Repression of cystathionine
g
-synthase in
Arabidopsis thaliana
produces partial methionine auxotrophy and developmental
abnormalities
Jungsup Kim, Thomas Leustek *
Biotechnology Center for Agriculture and the En6ironment,Rutgers Uni6ersity,Cook College,59Dudley Road,New Brunswick,
NJ08901-8520,USA
Received 2 July 1999; received in revised form 1 September 1999; accepted 1 September 1999
Abstract
Cystathionineg-synthase (CGS), a key enzyme in methionine biosynthesis, was repressed in transgenicArabidopsis thalianaby antisense expression of CGS RNA. CGS activity was reduced by 5 – 9-fold in the antisense plants resulting in severe growth stunting, morphological abnormalities and an inability to flower. Feeding the plants methionine (Met) or Met metabolites reversed the morphological effects of CGS repression. There was little change in the content of free Met andS-methylmethionine despite the need for exogenously applied Met for growth. The overall amino acid content was significantly increased. The CGS antisense transgene is inherited as a single recessive locus. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Antisense repression;Arabidopsis thaliana; Cystathionineg-synthase; Methionine biosynthesis
www.elsevier.com/locate/plantsci
1. Introduction
The sulfur containing amino acid methionine (Met) is a fundamental metabolite in plant cells. It is both a protein constituent and the precursor of
S-adenosyl-L-methionine (SAM) the primary
bio-logical methyl-group donor. Met is of equal im-portance to animals that lack the ability to synthesize this amino acid and must obtain it from their diet or from enteric bacteria. The nutritional value of some crops, legumes in particular, is
limited by low Met content [1]. Despite its bio-chemical and agronomic importance the regula-tion of Met synthesis in higher plants is not well understood.
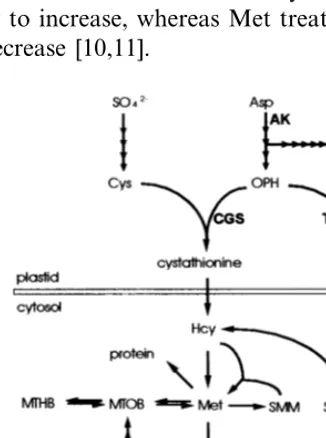
Met is a 4-carbon amino acid synthesized from independently derived components (Fig. 1). The sulfur atom is derived from Cys. The carbon skele-ton is derived from Asp as are the amino acids Lys and Thr. The immediate precursor of both Met and Thr is O-phosphohomoserine (OPH). The first Met-specific reaction is catalyzed by cys-tathionineg-synthase (CGS) which condenses Cys and OPH to form cystathionine. Next, cystathion-ine b-lyase carries out b-cleavage to form homo-cysteine (Hcy). Met is produced by transmethyl-ation of homocysteine. Aside from its incorpora-tion into proteins Met is the precursor of S -methylmethionine (SMM), a compound that is an intermediate in the synthesis of dimethylsulfonio-propionate (DMSP) in some angiosperms [2,3], and SAM, which is synthesized by Met adenosyl-transferase (SAM synthetase). The Met pathway
Abbre6iations:ACC, 1-aminocyclopropane-1-carboxylic acid; CGS, cystathionineg-synthase; DMS, dimethylsulfide; DMSP, dimethylsul-foniopropionate; Hcy, homocysteine; Kan®, kanamycin resistant;
MTA, 5-methylthioadenosine; MTHB, 4-methylthio-2-hydroxy bu-tyric acid; MTOB, 4-methylthio-2-oxobutanoic acid; OPH,O -phos-phohomoserine; PITC, phenylisothiocyanate; SAH, S
-adenosyl-L-homocysteine; SAM,S-adenosyl-L-methionine; SAT, serine acetyl-transferase; SMM,S-methylmethionine; TS, threonine synthase.
* Corresponding author. Tel.:+1-732-9320312, ext. 326; fax:+ 1-732-9328165, ext. 326.
E-mail address:[email protected] (T. Leustek)
enzymes are distributed between plastids and the cytosol [4]. Plastids contain all the Asp-family enzymes, the complete Cys pathway, CGS and cystathionineb-lyase. Met synthase and SAM syn-thetase are exclusively cytosolic as are the enzymes for SMM synthesis [5].
Met synthesis is regulated at multiple levels. A general mechanism for control of the Asp-family amino acids centers on feedback inhibition of Asp kinase (AK), the first enzyme of the Asp pathway, by Lys, Thr, and SAM [6]. Combined treatment with Lys and Thr is herbicidal because they re-press the Asp pathway blocking the synthesis of the carbon skeleton and causing Met starvation [7]. A Met-specific control mechanism centers on the competition between CGS and Thr synthase (TS) for their common substrate OPH. TS activity is stimulated by SAM and it has a much higher affinity for OPH than does CGS. Thus, it has been proposed that CGS may compete poorly for OPH when Met (hence SAM) is abundant [8,9]. By contrast, when Met is limiting and TS less active CGS has a greater ability to compete for OPH. There is also evidence that when Met is limiting CGS expression is induced. For example, com-bined treatment with Thr and Lys causes CGS activity to increase, whereas Met treatment causes it to decrease [10,11].
With the recent cloning of the CGS cDNA from
Arabidopsis thaliana [12] it became possible to study its function in transgenic plants. Repression of CGS activity was found to limit the ability of
A. thaliana to grow autonomously without exoge-nous application of Met. The CGS-repressed plants show an abnormal morphology that is in-herited as a recessive trait.
2. Materials and methods
2.1. Preparation of antibodies against recombinant CGS
Recombinant A. thaliana CGS was synthesized as an S-TAG and hexa-His fusion protein ex-pressed from vector pET30c (Novagen). A 1.4 kbp
XhoI fragment from the CGS cDNA [12] (Gen-Bank Accession Number U43709) was cloned into pET30c to produce the pET-CGS construct which was used to transform Escherichia coli strain BL21(DE3)pLysS (Novagen). Transformants were selected on LB medium with 40 mg/ml chloram-phenicol and 30 mg/ml kanamycin. The culture was grown in liquid LB medium with the antibi-otics at 37°C until an OD at 600 nm of approxi-mately 0.3 was achieved. Then 2.0 mM IPTG was added and the culture incubated further for 5 h at 30°C. The recombinant enzyme, purified by Ni-affinity chromatography, as described by the pET protocol from Novagen, showed a characteristic absorption peak at 415 nm associated with pyri-doxal phosphate enzymes. The ratio between ab-sorbency maxima at 282 and 415 nm was 3.88, identical to native CGS from spinach [13]. CGS activity was measured as described by Ravanel et al. [13] using O-succinylhomoserine. The pure en-zyme showed a specific activity of approximately 2.1 mmol cystathionine formed/min/mg protein at 24°C, pH 7.5. Although plant CGS uses OPH as the physiological substrate it can also use a variety of homoserine esters including O -succinylhomos-erine [13] which is commercially available from Sigma.
A New Zealand White rabbit was immunized subcutaneously with purified recombinant CGS protein (1 mg) in Freund’s Complete Adjuvant. The rabbit was boosted with 1 mg CGS in saline solution at intervals of 1 month. Serum samples were taken 7 days after boost immunization. The Fig. 1. Met metabolism and regulation in plants. The
sample taken after the third boost was used di-rectly on immunoblots.
2.2. Construction of transgenic plants
Antisense repression was used to reduce CGS activity in A. thaliana. A 1.1 kbp SalI fragment from the 5%end of the CGS cDNA [12] was cloned into pFF19 [14] placing the CGS gene in the antisense orientation with respect to the 35S pro-moter. The expression cassette was subcloned into the transformation vector pBI101 (Clonetech) as an approximately 2.0 kbp HindIII –EcoRI frag-ment to produce the CGS[−] construct. The CGS[−] construct was used to transform
Agrobacterium tumefaciens strain pGV2260, and thenA. thaliana (C24) by vacuum infiltration [15]. KanR plants were selected on medium with 50 mg/ml kanamycin.
2.3. Analysis of CGS[−] plants
All soil grown plants were raised in a 23°C growth chamber with a light intensity of 100 mE/
m2 per second and a photoperiod of 14 h light
followed 10 h of darkness. The plants were wa-tered with one-quarter strength Peters™ water-sol-uble (20:20:20) fertilizer (Grace-Sierra, Milpitas, CA) prepared in distilled water. Plants requiring exogenously supplied Met for growth in soil were, in addition, watered daily in the root zone with 1 ml 0.2 mM Met. The nutritional requirements of the CGS[−] plants were tested with axenically grown plants raised on agar solidified MS medium supplemented as described in the figure or table legends. The plants were incubated in a 21°C growth chamber with a light intensity of 90 mE/
m2/s, 13 h light/11 h dark period. Transgenic
plants were confirmed to carry the CGS[−] con-struct by a PCR method [16] using a 35S promoter primer (5% -TATCTCCACTGACGTAAGGGAT-GA-3%) and a CGS specific primer (5% -ATGGC-ATCTGGGATGTGTGC-3%) and by genomic DNA blotting using the CGS cDNA as a probe [17].
The content of the CGS protein was determined by immunoblotting carried out as described in Wang et al. [18]. Soluble protein extracts were prepared from the entire shoot of 40-day-old plants. Total protein (40 mg) was analyzed by SDS-PAGE in a gel containing 10% (w/v)
acry-lamide. The protein concentration was measured using the Bradford dye-binding assay with BSA as a standard (BioRad). Antisera against CGS or A.
thaliana serine acetyltransferase (SAT) [19] were used at a dilution of 1:2000. The SAT antibody served to control for protein loading. A secondary antibody was horseradish peroxidase-linked goat anti-rabbit diluted 1:8000 and immune complexes were detected with the Renaissance™ Kit (Dupont NEN).
Soluble amino acids were measured in the shoot of 19- and 40-day-old CGS[−] plants and mto1 plants [20] (provided by Dr Satoshi Naito, Hok-kaido University). Amino acids were analyzed by HPLC after alkylation with phenylisothiocyanate (PITC) [21]. Plant tissues were extracted and the amino acids purified by chromatography on AG 50W-8 [20]. After binding to the ion exchange resin and then washing, the column was eluted with 2 N NH4OH. The eluate was dried by
evapo-ration under a stream of nitrogen and the residue dissolved in a solution of 7:1:1:1 (v/v/v/v) ethanol – water – triethanolamine – PITC. Met and Thr were specifically measured. Their recoveries were estimated by comparing the amount of each amino acid from a typical tissue sample with that in a spiked tissue sample. The recoveries were, Thr approximately 81% and Met approximately 88%. Total amino acid content was calculated as the sum of all peak areas from a chromatograph divided by the fresh weight of the plant sample. SMM was measured as dimethylsulfide (DMS) released from fresh plant samples after alkaline treatment at 90°C. The assay was developed for algal samples [22] and was optimized here for A.
Fig. 2. Release of DMS from dimethyl sulfonium compounds and A. thaliana. DMSP (100 nmol) (), SMM (100 nmol)
(, ) or various amounts of A. thaliana tissue ( ) were
added to a vial containing 400ml 1 M NaOH. (A) Incubation was for the specified time at 23°C (,) or 90°C (, ). (B) Incubation was for 3 h at 90°C ( ).
The SMM assay is extremely simple and gives reliable measurements. Release of DMS from SMM requires strongly alkaline conditions and incubation at 90°C for about 2 h (Fig. 2A) [22]. GC analysis of the volatilized product from pure SMM subjected to these conditions shows a single peak of DMS with a retention time of 2.5 min. A.
thaliana leaf samples produce four volatile prod-ucts. The peak that migrates with DMS is pro-duced at a rate identical to that from pure SMM (Fig. 2A). Moreover, the DMS peak increases selectively when more sample is added (Fig. 2B) or pure SMM is added with the leaf sample. The three additional peaks have retention times of 0.77, 0.95 and 2.2 min. They are released from the leaf sample only after alkaline incubation at 90°C, but they appear more rapidly than does SMM-derived DMS or the peak area does not correlate with the amount of tissue added to the assay. Therefore, they are probably unrelated to SMM.
3. Results
Six KanR plants were isolated from a
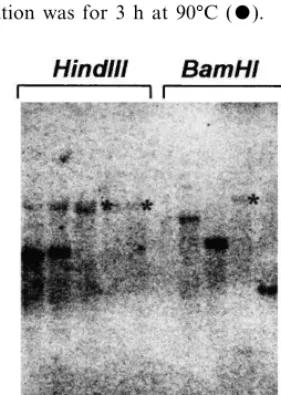
transfor-mation with the CGS[−] vector. All were confi-rmed to carry the CGS[−] transgene construct using a tissue PCR method (not shown). Four of the transformants, analyzed by genomic DNA blotting with the CGS cDNA as a probe showed a pattern of hybridization indicative of having arisen through independent single integration events. The transformants were analyzed with HindIII or
BamHI, enzymes that produce, respectively, either approximately 5 or approximately 15 kbp hy-bridizing fragments in wild type A. thaliana. The endogenous CGS fragment was observed in each of the transformants, but they showed in addition, hybridization to a second fragment of variable length, depending upon the transformant, corre-sponding to the transgene construct (Fig. 3).
Analysis of the T2 revealed that without Met feeding all the transgenic plants (KanR plants)
appeared normal up to 10 days after germination. However, after transfer to soil one-third developed severe growth stunting and where unable to repro-duce while the other two thirds developed nor-mally and produced viable progeny. Abnormal and normal siblings are shown in Fig. 4A, com-pare plant A1 with A2. In addition to growth stunting the abnormal plants developed a cluster of apical shoots and the oldest leaves, which ap-Fig. 3. Southern blot analysis of CGS[−] lines. Total DNA
was digested with HindIII (lanes 1 – 5) or BamHI (lanes 6 – 10). The plant samples were: 415-5 (lanes 1 and 7), 415-7 (lanes 2 and 8), 415-11 (lanes 3 and 9), 415-16 (lanes 4 and 10), untransformed A. thalianaecotype C24 (lanes 5 and 6). The blot was probed with a 1.1 kbp fragment from the 5%end
of the CGS1 cDNA. Faint bands are highlighted with aster-isks (*). The approximate 15 kbp fragment corresponding to the endogenous CGS gene was probably not detected due to inefficient transfer from the gel to the membrane. All the large fragments in this experiment show weak hybridization for the same reason.
peared normal early on, became thickened, curled and in some of the CGS[−] lines accumulated a red-brown pigment in the petioles. The changes in morphology became clearly evident within 40 days after germination. The abnormal plants were un-able to produce flowers and eventually died with-out reproducing (Fig. 4B, observe plants labeled B1). However, when watered with a solution of Met their growth was restored sufficiently to allow
them to flower and to set viable seed. Plant A1 was photographed before Met-feeding (Fig. 4A). The stimulation of growth is evident in the same plant 16 days after initiating Met-feeding (Fig. 4C, plant C1). Visible signs of growth became evident within 48 h after initiating Met-feeding. The pro-liferation of apical shoots observed in abnormal CGS[−] plants became clearly evident after Met was fed and each of the shoots developed into an independent inflorescence (Fig. 4C, plant C1).
The progenies derived from abnormal CGS[−] plants were all KanRand developed the abnormal
phenotype. By contrast, the morphologically nor-mal KanR plants produced KanR progeny at a
ratio of approximately 3:1. The KanRplants
segre-gated into normal and abnormal phenotypes at a ratio of 2:1 (Table 1). The segregation results indicate that the antisense transgene behaves as a single recessive locus that produces abnormality when in the homozygous state rather than the hemizygous condition. Similar results were ob-tained with all six independently isolated CGS[−] transgenic lines. The abnormal morphology has been stable over six generations.
The developmental progression of plants from abnormal homozygous CGS[−] lines was studied by recording the time of emergence of new leaves. Homozygous CGS[−] plants grew like wild-type and hemizygous siblings until 24 – 28 days after germination after which new leaves stopped emerging and the single apical shoot proliferated into a mass of apical shoots. The growth of the homozygous CGS[−] plants arrested prior to conversion to reproductive growth.
Testing a variety of sulfur compounds for the ability to suppress the abnormal phenotype pin-pointed the lesion in the CGS[−] lines. This ex-periment was carried out on agar medium under axenic conditions in order to eliminate the possi-bility of interference from or metabolism of the supplements by microorganisms. After growth on unsupplemented agar medium the abnormal phe-notype was clearly visible after 30 days (Fig. 5A). The phenotype differs slightly from plants grown on soil in that the plants become chlorotic and the apical shoot remains very small (Fig. 5A). The phenotype is suppressed by Met feeding (Fig. 5, compare A and B) similar to the results with soil grown plants. Cystathionine and Hcy also sup-pressed the abnormal phenotype but neither Cys or glutathione, a Cys-containing metabolite, were able to restore the normal phenotype (Table 2). Table 1
Inheritance of the abnormal phenotype in CGS[−] plantsa
KanR Phenotype
415-11c 137 1.00
65 45 20
415-16b 0.31
65 48 17
415-18b 0.26
aSegregation analysis was performed with self-pollinated
CGS[−] plants in the T3 generation. KanR plants were
selected, transferred to soil, and the plants scored for mor-phology after 45 days. The total number of KanR and the
number segregating into normal and abnormal plants are given.
bSeeds were from morphologically normal KanR plants. cSeeds were from abnormal KanR plants.
Table 2
Chemical supplements suppress the abnormal morphology of CGS[−] plantsa
D,L-homocysteine
A 4-Methylthio-2-hydroxy butyric acid B
(MTHB)
aCGS[−] plants were germinated and axenically grown for
Table 3
CGS activity inA.thalianaa
Specific activity (nmol/min per mg) Line
A.thaliana 0.33090.060
0.04090.010 415-5
0.03690.009 415-7
415-11 0.07390.011
aCGS enzyme activity was measured in the shoot of
40-day-old plants grown in soil. The transgenic lines analyzed were homozygous T3 plants. The averages9S.D. of three independent experiments are shown.
2) that plants can convert to Met without the aid of CGS (see Fig. 1). MTA is an intermediate in the Yang cycle that functions in recycling of Met from SAM [4]. MTHB and MTOB are hydroxy and oxo acids that can be converted to Met via ubiquitous amino acid transaminases and dehydrogenases [3]. Yet another indication that low CGS activity may account for the growth abnormalities of CGS[−] plants is that treatment of wild type A. thaliana
with the CGS inhibitor, propargylglycine (PAG) [10] produces similar growth abnormalities (Fig. 4D).
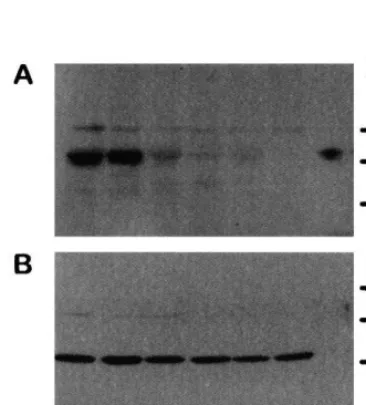
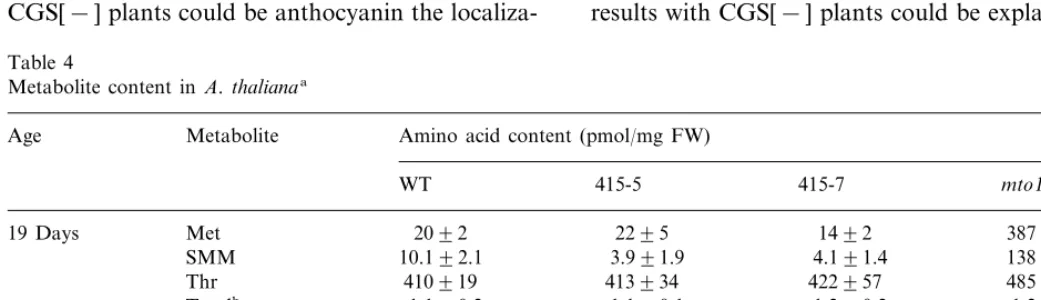
CGS activity is repressed in abnormal CGS[−] lines (Table 3). Compared with wild-type, the CGS[−] lines 415-5, 415-7 and 415-11 had 5 – 9-fold lower CGS activity and a comparably lower amount of CGS protein as measured by im-munoblotting (Fig. 6). Analysis of the amino acid content in the transgenic lines revealed that the level of free Met and SMM is similar to wild type at 19 and 40 days after germination (Table 4). The level of Thr and total amino acids was increased 3 – 5-fold in 40-day-old but not in 19-day-old plants. Therefore, the development of abnormality is correlated with the increase in total amino acids. However, Thr is not specifically increased. By comparison the mto1 mutant of A. thaliana shows 19 – fold higher Met and 14-fold higher SMM in 19-day-old plants but no higher level of total amino acids. By 40 days there was no greater Met than in the wild type. Similar results with mto1, an ethionine-resistant mutant, which accumulates Met in the early vegetative stage, have been previ-ously published [20].
4. Discussion
Gene families encode many amino acid biosyn-thetic enzymes in plants. CGS is unusual in that it exists as a single copy gene in A. thaliana [12]. Being a single copy gene there was a greater likelihood that its expression could be effectively repressed using an antisense RNA method. In-deed, all of the six independently isolated trans-genic A. thaliana lines transformed with the CGS[−] construct showed pronounced affects on growth and a 5 – 9-fold reduction in the level of CGS and CGS enzyme activity. Application of Met or a diverse collection of Met metabolites to the plants reversed the abnormal phenotype. How-Fig. 6. Immunoblot of CGS[−] plants. CGS (A) and SAT
(B) protein was measured in wild typeA.thaliana(C24), lanes 1 and 2; and in abnormal CGS[−] plants from line 415-5, lanes 3 and 4; or 415-7, lanes 5 and 6. Lane 7 contains 1 ng of pure recombinant CGS. A lower level of CGS protein was detected in CGS[−] line 415-11 (not shown). The faint band migrating above CGS is not consistently observed on blots, therefore it is not thought to be related to CGS. Immunoreac-tion with SAT antibodies was used as a protein loading control.
ever, Cys and glutathione, two sulfur-containing metabolites that must be acted upon by CGS in order to be converted to Met, were unable to reverse the abnormalities. Both Cys and glu-tathione produce physiological affects when sup-plied to A. thaliana [24], therefore, it is unlikely that the antisense plants were unable to import these compounds. Further evidence implicating the reduction of CGS activity as being responsible for the growth abnormalities is that application of the CGS inhibitor PAG to wild-type A. thaliana
produces similar growth defects. Just as with the CGS[−] plants the morphological changes associ-ated with PAG treatment could be reversed by exogenous application of Met [25]. Nearly identi-cal morphologiidenti-cal and developmental abnormali-ties are associated with repression of SAM synthetase in A. thaliana [26], which is accompa-nied by the massive accumulation of free Met in the leaves [27]. Thus, it is unlikely that the pheno-type of CGS[−] plants is due solely to a defi-ciency of Met for protein synthesis. A defidefi-ciency in SAM may also be partly responsible. SAM is necessary for a wide range of processes including the methylation of DNA and lignin precursors, ethylene production and polyamine synthesis. In-terestingly, some of the abnormalities observed in the CGS[−] lines, such as proliferation of the apical shoot (loss of apical dominance) and an inability to progress into the reproductive growth phase have been observed in A. thaliana plants in which DNA methylation was inhibited [28]. Al-though the red-brown pigment produced by CGS[−] plants could be anthocyanin the
localiza-tion near the vascular bundles is reminiscent of the brown midrib mutant of maize (bm3) which accu-mulates unmethylated lignin precursors [29]. Taken together the experimental results and simi-larities with other transgenic and mutant plant models suggest that the CGS[−] plants are unable to produce sufficient Met for growth beyond 24 – 28 days after germination.
Although the CGS[−] plants require exogenous application of Met to complete their life cycle the level of free Met and its metabolite SMM were found to be similar in wild type and the transgenic antisense lines. The expectation was that Met and SMM levels would be lower in the CGS antisense plants. To date, this counterintuitive result has not been satisfactorily explained, although it is un-likely to be the result of faulty analytical methods. The Met and SMM assay procedures were tested by analyzing themto1 mutant ofA.thalianathat is known to accumulate Met in young plants but not in older plants [20]. Our measurements were con-sistent with and confirmed the original study. Thus, it is more likely that the free Met and SMM measurements accurately reflect the condition in CGS[−] plants. There are several potential expla-nations for why the level of free Met and SMM are similar in wild type and CGS[−] plants. Al-though standard and widely accepted methods were employed for measurement of Met, perhaps the analytical results must be more critically inter-preted. For example, it is widely assumed that the measured level of an amino acid in plants repre-sents the metabolically active pool. However, the results with CGS[−] plants could be explained by
Table 4
Metabolite content in A.thalianaa
Age Metabolite Amino acid content (pmol/mg FW)
WT 415-5 415-7 mto1
Met 2092
19 Days 2295 1492 387945
10.192.1
SMM 3.991.9 4.191.4 138912 Thr 410919 413934 422957 485954 Totalb 1.190.2 1.190.1 1.290.2 1.290.2
Met 2098
40 Days 3599 4596 2997
SMM 11.391.7 10.192.2 22.992.3 71.8919.7 452962 23639515
Thr 31819791 481919
1.290.2 4.590.3 6.091.1
Totalb 1.390.2
aSoluble amino acids were measured in the shoot of 19 and 40-day-old plants. The transgenic lines analyzed were homozygous
T3 generation. The averages9S.D. of three independent experiments are shown.
the existence of a metabolically inactive pool that is not in equilibrium with the metabolically active pool. Another trivial explanation relates to the very small size of the free Met pool in plants compared with other free amino acids and with the bound Met in proteins. Thus, the analytical results could easily have been skewed by relatively minor amounts of protein hydrolysis during sam-ple processing.
Young antisense plants appear to be unaffected by the CGS[−] transgene whereas growth abnor-malities appear abruptly 24 – 28 days after germi-nation. This pattern suggests that young antisense plants produce sufficient Met for growth. How-ever, at a critical stage in development the rate of Met synthesis becomes insufficient. It is notewor-thy that the transition to abnormal growth occurs at precisely the time when wild type A. thaliana
and hemizygous antisense plants begin the transi-tion to reproductive growth. Perhaps a new and greater demand for Met develops at this time with which the CGS[−] plants are unable to cope. After the development of abnormality the anti-sense plants show an overall increase in amino acids, indicating that general amino acid metabolism is effected by the CGS[−] transgene. Little is known about the global regulation of amino acids in plants although a similar general increase in amino acids was observed in transgenic plants repressed for the branched chain amino acid enzyme acetolactate synthase (ALS) [30]. Like the CGS[−] plants, the ALS antisense plants show severe growth retardation and a wide range of morphological deformations. Therefore, it ap-pears that disruption of single amino acids can have pleiotropic effects on the homeostasis of all amino acids.
A decrease in CGS activity of as little as 5-fold was correlated with gross changes in development and morphology observed in the CGS[−] line 415-11. This result is an indication that the metabolic flux coefficient for CGS is quite high as is usually associated with a metabolic regulation point [31]. By comparison, a 7-fold reduction in ALS, a known control point in the branched chain amino acid pathway, produced severe growth stunting [30]. The early studies on Met biosynthe-sis in Lemna were the first to indicate that CGS is likely a key rate limiting step in Met biosynthesis [10,25]. The results with the CGS[−] plants are in agreement with this hypothesis and indicate that
similar processes operate for Met synthesis in A.
thaliana, a more complex vascular plant. Ho¨fgen et al. [30] proposed that antisense RNA experi-ments may be useful for the identification of po-tential enzyme targets for herbicide discovery, and they were able to demonstrate the concept by creating antisense plants with repressed ALS, a known target for the commercial sulfonylurea class of herbicides. The results reported here sug-gest that CGS may be a good potential target for herbicide discovery.
The analysis of CGS[−] lines raises another potentially significant finding. Up to now a dogma of the sulfur metabolism field has been that re-duced sulfur is transported throughout plants as glutathione [32]. However, application of Met or Met-derived metabolites to the roots of CGS[−] plants reverses the growth abnormalities in the plant shoot even when measures were taken to prevent Met from contacting the leaves. From this we can conclude that A. thaliana is capable of vascular transport of Met or a Met metabolite. The compound cannot be glutathione or Cys since both would require CGS for conversion to Met once transported to the shoot. This result suggests that Met or one of its metabolites has the poten-tial to play a heretofore unsuspected role in the transport of reduced sulfur. One candidate molecule is SMM, which has recently been shown to be phloem transported in vascular plants [33].
Acknowledgements
This work was supported by the National Sci-ence Foundation (Grant cMCB-9728661). We wish to thank Dr Satoshi Naito for providing
mto1 seed and Dr Andrew Hanson for many helpful discussions.
References
[1] S.W.J. Bright, P.R. Shewry, Improvement of protein quality in cereals, Crit. Rev. Plant Sci. 1 (1983) 49 – 93. [2] J. Giovanellli, S.H. Mudd, A.H. Datko, Sulfur amino
acids in plants, in: B.J. Miflin (Ed.), The Biochemistry of Plants, vol. 5, Academic Press, New York, 1980, pp. 453 – 505.
[4] J.W. Anderson, Sulfur metabolism in plants, in: B.J. Miflin (Ed.), The Biochemistry of Plants, vol. 16, Aca-demic Press, New York, 1990, pp. 454 – 500.
[5] C. Trossat, K.D. Nolte, A.D. Hanson, Evidence that the pathway of dimethylsulfoniopropionate biosynthesis be-gins in the cytosol and ends in the chloroplast, Plant Physiol. 111 (1996) 965 – 973.
[6] G. Galili, Regulation of lysine and threonine synthesis, Plant Cell 7 (1995) 899 – 906.
[7] C.E. Green, R.L. Phillips, Potential selection system for mutants with increased lysine, threonine and methionine in cereal crops, Crop Sci. 14 (1974) 827 – 830.
[8] G. Curien, R. Dumas, S. Ravanel, R. Douce, Character-ization of an Arabidopsis thaliana cDNA encoding an
S-adenosylmethionine-sensitive threonine synthase, FEBS Lett. 390 (1996) 85 – 90.
[9] S. Ravanel, B. Gakie`re, D. Job, R. Douce, The specific features of methionine biosynthesis and metabolism in plants, Proc. Natl. Acad. Sci. USA 95 (1998) 7805 – 7812. [10] G.A. Thompson, A.H. Datko, S.H. Mudd, J. Giovanelli, Methionine biosynthesis inLemna. Studies on the regula-tion of cystathionine g-synthase, O-phosphohomoserine sulfhydrase, and O-acetylserine sulfhydrase, Plant Phys-iol. 69 (1982) 1077 – 1083.
[11] S.E. Rognes, R.M. Wallsgrove, J.S.H. Kueh, S.W.J. Bright, Effects of exogenous amino acids on growth and activity of four aspartate pathway enzymes in barley, Plant Sci. 43 (1986) 45 – 50.
[12] J. Kim, T. Leustek, Cloning and analysis of the gene for cystathionineg-synthase fromArabidopsis thaliana, Plant Mol. Biol. 36 (1996) 1117 – 11124.
[13] S. Ravanel, M. Droux, R. Douce, Methionine biosynthe-sis in higher plants. I. Purification and characterization of cystathionine g-synthase from spinach chloroplasts, Arch. Biochem. Biophys. 316 (1995) 572 – 584.
[14] M.C.P. Timmermans, P. Maliga, J. Vieira, J. Messing, The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants, J. Biotechnol. 14 (1990) 333 – 344.
[15] N. Bechtold, J. Ellis, J.G. Pelletier,In Planta Agrobac
-terium mediated gene transfer by infiltration of adult
Arabidopsis plants, CR Acad. Sci. (Paris) 316 (1993) 1194 – 1199.
[16] M.W. Lassner, P. Peterson, J.I. Yoder, Simultaneous amplification of multiple DNA fragments by polymerase chain reaction in the analysis of transgenic plants and their progeny, Plant Mol. Biol. Rep. 7 (1989) 116 – 128. [17] P.A. Sabelli, P.R. Shewry, Gene characterization by
Southern analysis, in: H. Jones (Ed.), Plant Gene Trans-fer and Expression Protocols, Methods in Molecular Biology™, vol. 49, Humana, Clifton, NJ, 1995, pp. 161 – 180.
[18] H. Wang, M. Goffreda, T. Leustek, Characteristics of an Hsp70 homolog localized in higher plant chloroplasts similar to DnaK, the Hsp70 of prokaryotes, Plant Phys-iol. 102 (1993) 843 – 850.
[19] M. Murillo, R. Foglia, A. Diller, S. Lee, T. Leustek, Serine acetyltransferase from Arabidopsis thaliana can
functionally complement the cysteine requirement of a
cysE mutant strain of Escherichia coli, Cell. Mol. Biol. Res. 41 (1995) 425 – 433.
[20] K. Inaba, T. Fujiwara, H. Hayashi, M. Chino, Y. Komeda, S. Naito, Isolation of anArabidopsis thaliana
mutant,mto1, that overaccumulates soluble methionine, Plant Physiol. 104 (1994) 881 – 887.
[21] V. Fierabracci, P. Masiello, M. Novelli, E. Bergamini, Application of amino acid analysis by high-performance liquid chromatography with phenyl isothiocyanate derivatization to the rapid determination of free amino acids in biological samples, J. Chromatogr. 570 (1991) 285 – 291.
[22] R.H. White, Analysis of dimethyl sulfonium compounds in marine algae, J. Marine Res. 40 (1982) 529 – 536. [23] A.A. Bezzubov, N.N. Gessler,
Gazokhromatografiches-koe opredelenieS-metilmetionina (vitamin U) v rasteni-iakh, Prikl. Biokhim. Mikrobiol. 13 (1977) 301 – 309. [24] S. Lee, T. Leustek, The effect of cadmium on sulfate
assimilation enzymes in Brassica juncea, Plant Sci. 141 (1999) 201 – 207.
[25] A.H. Datko, S.H. Mudd, Methionine biosynthesis in
Lemna: inhibitor studies, Plant Physiol. 69 (1982) 1070 – 1076.
[26] F. de Carvalho, W. Boerjan, I. Ingelbrecht, A. Depicker, D. Inze´, M. Van Montagu, Post-transcriptional gene silencing in transgenic plants, NATO-ASI Ser. H 81 (1994) (1994) 437 – 452.
[27] W. Boerjan, G. Bauw, M. Van Montagu, D. Inze´, Dis-tinct phenotypes generated by overexpression and sup-pression of S-adenosyl-L-methionine synthetase reveal developmental patterns of gene silencing in tobacco, Plant Cell 6 (1994) 1401 – 1414.
[28] M.J. Ronemus, M. Galbiati, C. Ticknor, J. Chen, S.L. Dellaporta, Demethylation-induced developmental pleitropy inArabidopsis, Science 273 (1996) 654 – 657. [29] F. Vignols, J. Rigau, M.A. Torres, M. Capellades, P.
Puigdomenech, The brown midrib3 (bm3) mutation in maize occurs in the gene encoding acid O -methyltrans-ferase, Plant Cell 7 (1995) 407 – 416.
[30] R. Ho¨fgen, B. Laber, I. Schu¨ttke, A.-K. Klonus, W. Steber, H.-D. Pohlenz, Repression of acetolactate syn-thase activity through antisense inhibition. Molecular and biochemical analysis of transgenic potato (Solanum tuberosum L. c.v. De´sire´e) plants, Plant Physiol. 107 (1995) 469 – 477.
[31] M. Stitt, U. Sonnewald, Regulation of metabolism in transgenic plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 (1995) 341 – 368.
[32] H. Rennenberg, A. Polle, N. Martini, B. Thoene, Inter-action of sulfate and glutathione transport in cultured tobacco cells, Planta 176 (1988) 68 – 74.
[33] F. Bourgis, S. Roje, M.L. Nuccio et al., S -Methylme-thionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase, Plant Cell 11 (1999) 1485 – 1498.
![Fig. 4. Morphology of plants with repressed levels of CGS. (A), siblings from CGS[−] line 415-7 (T2 generation) showingabnormal (A1) and normal (A2) morphology](https://thumb-ap.123doks.com/thumbv2/123dok/1035219.929004/5.612.42.512.29.613/morphology-plants-repressed-levels-siblings-generation-showingabnormal-morphology.webp)