www.elsevier.nlrlocateraqua-online
Effects of ultraviolet irradiation on genetical
inactivation and morphological structure of sperm
of the Japanese scallop, Patinopecten yessoensis
Qi Li
a, Makoto Osada
a, Masaru Kashihara
b, Ken Hirohashi
b,
Akihiro Kijima
a,)a
Education and Research Center of Marine Bio-resources, Faculty of Agriculture, Tohoku UniÕersity,
Onagawa, Miyagi 986-2242, Japan b
Biomate, Higashikasai, Edogawa, Tokyo 134-0084, Japan
Accepted 9 December 1999
Abstract
Ž .
Effects of ultraviolet UV irradiation on genetic inactivation and morphological structure of sperms were examined in the scallop, Patinopecten yessoensis. Haploid gynogenesis of the scallop was successfully induced by 50–60 s UV irradiation of 720mW cmy2sy1. The fertilization rate
apparently decreased with increasing irradiation time, and the development of the eggs fertilized with the genetically inactivated sperms terminated before reaching the D-shaped larvae stage.
Ž .
Scanning electron microscopy SEM showed clear destruction of the sperm acrosome and flagellum in the UV-irradiated sperms. As the duration of UV irradiation increased, the acrosome of sperms tended to suffer greater damage, until the sperms eventually lost their flagella. Abnormalities in these structures have appeared to account, at least in part, for the decline of the fertilization rate of eggs inseminated with UV-irradiated sperms.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Japanese scallop; UV irradiation; Gynogenesis; Sperm morphology
1. Introduction
As a form of development in which eggs are activated by sperm that does not contribute genetically to the resulting embryo, artificial gynogenesis has been
success-)Corresponding author. Tel.:q81-225-53-2436; fax:q81-225-53-2303.
Ž .
E-mail address: [email protected] A. Kijima .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Q. Li et al.rAquaculture 186 2000 233–242
234
Ž
fully induced in many fish species and used for examining sex-determination Avtalion
. Ž .
and Don, 1990 and gene–centromere recombination Arai et al., 1991 , as well as
Ž
production of inbred line, mono-sexual broods or clones Naruse et al., 1985; Tabata and Gorie, 1988; Taniguchi et al., 1988; Fujino, 1989; Fujioka, 1993; Kobayashi et al.,
.
1994 .
In molluscs, however, studies on gynogenesis have been still preliminary. Although
Ž
induction of gynogenetic diploids has been reported for Haliotis discus hannai Fujino
. Ž . Ž .
et al., 1990 , Crassostrea gigas Guo et al., 1993 , Mytilus edulis Fairbrother, 1994
Ž .
and Mytilis galloproÕincialis Scarpa et al., 1994 , practical procedures for induction of
gynogenetic diploid have not been established for molluscs as for fishes. One of the plausible reasons for this is considered to be the decreasing fertilization rate with
Ž .
increasing ultraviolet UV irradiation time of sperm that has been observed in all these studies. Since the sperms of fish species do not lose their fertilizing ability at a dosage of
Ž
UV irradiation for genetic inactivation Onozato and Yamaha, 1983; Tabata et al., 1986;
. Ž .
Taniguchi et al., 1986 , Kijima 1992 supposed that UV irradiation affects not only the
Ž .
sperm genome, but also the acrosome structure which is absent in teleost fishes in the Pacific abalone. However, the possibility has not been evidenced.
The Japanese scallop, Patinopecten yessoensis, is one of the most commercially important bivalves in Japan. Numerous studies have been performed on propagation of
Ž .
the scallop Yamamoto, 1964; Kanno and Sato, 1980; Nagasaki, 1999 , but few studies have been undertaken on gynogenesis or polyploidy.
In the present study, the effect of UV irradiation on sperm morphology was examined
Ž .
by scanning electron microscopy SEM , and various durations of UV irradiation of sperm were also examined to determine the optimum conditions for induction of gynogenesis in the Japanese scallop, P. yessoensis.
2. Materials and methods
2.1. Gametes
Ž .
Specimens of sexually matured P. yessoensis shell length, 12.5"0.6 cm were
collected in late March and early April at Onagawa Bay, Miyagi Prefecture, Japan. They were separated into males and females and kept in running seawater tanks until used.
Spawning of eggs and sperms was induced by raising water temperature from 98C to
Ž .
148C and injecting 0.5 ml of 1 mM serotonin-creatinine sulfate Wako, Japan into the
gonad. Discharged eggs were collected by suction and rinsed in filtered seawater several
times. Sperm suspension was prepared at a concentration of 1=107 sperm
rml by
dilution with filtered seawater.
2.2. UV irradiation of sperm and insemination
Two milliliters of sperm suspension were spread on a 9.0-cm diameter plastic petri
Ž .
dish Nunclon dish; Nalge Nunc, Denmark . The dish was placed on a shaker 25 cm
Ž .
provided an UV intensity of 720 mW cmy2 sy1 as measured by a digital radiometer
ŽDRC-100X; Spectronics, USA . The sperm was exposed to the UV light for either 0.
Žcontrol , 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 70 or 90 s. During the UV.
irradiation, the sperm suspension was shaken at approximately 0.5 cyclers.
Ž 4 .
At completion of irradiation, 10 ml of egg suspension 1=10 eggrml was added
to each petri dish, mixed and finally transferred to a beaker for culture at 118C. The fertilization and development rates were calculated by counting the cleaved eggs and the D-shaped larvae at 8 h and day 4 after insemination, respectively. The experiment was repeated three times separately using gametes from different spawning.
2.3. Ploidy determination
The relative value of DNA content in larval cells was determined by DNA
microfluo-Ž .
rometry in accordance with Komaru et al. 1988 . Samples of veliger larvae were collected from each group on day 3 postinsemination, subjected to a hypotonic treatment
Ž
with 0.075 M KCl solution, fixed by cold Carnoy’s solution methanol:acetic acid, 3:1,
.
vrv and stored iny208C. Larvae were dissociated in 50% acetic acid by aspirating
Ž .
with a pipette. A sample of the cell suspension and control cells diploid veliger cells were placed on a warmed glass slide, air-dried and stained with 4X
,6-diamidino-2-phenyl-Ž .
indole dihydrochloride DAPI . The fluorescence intensity of nuclei was measured with a Nikon P1 photometer.
Q. Li et al.rAquaculture 186 2000 233–242
236
2.4. SEM of spermatozoa
The sperm suspension UV-irradiated for either 0, 30, 60, 90 or 120 s was placed on a
Ž 2.
small piece of slide glass approximately 5=5 mm coated with 0.1% poly-L-lysine
ŽWako , prefixed with 1% paraformaldehyde Wako and 2.5% glutaraldehyde EM Sci.,. Ž . Ž
. Ž .
USA in 0.2 M phosphate buffer pH 7.4 for 2 h at room temperature, washed three
Ž .
times in phosphate buffered saline PBS, pH 7.2, 0.85% NaCl , and then postfixed in
Ž .
2% Osmic acid solution Wako for 1 h at 48C. After washing three times with PBS, the
Ž .
samples were dehydrated in ethanol 50–100% , freeze-dried in t-butyl alcohol using an
Ž .
HITACHI ES2030 freeze dryer Hitachi, Japan , attached to larger metal discs, sputter-coated with Platinum–Paladium using an HITACHI E-1030 ion sputter, and observed with an HITACHI S-4200 scanning electron microscope.
3. Results
3.1. Effects of UV irradiation dosage on the rates of fertilization and deÕelopment
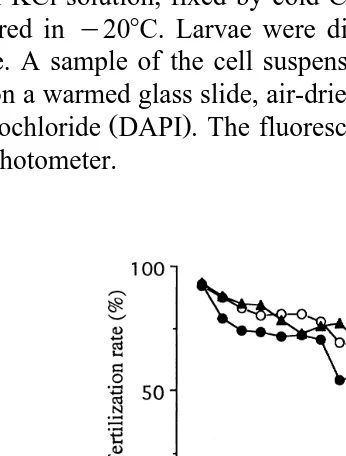
Relationships between the duration of UV irradiation and the rates of fertilization and development of D-shaped larvae are shown in Fig. 1. In experiments 1, 2 and 3, the fertilization rate for controls was more than 90%, but was apparently reduced with increasing exposure time, falling to 70%–80% by 30 s and to approximately 45%–60% by 60 s irradiation. As UV irradiation duration increased, the developmental rate of D-shaped larvae decreased sharply and became 0 by 30 s irradiation in each of the three experiments, at the same time more and more eggs developed into abnormal larvae and never reached D-larval stage.
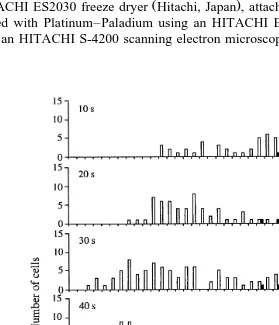
3.2. Microfluorometry
Fig. 2 shows the distribution of fluorescence intensity from veliger larvae cells of P. yessoensis by UV irradiation for 10, 20, 30, 40, 50 and 60 s. For the 10- and 20-s irradiation groups, the fluorescence intensity modal values of veliger larvae cells from eggs inseminated with UV-irradiated sperm were still observed at 40–55 FU, the same as those of diploid control cells, and cells having lower fluorescence intensity value apparently increased. For the 30- and 40-s irradiation groups, in spite of a small number of cells showing the diploid control values, the mode at around 20–27 FU, which was estimated to be the haploid values, was clearly seen. For the 50- and 60-s irradiation groups, the mode at around 20–27 FU became more distinct and almost no cells exhibited the diploid control values, and the mean fluorescence intensity values of larvae cells were, respectively, 25.2"5.4 and 24.5"5.6 FU, which were approximately half
Ž .
that of diploid control cells 48.9"3.0 and 48.7"3.2 FU , indicating that haploid gynogenesis was successfully induced.
Ž .
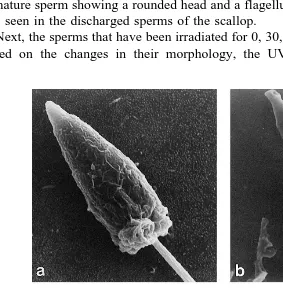
Fig. 3. SEM micrographs of normal spermatozoa of the scallop, P. yessoensis. a Whole spermatozoa
Žbars4mm . Arrowhead indicates a immature spermatozoon with a rounded head; b the nucleus region of a. Ž .
Ž .
Q. Li et al.rAquaculture 186 2000 233–242
238
3.3. Effect of UV irradiation on sperm morphology
The structure of normal untreated sperm was observed by SEM for comparison with
Ž .
the irradiated sperm Fig. 3a,b . The mature spermatozoon of P. yessoensis was
3.9"0.2mm long when measured from the tip of the acrosome to the distal end of the
Ž .
mid-piece ns30 . It was composed of a cone-shaped acrosome, an elongated nucleus,
Ž .
a short mid-piece and a single flagellum Fig. 3b . The nucleus was conical in form,
Ž .
3.0"0.2mm long and 1.3"0.1mm wide ns30 . The acrosome measured 0.5"0.1
Ž .
mm in length and 0.5"0.0 mm in maximum diameter ns5 . The midpiece showed
the typical primitive arrangement with mitochondrial spheres around a centriolar
appara-Ž .
tus. The flagellum had a total length of 50.2"2.9 mm ns10 . In addition, the
immature sperm showing a rounded head and a flagellum, but without an acrosome, was also seen in the discharged sperms of the scallop.
Next, the sperms that have been irradiated for 0, 30, 60, 90 and 120 s were examined. Based on the changes in their morphology, the UV-irradiated mature sperms were
Ž . Ž .
Fig. 4. SEM micrographs of UV-irradiated spermatozoa of P. yessoensis bars1mm . a A 30-s irradiated
Ž .
spermatozoon characteristic of group A, in which no morphological changes could be observed; b a 30-s
Ž .
irradiated spermatozoon characteristic of group B, in which the flagellum was lost; c a 60-s irradiated
Ž .
Table 1
Ž .
Distribution of spermatozoa from various UV irradiation doses, classified into groups A–D based on the changes in morphology as assessed by SEM
Figures in parentheses are number of spermatozoa observed.
Ž .
classified into the following four groups: Group A sperms showed no apparent change in
Ž . Ž .
morphology Fig. 4a ; Group B sperms had a missing flagellum Fig. 4b ; Group C
Ž .
sperms had an abnormal acrosome Fig. 4c ; and Group D sperms had both a missing
Ž .
flagellum and an abnormal acrosome Fig. 4d . For each irradiation duration, more than 50 randomly selected mature sperms were used to classify the sperms into groups A–D. Table 1 shows the changes in percentage of the four groups of sperms in different UV irradiation groups. The group A type accounted for 98% of the control group sperms, but this percentage decreased significantly with increasing exposure time. The percent-age of group B sperms was consistently low and changed little in all groups. Group C sperms were not observed in the control group but occurred in all irradiation groups, accounting for 29%, 44%, 28% and 23% of sperms in the 30, 60, 90 and 120 s irradiation groups, respectively. Group D sperms markedly increased with increasing irradiation duration, reaching a maximum proportion of 77% in the 120-s irradiation group.
4. Discussion
In this study, although the rates of fertilization and development of D-shaped larvae markedly decreased with the increase in exposure duration, haploid gynogenesis of the
Japanese scallop was successfully induced by 50–60 s UV irradiation of 720mW cmy2
sy1. In C. gigas, the threshold dosage was 5–6 min at an UV intensity of 1080 mW
y2 y1 Ž . Ž
cm s Guo et al., 1993 . In M. galloproÕincialis, the 2-min irradiation 620 mW
y2 y1. Ž
cm s was the most successful in achieving gynogenetic development Scarpa et
.
al., 1994 . In M. edulis, the 15-min irradiation provided the highest incidence of haploid
Ž .
embryos UV intensity unshown; Fairbrother, 1994 . In bivalve molluscs, the optimum dose for genetically inactivating sperm might vary from species to species and depends on the density and volume of sperm suspension and UV intensity.
Inactivation of sperm chromosomes by UV irradiation is suggested to be a continuous
Ž .
process Guo et al., 1993 . The viable larvae with around 30–40 FU shown here are
Ž
considered to be aneuploids, which have been also reported in the previous studies Arai
.
Q. Li et al.rAquaculture 186 2000 233–242
240
and measurement, andror existence of natural aneuploids, which occur in C. gigas
ŽThiriot Quievreux et al., 1988 ..
The decrease in fertilization rate with increasing UV irradiation time was reported
Ž . Ž .
also for H. discus hannai Arai et al., 1984; Kijima, 1992 , C. gigas Guo et al., 1993 ,
Ž .
and M. edulis Fairbrother, 1994 , indicating that the ability of sperm to activate eggs
Ž .
was reduced as irradiation duration increased. In H. discus hannai, Kijima 1992 assumed that the acrosome structure of sperm is damaged by UV irradiation, but did not submit any evidence.
The ultrastructural features of normal Japanese scallop spermatozoa observed here are
Ž
fundamentally similar to that described for other bivalve molluscs Hodgson and
Bernard, 1986; Dorange and Le Pennec, 1989; Peredo et al., 1990; Rocha and Azevedo,
.
1990; Nicotra and Zappata, 1991; Bozzo et al., 1993; Garrido and Gallardo, 1996 , demonstrating a cone-shaped acrosome, conical nucleus, short mid-piece, and a tail
flagellum about 50-mm long. The spermatozoon is of the primitive type usually
Ž .
associated with species having external fertilization Franzen, 1983 .
´
On the other hand, the UV-irradiated sperms exhibited the destruction of the acrosome and flagellum. As the duration of UV irradiation increased, the acrosome of sperms tended to suffer greater damage, until eventually the sperms lost their flagella. Because the flagellum and acrosome are essential for the sperm to move to and penetrate
Ž .
the egg envelope and then fertilize the egg Hylander and Summers, 1977 , abnormali-ties in these structures have appeared to account, at least in part, for the decline of the fertilization rate of eggs inseminated with UV-irradiated sperms. In tilapia Oreochromis niloticus, the loss of sperm flagellum due to UV irradiation has also been observed, and suggested that the UV irradiation might have caused depolymerization of the
microtubu-Ž .
lar fibres of the flagellum Don and Avtalion, 1993 . A small number of sperms in
Ž .
which only the flagellum was lost Group B sperms were retained in each treatment group, including the control group, and thus Group B sperms were considered to result from physical damage occurring during sample fixation rather than from the effects of UV irradiation.
The destruction of the sperm acrosome and flagellum resulting from UV irradiation
Ž .
has also been observed in the Pacific oyster Li et al., in press , suggesting that it might be a common problem in molluscs. Although the damages of sperm by UV irradiation decreased efficiency of gynogenetic induction, the fertilization rate could be improved
Ž .
by increasing insemination concentration of UV-irradiated sperm Li, unpublished , and successful production of viable gynogenesis was possible in the Japanese scallop.
Acknowledgements
Ž
This work was supported by a Grant-in-Aid National Project for Development of
.
Innovative Technology on Agriculture, Forestry and Fisheries from the Society for
Ž .
Techno-innovation of Agriculture, Forestry and Fisheries of Japan STAFF . The
experi-ments. We also wish to thank Mr. H. Matsuura of Miyagi Prefecture Fisheries Research and Development Center for technical assistance on the microfluorometry.
References
Arai, K., Fujino, K., Sei, N., Chiba, T., Kawamura, M., 1991. Estimating rate of gene–centromere recombination at eleven isozyme loci in the SalÕelinus species. Nippon Suisan Gakkaishi 57, 1043–1055.
Arai, K., Naito, F., Sasaki, H., Fujino, K., 1984. Gynogenesis with ultraviolet ray irradiated sperm in the Pacific abalone. Bull. Jpn. Soc. Sci. Fish. 50, 2019–2023.
Avtalion, R.R., Don, J., 1990. Sex-determination genes in tilapia: a model of genetic recombination emerging from sex ratio results of three generations of diploid gynogenetic Oreochromis aureus. J. Fish Biol. 37, 167–173.
Bozzo, M.G., Ribes, E., Sagrista, E., Poquet, M., Durfort, M., 1993. Fine structure of the spermatozoa of
Ž .
Crassostrea gigas Mollusca, Bivalvia . Mol. Reprod. Dev. 34, 206–211.
Don, J., Avtalion, R.R., 1993. Ultraviolet irradiation of tilapia spermatozoa and the Hertwig effect: electron microscopic analysis. J. Fish Biol. 42, 1–14.
Dorange, G., Le Pennec, M., 1989. Ultrastructural characteristics of spermatogenesis in Pecten maximus
ŽMollusca, Bivalvia . Invertebr. Reprod. Dev. 15, 109–117.. Ž .
Fairbrother, J.E., 1994. Viable gynogenetic diploid Mytilus edulis L. larvae produced by ultraviolet light irradiation and cytochalasin B shock. Aquaculture 126, 25–34.
˚
Franzen, A., 1983. Ultrastructural studies of spermatozoa in three bivalve species with notes on evolution of´ elongated sperm nucleus in primitive spermatozoa. Gamete Res. 7, 199–214.
Fujino, K., 1989. Historical aspects of research and development of genome manipulation technologies. In:
Ž .
Suzuki, R. Ed. , Chromosome Manipulation and its Application for Aquaculture. Koseisha koseikaku,
Ž .
Tokyo, pp. 9–20, in Japanese .
Fujino, K., Arai, K., Iwadare, K., Yoshida, T., Nakajima, S., 1990. Induction of gynogenetic diploid by inhibiting second meiosis in the Pacific abalone. Nippon Suisan Gakkaishi 56, 1755–1763.
Fujioka, Y., 1993. Induction of gynogenetic diploids and cytological studies in honmoroko Gnathopogon
caurulescens. Nippon Suisan Gakkaishi 59, 493–500.
Garrido, O., Gallardo, C.S., 1996. Ultrastructure of sperms in bivalve molluscs of the Mytilidae family. Invertebr. Reprod. Dev. 29, 95–102.
Guo, X., Hershberger, W.K., Cooper, K., Chew, K.K., 1993. Artificial gynogenesis with ultraviolet light-irradiated sperm in the Pacific oyster, Crassostrea gigas: I. Induction and survival. Aquaculture 113, 201–214.
Hodgson, A.N., Bernard, R.T.F., 1986. Ultrastructure of the sperm and spermatogenesis of three species of
Ž .
Mytilidae Mollusca, Bivalvia . Gamete Res. 15, 123–135.
Hylander, B.L., Summers, R.G., 1977. An ultrastructural analysis of the gametes and early fertilization in two bivalve molluscs, Chlama macerophylla and Spisula solidissima with special reference to gamete binding. Cell Tissue Res. 182, 469–489.
Kanno, H., Sato, S., 1980. Present aspects of commercial scallop culture in Japan. In: The Japanese Society of
Ž .
Fisheries Science Ed. , Systematic Approach to Mariculture and Ultilization of Scallop. Koseisha
Ž .
koseikaku, Tokyo, pp. 11–25 in Japanese .
Kijima, A., 1992. Effect of UV irradiation on genetic inactivation of sperm using marketing tissue culture petri dish in the Pacific abalone Haliotis discus hannai. Tohoku J. Agric. Res. 42, 73–81.
Kobayashi, T., Ide, A., Hiasa, T., Fushiki, S., Ueno, K., 1994. Production of cloned amago salmon
Oncorhynchus rhodurus. Fish. Sci. 60, 275–281.
Komaru, A., Uchimura, Y., Ieyama, H., Wada, K.T., 1988. Detection of induced triploid scallop, Chlamys
nobilis, by DNA microfluorometry with DAPI staining. Aquaculture 69, 201–209.
Q. Li et al.rAquaculture 186 2000 233–242
242
Nagasaki, M., 1999. The situation and problems of the scallop farming at southern coast of Funka Bay. Suisan
Ž .
Zoshoku 47, 145–150, in Japanese .
Ž
Naruse, K., Ijiri, K., Shima, A., Egami, N., 1985. The production of cloned fish in the medaka Oryzias
.
latipes . J. Exp. Zool. 236, 335–341.
Nicotra, A., Zappata, S., 1991. Ultrastructure of the mature sperm and spermiogenesis in Callista chione
ŽMollusca, Bivalvia . Invertebr. Reprod. Dev. 20, 213–218..
Onozato, H., Yamaha, E., 1983. Induction of gynogenesis with ultraviolet rays in four species of
salmoni-Ž .
formes. Nippon Suisan Gakkaishi 49, 693–699, in Japanese .
Peredo, S., Garrido, O., Parada, E., 1990. Spermiogenesis and sperm ultrastructure in the freshwater mussel
Ž .
Diplodon chilensis chilensis Mollusca, Bivalvia . Invertebr. Reprod. Dev. 17, 171–179. Ž
Rocha, E., Azevedo, C., 1990. Ultrastructural study of the spermatogenesis of Anodonta cygnea L. Bivalvia,
.
Unionidae . Invertebr. Reprod. Dev. 18, 169–176.
Scarpa, J., Komaru, A., Wada, K.T., 1994. Gynogenetic induction in the mussel, Mytilus galloproÕincialis.
Ž .
Bull. Natl. Res. Inst. Aquacult. Jpn. 23, 33–41.
Tabata, K., Gorie, S., 1988. Induction of gynogenesis diploids in Paralichthys oliÕaÕeus by suppression of the
1st cleavage with special reference to their survival and growth. Nippon Suisan Gakkaishi 54, 1867–1872. Tabata, K., Gorie, S., Nakamura, K., 1986. Induction of gynogenetic diploid in hirame Paralichthys oliÕaceus.
Ž .
Nippon Suisan Gakkaishi 52, 1901–1904, in Japanese .
Taniguchi, N., Kijima, A., Fukai, J., Inada, Y., 1986. Conditions to induce triploid and gynogenetic diploid in
Ž .
ayu Plecoglossus altiÕelis. Nippon Suisan Gakkaishi 52, 49–53, in Japanese .
Taniguchi, N., Seki, S., Fukai, J., Kijima, A., 1988. Induction of two types of gynogenetic diploids by hydrostatic pressure shock and verification by genetic marker in ayu. Nippon Suisan Gakkaishi 54, 1483–1491.
Thiriot Quievreux, C., Noeel, T., Bougrier, S., Dallot, S., 1988. Relationships between aneuploidy and growth rate in pair matings of the oyster Crassostrea gigas. Aquaculture 75, 89–96.
Ž .
Yamamoto, G., 1964. Studies on the propagation of the scallop, Patinopecten yessoensis Jay , in Mutsu Bay.
Ž .