beyond that volume it did not show any linearity.
Fig. 1c shows that the rate of product formation was linear with increasing reaction time up to 30 min. Further increase in incubation time did not increase the product formation linearly. It may be due to limitation of the substrate. Enzyme activity was linear up to 30 min incubation time.
Temperature affects the rate of enzyme
catalysed reaction. A rising temperature (Fig. 1d) increased the rate of reaction up to a certain limit (20 – 37°C) above which the enzyme activity was inhibited. However, higher temperature (\37°C) might lead to the denaturation of the enzyme which caused a decrease in catalytic activity.
3.3. Sensiti6ity of the present method
To examine the sensitivity of the present method for measuring amine oxidase activity, DAO was measured by the present H2O2– Ti method as
stan-dardized above (Fig. 2) and the activity was com-pared with the starch – iodide method under the assay conditions described by Smith [5], as well as under the assay conditions standardized for H2O2– Ti method reported here. It was observed
that DAO activity significantly increased (10 times) in terms of mM H2O2 produced/30 min if
measured under the present H2O2– Ti method over
the starch – iodide method. Furthermore, there was a significant improvement in DAO activity (about 2.5 times) over the method standardized by Smith [5], if measured by starch – iodide method under the assay conditions reported for H2O2– Ti method
instead of those reported by Smith. These results clearly reveal greater sensitivity of the H2O2– Ti
method than that of the starch – iodide method.
3.4. Effect of IBA (25 mM) on DAO/PAO
Table 4 shows that activity of both DAO and
PAO measured by the standardized H2O2– Ti
method in the hypocotyls of IBA (25 mM)-treated seedlings greatly increased over untreated controls in both treatment hours.
3.5. DAO/PAO in other plants
Table 5 shows that the H2O2– Ti method is
more sensitive and more reproducible than the starch – iodide method. Results show that activities of both DAO and PAO with the H2O2– Ti method
were more sensitive than with the starch – iodide method in the three separate plant samples. It has been also known that DAO activities are greater than PAO in all the plants studied with either method.
Results of the present enzyme kinetics with DAO/PAO confirm the general criteria of enzyme kinetics and hence the H2O2– Ti method is valid.
Acknowledgements
The authors are grateful to Dr D. Sengupta, Professor of Biochemistry, University of Calcutta for his valuable advice and suggestions for inter-preting the results in proper perspective.
References
[1] A.W. Galston, Polyamines as modulators of plant devel-opment, Bioscience 33 (1983) 382 – 388.
[2] T.A. Smith, Polyamines, Annu. Rev. Plant Physiol. 36 (1985) 117 – 143.
[3] B.I. Naik, R.G. Goswami, S.K. Srivastava, Rapid and sensitive colorimetric assay of amine oxidase, Annu. Biochem. 111 (1981) 146 – 148.
[4] B. Holmsted, L. Larsson, R. Tham, Further studies on spectrophotometric method for the of amine oxidase activity, Biochim. Biophys. Acta 48 (1961) 182 – 186. [5] T.A. Smith, Polyamine oxidase in higher plants,
Biochem. Biophys. Res. Commun. 41 (1970) 1452 – 1456. [6] T.A. Smith, Polyamine oxidation by enzymes from
Hordeum 6ulgare and Pisum sati6um seedlings,
Phyto-chemistry 13 (1974) 1075 – 1081.
[7] A. Rinaldi, G. Floris, A. Finazzi-Agro, Purification and properties of diamine oxidase fromEuphorbialatex, Eur. J. Biochem. 127 (1982) 417 – 422.
[9] C.H. Moncousin, T.H. Gaspar, Peroxidase as a marker for rooting improvement ofCynara scolymusL. cultured in vitro, Biochem. Physiol. Pflanzen 178 (1983) 263 – 271. [10] F.D. Snell, C.T. Snell, Colorimetric Method of Analysis IV AAA. Van Nostrand Reinhold Co., New York, 1971, pp. 26 – 27.
[11] A.K. Biswas, M.A. Choudhuri, Differential behaviour of the flag leaf of intact rice plant during ageing, Biochem. Physiol. Pflanz. 173 (1978) 220 – 228.
[12] M. Kar, D. Mishra, Catalase, peroxidase and
polyphe-nol oxidase activities during rice leaf senescence, Plant Physiol. 57 (1976) 315 – 319.
[13] N.G. Fick, C.O. Qualset, Genetic control of endosperm amylase activity. Gibberellin responses in standard height and short saturated wheat, Proc. Natl. Acad. Sci. USA 72 (1975) 892 – 895.
[14] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
.