www.elsevier.com / locate / bres
Interactive report
L-type calcium channels modulate glutamate-driven bursting activity in
1the nucleus accumbens in vivo
a ,1 b ,
*
Donald C. Cooper
, Francis J. White
a
Department of Neuroscience, Finch University of Health Sciences, The Chicago Medical School, 3333 Green Bay Rd, North Chicago, IL 60064,
USA
b
Department of Cellular and Molecular Pharmacology, Finch University of Health Sciences /The Chicago Medical School, 3333 Green Bay Rd,
North Chicago, IL 60064, USA Accepted 24 August 2000
Abstract
The majority of adult nucleus accumbens medium spiny neurons exhibit a bistable membrane potential that fluctuates between a relatively hyperpolarized (Down) state (average5276 mV) and a less hyperpolarized (Up) state (average5–60 mV) near firing threshold. During in vivo extracellular recordings from nucleus accumbens neurons, we used microiontophoresis to apply glutamate and selected neurons that fired in bursting patterns reflecting a subthreshold bistable membrane potential. The average frequency of bursts events was 0.85 Hz. The average burst duration was 39263.5 ms, with an average of 13.4 spikes and an average spike frequency of 30.663.1 Hz per burst. To determine the involvement of the L-type calcium channel in the bursting pattern, we applied the benzothiazepine L-type calcium channel blocker, diltiazem. Diltiazem rapidly (,2 min) and reversibly decreased the burst duration by 29% and the frequency of spikes within a burst by 30% without changing the overall burst event frequency. The results provide the first in vivo electrophysiological evidence implicating an L-type calcium channel that modulates glutamate-induced burst firing of nucleus accumbens neurons. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Postsynaptic mechanisms
Keywords: Nucleus accumbens; L-type calcium channels; Glutamate; Microiontophoresis; In vivo extracellular recording
1. Introduction sion [22]. While acute cocaine is capable of producing
short-lived, but significant changes in the extracellular The nucleus accumbens (NAc) is thought to be an firing pattern in self-administering rats, withdrawal from important interface between limbic and motor systems [19] cocaine has been shown in both animals and humans to and has been implicated in the reinforcing properties of produce long-lasting neuroadaptations [11]. Some of these drugs of abuse [24]. As in the dorsal striatum, the majority neuroadaptations profoundly reduce the ability of the of neurons within the NAc are GABAergic medium spiny neurons to fire action potentials and therefore, potentially projection neurons. These neurons normally fire at very disrupt normal information processing [31]. To further our slow rates (,0.1 Hz), but during cocaine self-administra- understanding of how the NAc processes information in tion, a subpopulation (30%) become more excitable and normal and drug-altered conditions, it is important to study increase their firing rate time-locked to cocaine self-infu- the factors that influence the generation of action potentials
by NAc neurons.
The major factors that determine NAc neuronal firing
1
Published on the World Wide Web on 7 September 2000. patterns are presynaptic glutamatergic innervation, post-*Corresponding author. Tel.: 11-847-578-3270; fax: 11-847-578- synaptic glutamatergic receptor subtypes (non-NMDA and 3268.
NMDA) and activation of postsynaptic voltage-sensitive
E-mail address: [email protected] (F.J. White).
1 ion channels. Glutamatergic, excitatory projections arising
Present address. Department of Neurobiology, Northwestern University,
Evanston, IL 60201, USA. from the thalamus, prefrontal cortex, ventral subiculum
and basolateral amygdala converge on the NAc neurons consequently decrease the duration of the bursts and the and elicit large membrane potential fluctuations between burst spike frequency without changing the frequency of two steady-state (bistable) membrane potentials, a hy- the burst events.
perpolarized Down state (¯275 mV) and a depolarized Up
(¯260 mV) state [20,8]. The origins of the in vivo
bistable membrane potential in striatal medium spiny 2. Materials and methods neurons are absolutely dependent on temporally
con-vergent synaptic input [28]. This input establishes a 2.1. Animals ‘network’ that drives medium spiny neurons to depolarize
with a frequency of about 0.8 Hz into an Up state for 80 to Male Sprague–Dawley rats (Harlan, Indianapolis, IN) 1000 ms during which burst firing activity is prominent weighing 200–250 g were group housed with food and [20,28]. Within the NAc, initiation of the Up state is water available ad libitum. The colony room was main-thought to be gated by excitatory inputs from the hip- tained at constant temperature and humidity with a 12 h pocampal formation carried by the fimbria / fornix [20], but light / dark cycle. Rats were anesthetized with chloral as in striatal neurons, the Up state is likely maintained by hydrate (400 mg / kg, i.p.), mounted in a stereotaxic postsynaptic voltage-sensitive potassium and calcium apparatus, and prepared for recording as detailed
previous-21 21
(Ca ) currents [28,3]. It is possible that an L-type Ca ly [15]. Body temperature was maintained at 378C with a current modulates the duration of the Up state because it is thermostatically controlled heating pad. A 25-gauge hypo-activated at membrane potentials (¯255 mV) near the dermic needle was inserted into the lateral tail vein for
spike threshold [2,6] and therefore it is likely to influence administration of supplemental anesthetic. The scalp was
burst firing in vivo. removed and burr holes were drilled through the skull
During an in vitro experiment designed to mimic in vivo overlying the NAc [21]. All experiments were carried out
21
conditions in the striatum, the L-type Ca channel in accordance with the NIH guidelines for care and use of activator Bay K 8644 was shown to prolong a plateau laboratory animals.
potential in response to a brief current depolarizations [12].
21
In brain slices, L-type Ca channel blockers decrease 2.2. Recording procedure excitatory postsynaptic potentials in striatal neurons, most
likely by limiting depolarization and decreasing removal of Intracellular current–clamp recordings were performed
21
Mg -dependent block of NMDA channel [9]. Under in using 1 mm o.d. borosilicate electrodes (Sutter Inst.) filled vivo conditions, medium spiny neurons frequently enter with 2 M K-Acetate and 2% fast green dye, pulled on a the Up state for long durations in the voltage range where horizontal Flaming / Brown puller (Sutter Instruments) to
21 21
L-type Ca channels activate. Accordingly, L-type Ca between 70 and 130 MV. Voltage was amplified with a channels could increase voltage-dependent removal of SEC-05L amplifier in bridge mode (ALA Instruments).
21
Mg from the NMDA channel, thereby potentiating Extracellular recordings were made using the center glutamatergic input. Although in vitro experiments suggest barrel of a five barrel glass microelectrode assembly (¯7
21
a role for an L-type Ca conductance in modulation of mm in diameter) which was filled with 2 M NaCl saturated glutamate-induced excitation within the NAc and striatum, with Fast Green dye (impedance ¯1–2 MV). One side
this has not been verified in vivo. To characterize the barrel was filled with 2 M NaCl and used for automatic
21
influence of L-type Ca channels on the neuronal ex- current-balancing and NaCl control tests. The other three citability within the NAc in vivo, we performed extracellu- side barrels were filled with either l-glutamic acid mono-lar recordings from bistable neurons driven to fire in sodium salt (100 mM, in 50 mM NaCl, pH 8) or the
21
discrete bursts by microiontophoretic application of gluta- selective L-type Ca channel blocker, diltiazem (5 mM,
mate. in 145 mM NaCl, pH 4). Glutamate was ejected as an
For these experiments, we recorded only from NAc anion and diltiazem was ejected as a cation. Retaining neurons that responded to glutamate (30 s) with discrete 40 currents were maintained between 8 and 20 nA during to 1000 ms burst durations and a burst event frequency drug off periods to minimize passive diffusion. The between 0.5 and 1.2 Hz, (i.e. neurons that fluctuated impedance of the side barrels was usually between 20 and between the Up and Down states). Using these criteria, we 80 MV
recorded from a population of bistable neurons that burst NAc recordings were performed using a hydraulic fire, timed by a ‘network’ of temporally convergent microdrive. The coordinates for recording were: 1.9 an-excitatory presynaptic input. We assume that this ‘network’ terior to Bregma, 1.2 lateral to the sagittal sinus and 6.5 to determines the burst event frequency, whereas the burst 7.5 below the cortical surface. Because most NAc neurons duration and burst spike frequency are more influenced by were quiescent, glutamate (220 to2125 nA) was continu-postsynaptic conductances. Therefore, we hypothesized ously ejected from one side barrel while searching for
21
Digidata 1200A (Axon Instruments) interface with a 2.6. Statistics desktop computer running pCLAMP7 software (Axon
Instruments). Digital counts were obtained for off-line Data were analyzed using either paired or unpaired
analysis. two-tailed Student’s t-tests where appropriate. No more
than three neurons per rat were included in the analysis. 2.3. Definition of bursting
2.7. Materials
For the purposes of data analysis, a burst was defined as
Diltiazem and all other reagents were obtained from an abrupt action potential discharge of at least two spikes
Sigma (St. Louis). Five-barrel electrode assemblies for the with an interspike interval of less than 40 ms. Burst
extracellular recording and microiontophoresis were ob-termination was defined as an interspike interval of greater
tained from ASI Instruments (Warren, MI) and intracellu-than 80 ms. The burst event frequency was between 0.5
lar electrodes were obtained from World Precision Instru-and 1.2 Hz. Burst duration was defined as the time
ments (Sarasota, FL). between the first and last spike in a burst. Once a neuron
was detected, the current for glutamate was adjusted to determine if it exhibited a ‘network’ bursting pattern
3. Results (between 0.5 and 1.2 Hz). Most neurons (¯60%) exhibited
such bursting patterns. Once a bursting neuron was
iden-We conducted in vivo intracellular recordings from NAc tified, glutamate was iontophoresed every 30 sec and the
neurons such that we could compare burst firing obtained signal-to-noise ratio was monitored (greater than 4:1) for
with glutamate during extracellular recordings to that at least 5 min before beginning the experiment. For
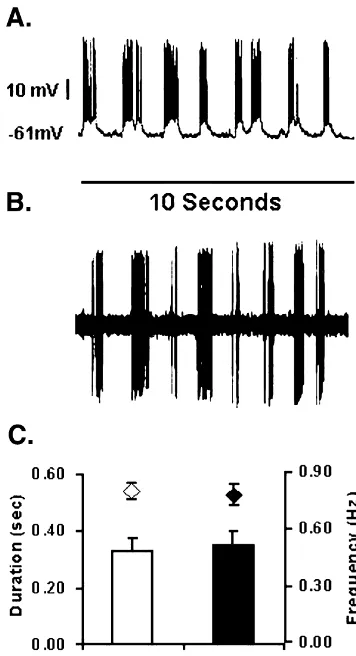
obtained with direct membrane depolarization. Fig. 1 A diltiazem recording, the current applied to the barrel was
shows an in vivo intracellular recording example of the Up continuously applied and doubled (beginning with11 nA)
and Down state membrane potential transitions from a every min until 164 nA was reached, at which time the
NAc neuron. Burst firing was produced by a depolarizing retaining current was re-applied. All neurons exhibited at
0.1 nA constant current injection. Although this neuron least 95% recovery to baseline levels of firing and bursting
had the same frequency, duration and amplitude of the Up within 5 min after cessation of diltiazem application.
state before direct current injection, the neuron was more Based on preliminary current–response curves, a diltiazem
hyperpolarized (273 mV Down state) and fired sponta-current of 120 nA was maximally effective at decreasing
neously at a very slow rate ,0.1 Hz (data not shown). burst duration without altering spike amplitude and was
After 0.1 nA depolarizing current injection, the neuron continuously applied for at least 2 min following a 5 min
continued the 0.8 Hz Up state transitions but depolarized to baseline.
a Down state potential of 261 mV and fired bursts of action potentials at an average frequency of 27 Hz
2.4. Burst analysis throughout the duration of the Up state. At no time did the
neuron fire during the Down state (data not shown). The After at least 5 min of stable (less than a 15% variation average Down state potential was 27666 mV and the Up in firing rate) firing rates in response to glutamate mi- state potential was26363 mV. Constant current injections croiontophoresis, data were acquired and digitized at 10 between 0.1 and 0.8 nA were used to depolarize the kHz. Burst event frequency and burst spike frequency were bistable neurons to fire in bursts for the duration of their determined from 10-s traces downsampled to 1000 kHz by Up states (Fig. 1C).
taking the minimum and maximum value between every 1 Fig. 1B shows an in vivo extracellular recording from a ms (i.e. Min / Max reduction factor of 10) using pCLAMP NAc neuron during microiontophoresis of glutamate for 30 7 software. A custom-made burst program was used to s. Before glutamate microiontophoresis (2122 nA) this determine the spike amplitude threshold, burst duration, neuron fired at less than 0.1 Hz (data not shown). Less than and frequency during the burst. 2 s after glutamate application the neuron began to fire in a
burst pattern with an average frequency of spikes in the
2.5. Histology burst of 33 Hz. After 30 s of glutamate microiontophoresis,
positive retaining current (10 nA) was applied and the After the last neuron was tested, the location of the neuron immediately (,1 s) stopped firing and resumed low electrode tip was marked by iontophoretic ejection of Fast frequency activity (,0.1 Hz) (data not shown). There were Green dye, which was subsequently localized on 60 mm no cumulative effects of repeated glutamate application on slices using routine histological methods. Reconstruction any parameters.
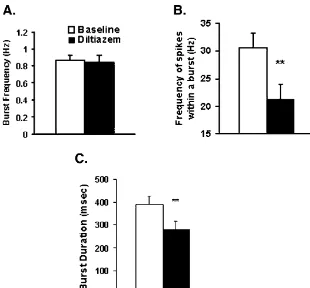
the burst by 31% (Fig. 2B), from 3163 Hz to 2162.6 Hz (t58.26, df57,P50.004). All neurons included in the analysis fully (.95%) recovered from the inhibitory effects of diltiazem within 5-min post application. There were no differences in the duration of the waveform before and after the 120 nA of current (baseline duration5 1.4860.07 ms; after diltiazem51.4160.03 ms; n58, t5 0.98, df511 P50.35). In some neurons, diltiazem currents higher than 132 nA reduced the amplitude of the wave-form by about 20%. Therefore we restricted our analysis to currents5120 nA, where signal attenuation did not inter-fere with the analysis. Fig. 3 is a representative trace showing that diltiazem decreases burst duration without changing burst event frequency.
4. Discussion
We have identified a method of glutamate iontophoresis during extracellular recording that generates a burst pattern of activity in a subpopulation (|60%) of NAc neurons that is highly consistent with the proportion of bistable NAc neurons observed during in vivo intracellular recording [20]. The timing of action potential bursts (burst event frequency) we presume to result from temporally con-vergent glutamatergic input arising from the thalamus, prefrontal cortex, amygdala and ventral subiculum [20,8].
21
We have also shown that the L-type Ca channel
Fig. 1. Examples of intracellular and extracellular recordings of the Up blocker diltiazem reduces both the duration of burst firing and Down state membrane potential transitions and burst firing of NAc and the number of spikes within bursts but does not alter neurons. A. Depolarizing current injection (0.1 nA) into the neuron
burst event frequency. The latter finding suggests that the
depolarized the neuron to261 mV (Down state potential) and revealed a
burst duration is closely associated with postsynaptic
bursting action potential pattern. Note the lack of action potentials in the
conductance changes in the recorded neuron. This is also
Down states. B.In vivo extracellular recording of a bursting NAc neuron
depolarized by continuous glutamate microiontophoresis (10 s duration). predicted by the fact that the tip of the five-barrel C. Burst duration (bars) and burst event frequency (diamonds) of micropipette is less than 7 mm in diameter and is most intracellular (n54) and extracellular recordings (n56).
likely located on or near the soma. This makes rapid drug diffusion to the spines of dendrites, where most of the 0.8 nA) was injected to induce bursting. For the intracellu- presynaptic glutamatergic inputs converge, unlikely.
Fur-21 lar and extracellular recording there were no significant thermore localization studies have shown that L-type Ca differences in average frequency of burst events (intracel- channels are largely somatic, whereas N and P/ Q-type
21
lular) 0.8 Hz60.04 and (extracellular) 0.78 Hz60.06, (t5 Ca channels are more predominant on dendrites and 20.23,df56, P50.83), burst duration (intracellular) terminals [27,10] For these reasons, we believe that in 330647 ms and (extracellular) 350651 ms (t50.24, df56, NAc neurons exhibiting a bistable membrane potential, the
21
P50.81) or spike frequency within a burst (intracellular) somatic L-type Ca channels modulate the duration of the 26.364.7 ms and (extracellular) 27.562.2 ms (t520.25, Up state and consequently the glutamate-induced burst df59, P50.84). Clearly our method of glutamate adminis- duration.
tration induced burst firing that was highly similar to that Although we believe that alterations in postsynaptic caused by direct membrane depolarization. conductances are the most likely explanations for our Fig. 2 shows the effects of diltiazem on burst event findings, it is possible that specific glutamate receptor frequency (Fig. 2A), burst spike frequency (Fig. 2B) and subtypes contribute to the burst pattern. Levine and burst duration (Fig. 2C). Diltiazem did not change burst colleagues, using whole-cell patch clamp recordings from event frequency (baseline50.8660.55 Hz, after striatal neurons, reported that NMDA receptors are in-diltiazem50.8560.75 Hz; n58; t50.31,df57, P50.76), volved in bursting [4] and that dopamine (DA) activation but decreased burst duration by 29% (Fig. 2C), from of D1 receptors enhances NMDA currents via a cAMP-39263.4 ms to 28063.8 ms (t53.3, df57, P50.01). dependent cascade. This D1 receptor-induced potentiation
21
Fig. 2. Effects of the L-type Ca channel blocker diltiazem on glutamate driven burst firing in the NAc. (A) Diltiazem failed to alter burst event frequency. (B and C) Diltiazem significantly reduced burst duration and burst spike frequency Asterisks (**) indicate significant difference between diltiazem and baseline (Student’s t-test, n58, P,0.005).
channel blockers [5]. Similarly, we have preliminary In recent years, there has been a considerable expansion evidence indicating that local (iontophoretic) administra- of our knowledge of how DA interacts with glutamate and tion of a DA D1 class receptor agonist potently increases voltage-gated calcium channels to influence the excitability burst durations in NAc neurons, an effect that is blocked of medium spiny neurons in vitro [14]. Characterization of
21 21
by an L-type Ca channel blocker [7]. the voltage-sensitive Ca channels in acutely dissociated medium spiny neurons has revealed (L, N, P) currents [2,6,25] that are differentially modulated by D1 class receptor agonists [26]. Whereas D1 receptor agonists and cAMP analogs inhibit both the N and P-type currents, the
21
L-type Ca currents are enhanced. Under current–clamp
21
conditions in brain slices, the L-type Ca channel ac-tivator, Bay K 8644 or the D1 agonist, SKF 81297 prolonged a slow depolarizing plateau potential thought to
21
result primarily from L-type Ca current activation [26,12].
Our findings verify the functional significance of L-type
21
Ca channels on the NAc neurons in vivo. Consistent with in vitro findings, we report that L-type conductances modulate the excitability of the majority of NAc neurons in vivo and prolong glutamate-induced bursting. It is possible that this is accomplished by enhancing the duration and / or the amplitude of the Up states, by
21 relieving NMDA channels from voltage-dependent Mg block and enhancing NMDA currents.
Fig. 3. Representative traces showing the reduction in burst duration 21
L-type Ca channel modulation of glutamate-induced
without changes in burst event frequency caused by iontophoretic
the rat prefrontal cortex, amygdala, midline thalamus, and
hip-processing in this nucleus which has implication for
pocampal formation onto single neurons of the caudate / putamen
reward-related learning and pathologies. In particular,
and nucleus accumbens, Hippocampus 6 (1996) 495–512. 21
dopaminergic modulation of L-type Ca channels may [9] E. Galarraga, S. Hernandez-Lopez, A. Reyes, J. Barral, J. Bargas, play a role in drug addiction or drug craving processes. For Dopamine facilitates striatal EPSPs through an L-type Ca21
example, cocaine’s psychomotor stimulant effects are conductance, Neuroreport 8 (1997) 2183–2186.
[10] J.W. Hell, R.E. Westenbroek, C. Warner, M.K. Ahlijanian, W.
known to be dependent on functional dopamine D1
21 Prystay, M.M. Gilbert, T.P. Snutch, W.A. Catterall, Identification and receptors [29]. L-type Ca channel blockers are capable
differential subcellular localization of the neuronal class C and class
of blocking D1 receptor-mediated enhancement of L-type D L-type calcium channel alpha 1 subunits, J. Cell Biol. 123 (1993)
21
Ca currents in vitro [12]. Furthermore, D1 receptor 949–962.
function in the NAc is known to be persistently enhanced [11] D.J. Henry, F.J. White, The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons,
after repeated psychostimulant treatment [11,14] and is
J. Neurosci. 15 (1995) 6287–6299.
associated with behavioral sensitization [11], the induction
21 [12] S. Hernandez-Lopez, J. Bargas, D.J. Surmeier, A. Reyes, D1
of which is prevented by diltiazem [16]. L-type Ca receptor activation enhances evoked discharge in neostriatal medium channel blockers also decrease cocaine’s locomotor stimul- spiny neurons by modulating an L-type Ca21 conductance, J. ant [23], discriminative stimulus [23] and reinforcing Neurosci. 17 (1997) 3334.
[14] H. Higashi, K. Inanaga, S. Nishi, N. Uchimura, Enhancement of
effect [17,18]. Our present findings suggest that these
21 dopamine actions on rat nucleus accumbens neurons in vitro after anti-cocaine effects of L-type Ca channel blockers may
methamphetamine pre-treatment, J. Physiol. 408 (1989) 587–603.
result from modulation of NAc bursting activity.
[15] X.-T. Hu, F.J. White, Loss of D1 / D2 dopamine receptor synergisms following repeated administration of D1 or D2 receptor selective antagonists: electrophysiological and behavioral studies, Synapse 17 (1994) 43–61.
Acknowledgements [16] R. Karler, S.A. Turkanis, L.M. Partlow, L.D. Calder, Calcium channel blockers and behavioral sensitization, Life Sci. 49 (1991)
We thank Drs. Michela Marinelli, Xiu-ti Hu and James 165–170.
[17] A. Kuzmin, E. Zvartau, G.L. Gessa, M.C. Martellotta, W. Fratta,
Surmeier for helpful discussions and comments during the
Calcium antagonists isradipine and nimodipine suppress cocaine and
preparation of the manuscript. We are grateful to Lorinda
morphine intravenous self-administration in drug-naive mice,
Phar-Baker her superb technical assistance with the histology. macol. Biochem. Behav. 41 (1992) 497–500.
This work was supported by USPHS grant DA04093 and [18] A. Kuzmin, S. Semenova, N.F. Ramsey, E.E. Zvartau, J.M. Van Ree, Senior Scientist Award (FJW) National Research Service Modulation of cocaine intravenous self-administration in drug-naive animals by dihydropyridine Ca21 channel modulators, Eur. J.
Award DA05794 (DCC) from the National Institute on
Pharmacol. 295 (1996) 19–25.
Drug Abuse.
[19] G.J. Mogenson, D.L. Jones, C.Y. Yim, From motivation to action: functional interface between the limbic system and the motor system, Prog. Neurobiol. 14 (1980) 69–97.
[20] P. O’Donnell, A.A. Grace, Synaptic interactions among excitatory
References afferents to nucleus accumbens neurons: Hippocampal gating of
prefrontal cortical input, J. Neurosci. 15 (1995) 3622–3639. [1] T.A. Ansah, L.H. Wade, D.C. Shockley, Effects of calcium channel [21] G. Paxinos, P. Watson, The Rat Brain in Stereotaxic Coordinates,
entry blockers on cocaine and amphetamine-induced motor activities Academic Press, Sydney, 1986.
and toxicities, Life Sci. 53 (1993) 1947–1956. [22] L.L. Peoples, A.J. Uzwiak, F.X. Guyette, M.O. West, Tonic inhibi-[2] J. Bargas, A. Howe, J. Eberwine, Y. Cao, D.J. Surmeier, Cellular and tion of single nucleus accumbens neurons in the rat: a predominant molecular characterization of Ca21 currents in acutely isolated, but not exclusive firing pattern induced by cocaine self-administra-adult rat neostriatal neurons, J. Neurosci. 14 (1994) 6667–6686. tion sessions, Neuroscience 86 (1998) 13–22.
[3] P. Calabresi, N.B. Mercuri, A. Stefani, G. Bernardi, Synaptic and [23] M.D. Schechter, Cocaine discrimination is attenuated by isradipine intrinsic control of membrane excitability of neostriatal neurons. I. and CGS 10746B, Pharmacol. Biochem. Behav. 44 (1993) 661–664. An in vivo analysis, J. Neurophysiol. 63 (1990) 651–662. [24] D.W. Self, L.M. Genova, B.T. Hope, W.J. Barnhart, J.J. Spencer, E.J. [4] C. Cepeda, N.A. Buchwald, M.S. Levine, Neuromodulatory actions Nestler, Involvement of cAMP-dependent protein kinase in the of dopamine in the neostriatum are dependent upon the excitatory nucleus accumbens in cocaine self-administration and relapse of amino acid receptor subtypes activated, Proc. Natl. Acad. Sci. USA cocaine-seeking behavior, J. Neurosci. 18 (1998) 1848–1859. 90 (1993) 9576–9580. [25] W.J. Song, D.J. Surmeier, Voltage-dependent facilitation of calcium [5] C. Cepeda, C.S. Colwell, J.N. Itri, S.H. Chandler, M.S. Levine, channels in rat neostriatal neurons, J. Neurophysiol. 76 (1996)
Dopaminergic modulation of NMDA-induced whole cell currents in 2290–2306.
neostriatal neurons in slices: Contribution of calcium conductances, [26] D.J. Surmeier, J. Bargas, H.C. Hemmings Jr., A.C. Nairn, P. J. Neurophysiol. 79 (1998) 82–94. Greengard, Modulation of calcium currents by a D dopaminergic1 [6] D. Churchill, B.A. MacVicar, Biophysical and pharmacological protein kinase / phosphatase cascade in rat neostriatal neurons,
characterization of voltage-dependent Ca21 channels in neurons Neuron 14 (1995) 385–397.
isolated from rat nucleus accumbens, J. Neurophysiol. 79 (1998) [27] R.E. Westenbroek, M.K. Ahlijanian, W.A. Catterall, Clustering of
635. L-type Ca21channels at the base of major dendrites in
hippocam-[7] D.C. Cooper, F.J. White, Dopamine D1 receptor signaling in the pal pyramidal neurons, Nature 347 (1990) 281–284.
[29] M. Xu, X.T. Hu, D.C. Cooper, R. Moratalla, A.M. Graybiel, F.J. ‘accumbens’ part of the rat ventral striatum, Neuroscience 50 (1992) White, S. Tonegawa, Elimination of cocaine-induced hyperactivity 751–767.