www.elsevier.nlrlocateraqua-online
The effects of dietary retinoic acid on body lipid
deposition in juvenile red sea bream
ž
Pagrus major ; a preliminary study
/
Hiroshi Y. Ogata
), Hiromi Oku
Fish Nutrition DiÕision, National Research Institute of Aquaculture, Nansei, Mie 516-0193, Japan
Received 23 May 2000; received in revised form 27 July 2000; accepted 28 July 2000
Abstract
A 6-week feeding trial was conducted to investigate the effects of dietary retinoids, especially retinoic acid, an anti-adipogenic factor in mammals, on lipid deposition in juvenile red sea bream
Žinitial body weight: 8.5 g . The fish were fed either a commercial diet control: diet 1, crude. Ž
. Ž .
protein 61.7%, crude fat 13.9% , or diets supplemented with 100 mg retinyl acetate diet 2 , 10
Ž . Ž .
mg all-trans-retinoic acid diet 3 , or 100 mg all-trans-retinoic acidrkg diet diet 4 for the test
Ž .
period. A low mortality 2.5% was observed with diets 3 and 4 through the period. Weight gain, daily feed intake, feed efficiency, whole body crude protein, and crude fat content were not affected by the treatments. Visceral adiposomatic index of the fish was highest in fish fed diet 4 as follows: 2.90 in diet 1 fish, 3.46 in diet 2 fish, 3.28 in diet 3 fish, and 4.19 in diet 4. The treatments did not affect hepatosomatic index. Lipid in the dorsal white muscle was lowest and those in the visceral adipose tissue highest in fish fed diet 4. Liver lipid content was not affected
Ž .
by the treatments. Average plasma free fatty acid levels in fish fed diet 4 60mEqrl were lower
Ž .
than those of the other groups 113–148mEqrl . Plasma triacylglycerol levels in fish fed diet 2
Ž1845 mgrl were lower than those of the other groups 2944–3130 mg. Ž rl . Subsequently, to.
confirm that dietary retinoic acid was internally absorbed, plasma retinoic acid levels at 6-h post-feeding were determined by HPLC. These were 4"0 ngrml in diet 1, 6"0 ngrml in diet 2, 20"0 ngrml in diet 3, and 223"34 ngrml in diet 4. The present results suggest that dietary retinoic acid was directly incorporated into the body, and that retinoic acid up-regulated develop-ment of the visceral adipose tissue and down-regulated lipid deposition in the dorsal white muscle.
q2001 Elsevier Science B.V. All rights reserved.
Keywords: Pagrus major; Retinoic acid; Retinol; Retinoids; Fat; Lipid
)Corresponding author. Tel.:q81-599-66-1830; fax:q81-599-66-1962.
Ž .
E-mail address: [email protected] H.Y. Ogata .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
In Japan, red sea bream is one of the most popular food fish and a large culture industry has grown to meet the demand. However, this species can build up remarkable
Ž .
body fat depots Oku and Ogata, 2000 , resulting in higher lipid content of cultured red
Ž .
sea bream flesh compared to wild fish Morishita et al., 1988; Osato et al., 1991 .
Ž . Ž
Indeed, muscle lipid contents in cultured red sea bream 1.2–1.5 kg and wild fish 1.3
. Ž .
kg were 4.7–9.0% and 1.3%, respectively Morishita et al., 1988 . This has been associated with lower consumer satisfaction. The preferred lipid content depends on
Ž
local customs, yet a high level of fat leads to poor appearance and taste Fauconneau et
.
al., 1995 .
The extent of marbling, intramuscular fat deposition in beef cattle has been related to
Ž .
blood retinol level Torii et al., 1996 . Retinol action on muscular fattening appears to be mediated by retinoic acid, which has an antiadipogenic action on preadipocytes present
Ž .
in muscle tissue Keller et al., 1993; Kamei et al., 1994 . A recent trend in the beef cattle industry in Japan has been for farmers to feed vitamin A-deficient diets to produce
Ž
richly marbled beef, which is valued as a high-quality product in Japan Takeyama et al.,
.
1996a,b . There is little information on the effects of retinoids on body lipid deposition in fish. Therefore, the present study was conducted to investigate the effects of dietary retinoids, especially retinoic acid, on body fat deposition of juvenile red sea bream. A 6-week feeding trial was carried out with diets containing different retinoid sources to examine the effects of these compounds on tissue and plasma lipid contents. Subse-quently, to confirm that dietary retinoic acid was internally absorbed, plasma retinoic acid levels were determined at 6-h post-feeding.
2. Materials and methods
Ž .
Juvenile red sea bream Pagrus major were obtained from a private hatchery
ŽNisshin Marine Tech, Aichi, Japan and transported to the indoor facilities of the.
National Research Institute of Aquaculture at Nansei, Mie. For 2 months prior to the start of the experiment, the fish were held in indoor tanks and fed commercial dry feeds
ŽC-series, Kyowa Hakko, Tokyo, Japan under a natural photoperiod. Fish were selected.
to be of uniform size and randomly assigned to eight tanks, 20 fish per tank. The fish were acclimated to the tank conditions for 1 week prior to the commencement of the feeding trial. At the beginning of the experiment, mean body weight of the fish was 8.6"0.0 g. A sample of 16 fish was taken from the common pool at the start of the
Ž .
experiment and frozen y808C for later proximate analysis.
Each treatment was assigned to duplicate groups in a completely random design, and the treatments consisted of four diets. The control diet was a commercial dry feed
ŽC-series, Kyowa Hakko, Tokyo, Japan and retinoid diets were prepared by supplement-.
Ž 6 .
ing the control diet with either retinyl acetate 1.42–1.80=10 IUrg or crystalline
Ž . Ž . Ž
all-trans-retinoic acid retinoic acid Wako, Osaka, Japan . Retinyl acetate 100 mgrkg
. Ž .
amount of the carrier acetone was added to the control diet. All four diets were dried at 508C under vacuum in a rotary evaporator to remove acetone and then stored aty208C. Crude protein and crude fat contents of the test diets were 61.7% and 13.9% on a dry
Ž
basis, respectively. The control diet contained 5.8 mg retinyl palmitaterkg diet 10,500
.
IUrkg diet as a basal level of the dietary retinoid.
The fish were hand-fed to apparent satiation twice a day, 6 daysrweek for 6 weeks.
Ž 3.
The tanks used in this study were square polyvinylchloride tanks 60=25=35 cm with a 52.5 l capacity, filled to 40 l. Each tank was supplied with seawater drawn from Gokasho Bay, Mie at the rate of 2 lrtankrmin. The water temperature ranged from 19.38C to 23.58C during the feeding trial. The fish were weighed individually after being anesthetized with 0.01% ethyl 3-aminobenzonate methansulfonic acid salt solution
ŽAldrich Chemical Company, Milwaukee, WI, USA , at the beginning, at 3 weeks and.
the end of the feeding test. At the end of the feeding test, fish were starved for 48 h and then were sampled from each tank. Ten fish from each tank were frozen and stored for whole body analysis. The remaining fish in each tank were bled from the caudal vein with a heparinaized syringe. Plasma was separated by centrifugation, and the plasma and the bled bodies were stored aty808C for subsequent analysis.
Following the feeding trial, determination of plasma retinoic acid levels was
con-Ž .
ducted using fish mean body weight 60 g from the same source as the fish in the feeding trial. The fish for plasma retinoic acid determination was fed only one time the
Ž .
test diets, that is, blood samples were taken from eight fish starved for 48 h initial and
Ž .
then the remaining fish eight fish per tank and four tanks were fed either the control or the respective retinoid diets. Blood samples were taken 6 h post-feeding by the protocol as described earlier. Plasma was separated by centrifugation and stored aty808C for subsequent analysis.
Crude protein and crude fat of the test diets and the whole body were determined by
Ž
the Kjeldahl method and diethyl ether extraction, respectively two pooled samples of
.
five fish from each tank . The bled fish was stored at y808C, and then prior to lipid analysis, these were dissected and liver, visceral adipose tissue, and dorsal white muscle samples were taken. Hepatosomatic and visceral adiposomatic indices were calculated
Ž
according to the formula: organ indexs100= liver or visceral adipose tissue
.
weightrbody weight . Lipid contents of dorsal white muscle, liver, and visceral adipose
Ž
tissue were determined by chloroform–methanol extraction two pooled samples of five
. Ž . Ž .
fish from each tank Folch et al., 1957 . Plasma non-esterified fatty acid NEFA and
Ž .
triacylglycerol levels individual fish were determined by diagnostic kits, NEFA C-Test
Ž .
Wako and Triglyceride E-Test Wako Wako , respectively.
Ž .
Plasma from four fish 0.25 ml plasma from each fish was pooled to one and duplicate samples for each dietary treatment were used for plasma retinoid determina-tion. Plasma retinol and retinoic acid were extracted according to the method of Takeda
Ž .
and Yamamoto 1994 . Retinoids were analyzed using a high-performance liquid
Ž .
chromatograph Pharmacia LKB Biotechnology, Uppsala, Sweden with gradient pump
Ž
2249 and VWM 2141 spectrophotometric detector, and Wakosil-II5C18 HG ø 4.6=250
. Ž .
mm; Wako column. As the mobile phases, mixtures of acetonitrile A , methyl
Ž . Ž .
alcohol–0.3% ammonium acetate solution 16:28, by vol.: B , and 2-propanol C were
Ž .
rate of 0.6 mlrmin at 308C and detected at 345 nm. Retinol was separated using a
Ž .
mixture of B and C 15:85, by vol. at a flow rate of 0.4 mlrmin at 308C and detected at
Ž
325 nm. A vitamin-A–alcohol solution purity )98%, Fluka Chemie, Buchs,
Switzer-. Ž
land and all-trans-retinoic acid purity )95%, Biomol Research Lab., Plymouth
.
Meeting, PA, USA were used as the authentic standards for plasma retinoid determina-tion.
Data were statistically compared between the control and the fish fed retinoids using
Ž .
Student’s t-test P-0.05 , without any transformations of percentage data prior to the analysis.
3. Results
Mortality during the feeding trial was low and limited to one fish in one tank in both
Ž .
the retinoic acid groups Table 1 . The final body weight of the 100 mgrkg retinoic acid
Ž . Ž
group 42.9 g, P-0.05 compared to control was slightly lower than weights 45.3–45.5
. Ž .
g of the other groups, but the weight gain 400% was not significantly different from
Ž . Ž .
that of the control fish 419% . Feed efficiency 1.09–1.12 and daily feed intake
Ž3.03–3.17% of wet biomassrday were not affected by the treatments. No signs of.
hypervitaminosis A were observed in the test fish.
The dietary treatments did not affect the whole body crude protein and fat contents.
Ž .
Hepatosomatic index of the fish fed the 100 mgrkg retinyl acetate diet 1.50 tended to
Ž . Ž .
be higher but not significantly different from that of the control fish 1.35 Table 2 . Visceral adiposomatic index was highest in the fish fed the 100 mgrkg retinoic acid diet
Table 1
Ž .
The effects of dietary retinoids on growth performance of juvenile red sea bream mean value"se
Diet Control Retinyl acetate Retinoic acid Retinoic acid
Ž100 mgrkg diet. Ž10 mgrkg diet. Ž100 mgrkg diet. Ž .
Initial body weight g 8.72"0.02 8.57"0.15 8.61"0.24 8.58"0.02
) Ž .
Final body weight g 45.28"0.47 45.31"0.80 45.49"0.52 42.89"0.17 % weight gain 419"5 429"0 429"9 400"3
a
Feed efficiency 1.12"0.01 1.09"0.01 1.10"0.00 1.12"0.01 b
Daily feed intake 3.07"0.03 3.17"0.03 3.14"0.09 3.03"0.02
Ž .
Data were statistically different when compared between the control and retinoid diets using Student’s
Ž .
Feed intake g=100r initial weightqfinal weightr2=rearing period days . c
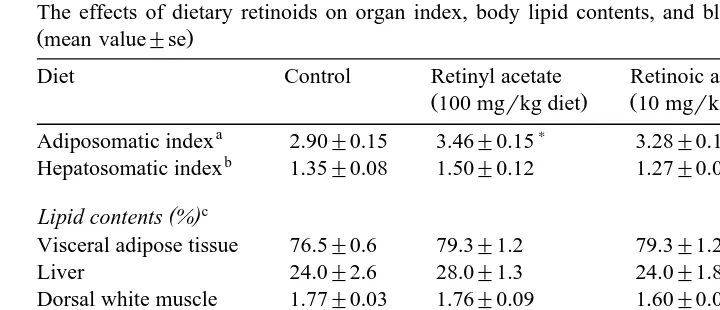
Table 2
The effects of dietary retinoids on organ index, body lipid contents, and blood parameters of red sea bream
Žmean value"se.
Diet Control Retinyl acetate Retinoic acid Retinoic acid
Ž100 mgrkg diet. Ž10 mgrkg diet. Ž100 mgrkg diet.
a ) )
Adiposomatic index 2.90"0.15 3.46"0.15 3.28"0.11 4.19"0.19 b
Hepatosomatic index 1.35"0.08 1.50"0.12 1.27"0.08 1.28"0.07 c
( ) Lipid contents %
)
Visceral adipose tissue 76.5"0.6 79.3"1.2 79.3"1.2 83.4"1.7 Liver 24.0"2.6 28.0"1.3 24.0"1.8 24.6"0.9
)
Dorsal white muscle 1.77"0.03 1.76"0.09 1.60"0.09 1.55"0.04 Blood plasma
Triacylglycerol mgrl 3130"193 1845"119 2944"202 3061"152
e )
Data were statistically different when compared between the control and retinoid diets using Student’s
Ž .
t-test P-0.05 .
aŽ .
Visceral adipose tissue weightrbody weight=100.
bŽ .
Liver weightrbody weight=100.
c Ž .
Chloroform–methanol 2:1, by vol. extracts. d
Non-esterified fatty acids.
e Ž .
Plasma retinoid levels at 6-h post-feeding. Initial levels 48 h-starvation of plasma retinol and retinoic acid levels were 762"10 and 5"0 ngrml, respectively.
Ž4.19, P-0.05 for control , and the index of the fish fed the retinyl acetate diet 3.46,. Ž
. Ž .
P-0.05 for control was significantly higher than that of the control fish 2.90 . Liver
Ž .
lipid contents were not related to the dietary treatments 24.0–28.0% . However, in the
Ž
fish fed the 100 mgrkg retinoic acid diet, lipid in the visceral adipose tissue 83.4%,
. Ž
P-0.05 for control was highest and that of the dorsal white muscle 1.55%, P-0.05
. Ž
for control was lowest. Plasma NEFA level of the 100 mgrkg retinoic acid group 61
. Ž
mEqrl, P-0.05 for control was lower than those of the other groups 113–148
. Ž
mEqrl . Plasma triacylglycerol level of the 100 mgrkg retinyl acetate group 1845
. Ž
mgrl, P-0.05 for control was lower than those of the other groups 2944–3130
.
mgrl . Plasma retinoic acid levels at 6-h post-feeding of the fish fed the 10 and 100
Ž .
mgrkg retinoic acid diets 20 and 223 ngrml, respectively, P-0.05 for control were
Ž . Ž .
higher than those of the control 4 ngrml and retinyl acetate group 6 ngrml . The
Ž
plasma retinol level at 6-h post-feeding of the retinyl acetate group 809 ngrml,
. Ž .
P-0.05 for control was higher than those of the other groups 601–714 ngrml .
4. Discussion
The effect of retinoic acid on fish body fat deposition in vivo has not been reported
Ž .
metabolism of brook trout, and reported that a supplementation of 250,000 USP units of vitamin A per 100 g diet significantly decreased carcass fat content. Overall feeding diets with vitamin A supplementation appears to lower whole body fat, though in most
Ž .
cases, the differences were not significant for brook trout Poston and Livingston, 1971 ,
Ž . Ž .
rainbow trout Hilton et al., 1978 , or guppy Shim and Tan, 1989 . When the effect of dietary retinoid supplementation on body fat content is assessed on a whole body basis, the effect seems equivocal. On the other hand, in the present study in which lipid content was determined in several tissues, the results clearly show that the fish fed the 100 mgrkg retinoic acid diet had lower dorsal white muscle lipid, and that in particular, the fish had both increased visceral adipose lipid and visceral adipose mass. This result indicates that retinoic acid drastically increases lipid deposition capacity of the visceral adipose tissue in red sea bream in vivo.
Retinoic acid has been recognized as a potent controller of adipocyte differentiation
Žreview by Villarroya et al., 1999 . In most cases, retinoic acid impairs preadipocyte.
differentiation. When preadipocytes from cell lines such as 3T3-L1 or 3T3-F442A, and
Ž
preadipocytes prepared from porcine dorsal subcutaneous adipose tissues Suryawan and
. Ž .
Hu, 1997 and ovine perirenal adipose tissues Ohyama et al., 1998 are exposed to all-trans-retinoic acid at concentrations ranging from 3 nM to 10 mM, the appearance of
Ž
the characteristic features of the white adipose cell phenotype is blunted Villarroya et
.
al., 1999 . Nevertheless, the physiological role of the inhibition of preadipocyte differen-tiation by retinoic acid has been questioned by observations that retinoic acid at
Ž .
physiological concentrations 1–10 nM , acted as an adipogenic factor that stimulated differentiation of Ob 1771 preadipocytes established from mouse periepididymal adipose
Ž
tissues and preadipocytes prepared from rat periepididymal fat pads Safonova et al.,
.
1994 . More recently, it has been shown that depending on the concentration and the period of treatment, retinoic acid is able to induce or to repress the differentiation program. A complex balance between retinoic acid metabolism and relative retinoic acid
Ž . Ž .
receptor RAR and retinoid-X receptor RXR availability in the cell seems to control the resulting effects, either negative or positive, of retinoids on preadipocyte
differentia-Ž .
tion Villarroya et al., 1999 . In the present study, lipid deposition responded differently to dietary retinoic acid in the dorsal white muscle and visceral adipose tissues. Although the molecular mechanism of up-regulation on the visceral adipose tissue and down-regu-lation on white muscle adiposity by retinoic acid was not elucidated in the present study, the findings suggest that preadipocyte differentiation to adipocytes that was mediated by retinoic acid may have been under different control in the dorsal white muscle and the visceral adipose tissue.
As there is little information available on the effects of retinoic acid on lipid metabolism in fish, it is difficult to compare the present results on plasma NEFA and triacylglycerol levels with those of mammals. Usually, feeding excess retinol or retinoic
Ž
acid seems to induce hypertriglyceridemia in rats Gerber and Erdman, 1980, 1981;
.
Gerber et al., 1981 . By contrast, plasma triacylglycerol levels in the fish fed the retinoic acid diet for 6 weeks did not differ from those of the control, and the fish fed the retinyl acetate diet had a lower plasma triacylglycerol level compared to the other groups.
Ž .
Short-term feeding 2 days of excess retinol elevated plasma NEFA levels in rats
the retinoid diet, especially the 100 mgrkg retinoic acid diet, than in the control. Fasting period appears to have quite a different influence on the relationship between the liver
Ž .
free fatty acid content and vitamin A status in rats Wiss and Wiss, 1980 . Considered all together, the inconsistency in plasma NEFA and triacylglycerol changes between rats and red sea bream might stem from differences not only between species but also in the
Ž . Ž
duration of the feeding 6 weeks in the present study and fasting periods 48 h in the
.
present study .
Dietary retinyl acetate and retinoic acid produced different effects on triacylglycerol metabolism in red sea bream. Although hepatic retinoid levels were not determined in the present study, it may be possible that unlike the case of retinoic acid, retinol provided in the form of retinyl acetate was stored at a high level as retinyl esters in the liver, which secondarily affected triacylglycerol metabolism. Retinoic acid is rapidly turned over in the whole animal, and this retinoid, unlike retinol, is not excessively
Ž .
stored in tissues Kurlandsky et al., 1995 . Hepatic accumulation of excess retinyl esters, due to a long-time feeding of retinyl acetate, might have induced a decrease in triacylglycerol production in the liver andror a decrease of triacylglycerol release from this organ into circulation. It seems possible that under in vivo conditions, retinol and retinoic acid fed in excess may produce different effects on the lipid metabolism of red sea bream. Fish fed the retinoid diet tended to have lower plasma NEFA levels than the control. Since adipose tissue is the major depot of circulating NEFA and the main organ to provide fatty acids to tissues through blood circulation, the decreased plasma NEFA levels could possibly be ascribed to an elevation of lipoprotein lipase activity andror a decline of hormone-sensitive lipase in the organs. Fluctuation of both lipase activities, possibly mediated by retinoic acid, may be related to the increase of visceral adipose mass of the fish fed this retinoid.
Endogenous retinoic acid levels have been reported to be around 1–2 ngrml plasma
Ž .
in healthy volunteers fasted overnight Meyer et al., 1994 and 10 ngrml serum in
Ž .
healthy subjects Lo et al., 1996 . Tissue retinoic acid levels range from 1.9 ngrg in
Ž .
brain tissue to 8.8 ngrg in pancreas tissue in rats Kurlandsky et al., 1995 . Our plasma
Ž .
data, at the start starved for 48 h and at 6-h post-feeding of the control and 100 mgrkg retinyl acetate groups, ranged within these values. At 6-h post-feeding, the fish fed the retinoic acid diets clearly had higher plasma retinoic acid levels compared with the control fish, indicating that dietary retinoic acid was directly incorporated into the body. The level detected in plasma, especially in the 100 mgrkg retinoic acid group, was
Ž .
above the physiological range for humans reported by Meyer et al. 1994 and Lo et al.
Ž1996 . The administration of high levels of retinoic acid may be toxic to animals..
Ž .
However, Gerber and Erdman 1980 reported that the use of 205–310 mgrkg diet of all-trans-retinoic acid may represent the lower limits of toxicity for rats. The 100 mgrkg
Ž
retinyl acetate diet contained 260,000 IU vitamin Arkg diet basal level of retinyl
.
palmitate in dietqaddition level of retinyl acetate , which was lower than the maximum
Ž . Ž .
tolerable level of vitamin A 904,000 IUrkg diet in rainbow trout Hilton, 1983 . Thus, we can rule out the possibility that the dietary retinoid levels used in this study resulted in an overdose of vitamin A sufficient to induce hypervitaminosis A.
observed here might be an extension of the physiological role of retinoic acid. Further study may provide effective methods to control adiposity in farmed fish.
Acknowledgements
We would like to thank Dr. K.D. Shearer, Northwest Fisheries Science Center, NMFS, USA and Dr. J.T. Silverstein, Catfish Genetics Research Unit, USDA, USA, for their critical reading of the manuscript. Financial support for this study was provided by
Ž .
the Ministry of Agriculture, Forestry and Fisheries of Japan BDP-00-IV-1-9 .
References
Fauconneau, B., Alami-Durante, H., Laroche, M., Marcel, J., Vallot, D., 1995. Growth and meat quality relations in carp. Aquaculture 129, 265–297.
Folch, J., Lee, M., Sloane Stanley, G.H., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Gerber, L.E., Erdman, J.W. Jr., 1980. Comparative effects of all-trans-retinoic acid administration on serum and liver lipids in rats. J. Nutr. 110, 343–351.
Gerber, L.E., Erdman, J.W. Jr., 1981. Hyperlipidemia in rats fed retinoic acid. Lipids 16, 496–501. Gerber, L.E., Wasserman, L.S., Cho, B.H.S., Erdman, J.W. Jr., 1981. Changes in serum triglycerides, retinoic
acid, and retinol associated with withdrawal of retinoic acid from the diet of rats. Nutr. Res. 1, 509–517.
Ž .
Hilton, J.W., 1983. Hypervitaminosis A in rainbow trout Salmo gairdneri : toxicity signs and maximum tolerable level. J. Nutr. 113, 1737–1745.
Hilton, J.W., Cho, C.Y., Slinger, S.J., 1978. Effect of hypervitaminosis A on the development of ascorbic acid
Ž .
deficiency in underyearling rainbow trout Salmo gairdneri R. . Aquaculture 13, 325–330.
Kamei, Y., Kawada, T., Mizukami, J., Sugimoto, E., 1994. The prevention of adipose differentiation of 3T3-L1 cells caused by retinoic acid is elicited through retinoic acid receptor alpha. Life Sci. 55, PL307–PL312.
Keller, H., Dreyer, C., Medin, J., Mahfoudi, A., Ozato, K., Wahli, W., 1993. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferation-activated receptor–retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. U. S. A. 90, 2160–2164.
Kurlandsky, S.B., Gamble, M.V., Ramakrishnan, R., Blaner, W.S., 1995. Plasma delivery of retinoic acid to tissues in the rat. J. Biol. Chem. 270, 17850–17857.
Lo, C.S., Wahlqvist, M.L., Horie, Y., 1996. Determination of retinoic acid and retinol at physiological concentration by HPLC in Caucasians and Japanese women. Asia Pac. J. Clin. Nutr. 5, 173–174. Meyer, E., Lambert, W.E., De Leenheer, A.P., 1994. Simultaneous determination of endogenous retinoic acid
isomers and retinol in human plasma by isocratic normal-phase HPLC with ultraviolet detection. Clin. Chem. 40, 48–50.
Morishita, T., Uno, K., Matsumoto, Y., Takahashi, T., 1988. Comparison of the proximate compositions in
Ž .
cultured red sea bream differing the localities and culture methods, and of the wild fish in Japanese . Nippon Suisan Gakkaishi 54, 1965–1970.
Ohyama, M., Matsuda, K., Torii, S., Matsui, T., Yano, H., Kawada, T., Ishihara, T., 1998. The interaction between vitamin A and thiazolidine on bovine adipocyte differentiation in primary culture. J. Anim. Sci. 76, 61–65.
Oku, H., Ogata, H.Y., 2000. Body lipid deposition in juveniles of red sea bream Pagrus major, yellowtail Seriola quinqueradiata, and Japanese flounder Paralichthys oliÕaceus. Fish. Sci. 66, 25–31.
Osato, S., Miyata, K., Matsuo, S., Itou, T., Kora, H., Misima, T., Tachibana, K., Tsuchimoto, M., 1991.
Ž .
Poston, H.A., 1971. Effect of feeding excess supplemental vitamin A on the carbohydrate and lipid metabolism and growth of brook trout. Fish. Res. Bull., N. Y. 34, 22–26.
Poston, H.A., Livingston, D.L., 1971. The influence of dietary levels of protein and vitamin A on the liver vitamin A level, lipid metabolism, and growth of brook trout. Fish. Res. Bull., N. Y. 34, 27–34. Safonova, I., Amri, E., Ailhaud, G., 1994. Retinoids are positive effectors of adipose differentiation. Mol. Cell.
Endocrinol. 104, 201–211.
Ž .
Shim, K.F., Tan, C.H., 1989. The dietary requirement of vitamin A in guppy Poecillia reticulata Peters . In:
Ž .
Takeda, M., Watanabe, T. Eds. , Proc. Third Int. Symp. on Feeding and Nutr. in Fish Toba Aug. 28–Sep. 1, Japan. pp. 133–140.
Singh, V.N., Singh, M., Venkitasubramanian, T.A., 1969. Early effects of feeding excess vitamin A: mechanism of fatty liver production in rats. J. Lipid Res. 10, 395–401.
Suryawan, A., Hu, C.Y., 1997. Effect of retinoic acid on differentiation of cultured pig preadipocytes. J. Anim. Sci. 75, 112–117.
Takeda, N., Yamamoto, A., 1994. Simultaneous determination of 13-cis- and all-trans-retinoic acids and retinol in human serum by high-performance liquid chromatography. J. Chromatogr. B 657, 53–59. Takeyama, M., Funagayama, Y., Kondou, M., Tokumoto, K., Nagatomo, K., Tsuchiya, K., 1996a. Effects of
Ž . Ž .
feeding Vitamin A on fattening of Japanese Black steers-I in Japanese . Bull. Miyazaki Livest. Ex. St. 9 , 74–78.
Takeyama, M., Funagayama, Y., Kondou, M., Tokumoto, K., Nagatomo, K., Tsuchiya, K., 1996b. Effects of
Ž .
feeding Vitamin A on fattening of Japanese Black steers-II in Japanese . Bull. Miyazaki Livestock Ex. St.
Ž .9 , 79–83.
Torii, S., Matsui, T., Yano, H., 1996. Development of intramuscular fat in Wagyu beef cattle depends on adipogenic or antiadipogenic substances present in serum. Anim. Sci. 63, 73–78.
Villarroya, F., Giralt, M., Iglesias, R., 1999. Retinoids and adipose tissues: metabolism, cell differentiation and gene expression. Int. J. Obes. 23, 1–6.