The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
1
Synthesis Organonitrogen Compounds from Patchouli Alcohol
1
Through Ritter Reaction with Acetonitrile and Its Toxicity to
2
Artemia salina
Leach.
3 4
Khoirun Nisyak*, M. Farid Rahman, dan Sutrisno 5
6
Chemistry Department, Brawijaya University 7
Jln. Veteran, Malang 65145, Indonesia 8
email : [email protected] 9
10
Received day moth year; Accepted day moth year (will be given) 11
12
ABSTRACT
13
Patchouli oil contains a compound with biological activities to human body called the 14
patchouli alcohol that can be further developed in medical field. This research aimed to 15
synthesize organonitrogen compound from patchouli alcohol through Ritter reaction with 16
acetonitrile and discover its toxicity towards Artemia salina Leach. The isolation of patchouli
17
alcohol from patchouli oil using fractional distillation under reduced pressure method. The 18
synthesis of organonitrogen compound is done at room temperature with the mol ratio of 19
patchouli alcohol: acetonitrile: sulfuric acid is 1:1,5:4 for 24 hours. The result showed that the 20
amount of patchouli alcohol produced from fractional distillation is 65,25%. The main 21
product yielded from the synthesis between patchouli alcohol and acetonitrile through Ritter 22
reaction is 36,93% of N-(4,8a,9,9-tetramethyl decahydro-1,6-metanonaftalen-1-il) acetamide. 23
Starting material used have LC50 of 77,39 ppm. The product of synthesis have higher toxicity 24
level than starting material, which have LC50 value is 10,39 ppm with the potential as medical 25
compound. 26
Key word: patchouli oil, patchouli alcohol, synthesis organonitrogen compound, Ritter 27
reaction, toxicity 28
29 30
INTRODUCTION 31
Indonesia is the largest producer of patchouli oil in the world, where 90 % of the world’s 32
patchouli oil needs are supplied from Indonesia [1]. Given the scale abundance of patchouli 33
oil is a challenge for people to explore it further into new products and to increase the resale 34
value. One is the use of patchouli oil in the development of therapeutic areas. 35
Various studies have shown that the potential of patchouli oil as a base for medicine. 36
Patchouli oil is shown to have pharmacological activity as an agent inhibiting platelet 37
activating factor (PAF) [2], anti microbial, sedative agents, antiseptic [3], antiviral [4], and 38
antifungal [5]. Pharmacological activity is influenced by the content patchouli alcohol is the 39
mayor compound in patchouli oil [3]. Patchouli alcohol can inhibit influenza virus [4]. This 40
encourages the optimation efforts patchouli alcohol as a pro drug compounds through 41
molecular modification of the structure to be beneficial in the medicine world. 42
Research have been done to modify the structure of patchouli alcohol during the 43
formation ester compound is carried out using acetic acid and acid catalyst producing 44
patchouli acetate compound [6] and dehydration becomes patchoulene by using a strong acid, 45
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
2
formation of organonitrogen compounds, i.e hydrocarbons containing N atom in a variety of 47
bonds. Organonitrogen compounds have properties related to biological and physiological 48
potential as a cure for diseases related to disorders of the central nervous system and its 49
ability to interact with receptors of the body [8]. Organonitrogen compounds have biological 50
activity as vasodilator, anti-inflammatory, antiviral, antimicrobial, analgesic, antidepressants, 51
antischistosoma, antitumor, and anticonsvulsan [9]. 52
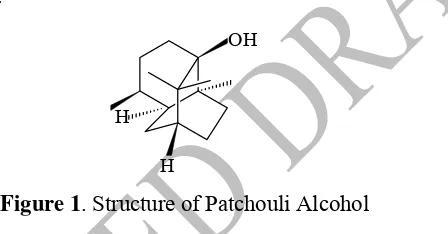
Patchouli alcohol has a rigid structure and including the tertiary alcohol group, which has 53
activity related to the formation of a stable carbocation. Reaction that can be used in the 54
formation organonitrogen compound with alcohol compound as the starting material through 55
formation of carbocations is the Ritter reaction. Ritter reaction is a reaction to the formation 56
N-alkyl carboxyamide from aliphatic or aromatic nitrile and carbocations in strong acid 57
media [10]. In the Ritter reaction will be produced carbocations as intermediates. Formation 58
of a stable carbocation is due to the protonation of a strong acid, which carbocations are later 59
attacked by the nucleophilic nitrogen atom. Acetonitrile used in this study as a nitrile reagent 60
that attacks the carbocations. 61
Figure 1. Structure of Patchouli Alcohol 69
70
Organonitrogen compounds have the ability to interact with receptors of the body 71
because of the hetero group that has a certain affinity and polarity that resulted in the 72
occurrence of molecular interactions through biochemical mechanisms in the body [11]. 73
Further studies to determine the ability of the products synthesized as a drug compound to be 74
done through the toxicity test by brine shrimp lethality test (BSLT). The results of toxicity 75
test the method BSLT reflects its potential as a drug compound. Wijdharti, et al [12] states 76
that BSLT method is a method that is simple, rapid, inexpensive, and reliable and usually 77
done at the preliminary stage of the screening materials that are thought to have anticancer 78
properties before stepping to the test in vitro against tumor cell sustainably. 79
80 81
EXPERIMENTAL SECTION 82
and DMSO, was purchased from MERCK, Artemia salina eggs was purchased from UIN 86
Malang, and patchouli alcohol (65,25%) was obtained from fractional distillation under 87
reduced pressure towards patchouli oil that purchased from Blitar, East Java. 88
89
Instrumentation 90
91
The journal homepage www.jpacr.ub.ac.id
Isolation Patchouli Alcohol from Patchouli Oil
96
A number of 75 mL of patchouli oil that has been analyzed compounds with GC-MS, 97
dried with Na2SO4 anhydrous and then inserted into the flask capacity 100 mL and put in a 98
series distillation apparatus with condenser column of 60 cm and vigreux column of 30 cm. 99
The process is then performed by fractional distillation under reduced pressure, the residue 100
was obtained then analyzed with GC-MS and FT-IR 101
102
Synthesis of Patchouli Acetamide Compound Through Ritter Reaction
103 104
A number of 28,2 mL of (contain 0,1 mol patchouli alcohol) is inserted into the flask 105
100 mL three-neck equipped with a thermometer. A number of 7,8 mL of acetonitrile (0,15 106
mol) is added to the flask. The mixture was cooled to 0 °C, cold condition are maintained 107
with the addition of salt around the flask. A number of 22 mL of 97% sulfuric acid (0,4 mol) 108
was added slowly dropwise to the mixture while stirring with a magnetic stirrer. Having run 109
out of concentrated sulfuric acid, the mixture is left at room temperature while stirring with a 110
magnetic stirrer for 24 hours. Synthesis mixture was poured into the erlenmeyer flask 111
containing 100 mL of cold distilled water. Then added 100 mL of diethyl eter, stirred, and 112
separated by a separating funnel. 113
Obtained organic phase was neutralized with saturated sodium carbonate solution and 114
saturated sodium chloride solution was added. Neutral organic phase was dried with Na2SO4 115
anhydrous, then filtered. The solution was concentrated with nitrogen gas. The compounds 116
synthesized were analyzed functional groups with FT-IR and analyzed the content of its 117
components by GC-MS. 118
119 120
Toxicity Test to Artemia salina Leach.
121 122
Artemia salina eggs are incubated in brine at pH 7-8 (48 h). Then, series of solutions of 123
test substances at varying concentrations and progressive were prepared in DMSO (dimethyl 124
sufoxide) and brine. A defined number of larvae introduced into each solution. All solution 125
sand control solutions containing no active substance were left stirring for 24 hours. Counting 126
under a microscope the number of death larvae in each solution used to evaluate the toxicity 127
of the solution. Tests were carried out in triplicate. 128
Percent of lethality shrimp Artemia salina calculated at each concentration by the 129
formula [13]: 130
131
where Nt is the number of shrimp larvae that died after incubation for 24 hours and No is the 132
total number of shrimp larvae are included. LC50 value is then determined by linear 133
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
4
RESULT AND DISCUSSION 140
141
Isolation Patchouli Alcohol from Patchouli Oil 142
143
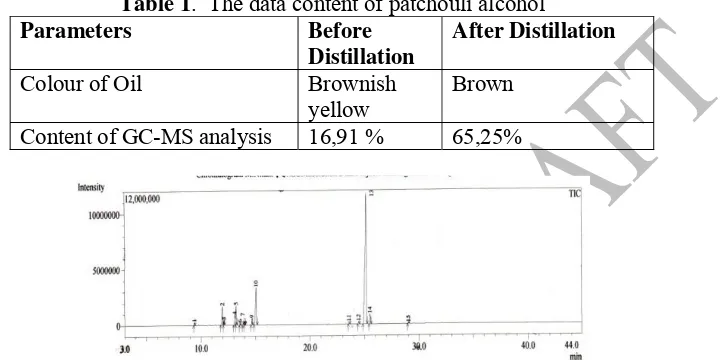
The yield of patchouli alcohol was obtained from fractional distillation under reduced 144
pressure 65,25 % at 100 mmHg. 145
146
Table 1. The data content of patchouli alcohol 147
Parameters Before Distillation
After Distillation
Colour of Oil Brownish
yellow
Brown
Content of GC-MS analysis 16,91 % 65,25% 148
149
Figure 2. The chromatogram of patchouli alcohol was obtained from fractional distillation 150
151
Synthesis Patchouli Acetamide Through Ritter Reaction 152
153
Based on the analysis using GC-MS using a column Rtx-wax, the chromatogram 154
obtained compounds synthesized as shown in Figure 3. Chromatogram compounds 155
synthesized showed a peak that has 14% area of more than 1% and 12 peaks that have M+ = 156
263 with a retention time sequence, shown in Table 2. Peak with M+ = 263 major expected 157
outcome is a product synthesis, namely N-(4,8a,9,9-tetramethyldecahydro-6,1-1-yl 158
methanonaphtalena) acetamide, or better known as patchouli acetamide. 159
160
161
Figure 3. The chromatogram compound synthesis 162
163
Table 2. Data Synthesis The Compound Allegations 164
Peak Retention Time
(minute)
% Area m/z
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
5
149, 163, 189, 206, 263
30 22,733 0,49% 30, 41, 43, 60, 79, 91, 98, 120, 137, 147, 163, 191, 206, 265
31 22,900 1,61% 30, 41, 43, 56, 81, 91, 107, 122, 136, 148, 163, 175, 189, 204, 263
32 23,325 1,71% 41, 43, 67, 91, 107, 121, 136, 148,
161, 175, 189, 204, 248, 263
33 23,625 0,81% 30, 41, 43, 67, 79, 91, 107, 120, 134, 149, 161, 189, 204, 263
34 23,867 1,38% 30, 41, 43, 67, 81, 91, 105, 124, 132, 152, 161, 178, 190, 206, 220, 248, 263
35 24,308 9,32% 30, 41, 43, 67, 79, 91, 107, 121, 133, 147, 161, 177, 189, 204, 220, 263
36 24,692 0,38% 30, 41, 43, 67, 84, 107, 122, 136,
148, 161, 189, 204, 263
37 24,917 0,99% 30, 41, 43, 67, 79, 91, 107, 120, 133, 148, 163, 177, 189, 204, 263
38 25,567 7,98% 30, 41, 43, 60, 81, 95, 114, 129, 151, 163, 177, 191, 206, 222, 250, 265 39 25,800 34,31% 30, 41, 43, 67, 79, 91, 107, 121, 133,
147, 161, 177, 189, 204, 220, 248, 263
165
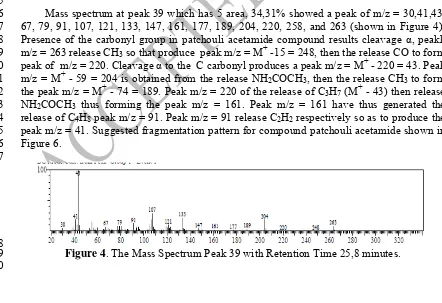
Mass spectrum at peak 39 which has 5 area, 34,31% showed a peak of m/z = 30,41,43, 166
67, 79, 91, 107, 121, 133, 147, 161, 177, 189, 204, 220, 258, and 263 (shown in Figure 4). 167
Presence of the carbonyl group in patchouli acetamide compound results cleavage α, peakk 168
m/z = 263 release CH3 so that produce peak m/z = M+ -15 = 248, then the release CO to form 169
peak of m/z = 220. Cleavage α to the C carbonyl produces a peak m/z = M+ - 220 = 43. Peak 170
m/z = M+ - 59 = 204 is obtained from the release NH2COCH3, then the release CH3 to form 171
the peak m/z = M+ - 74 = 189. Peak m/z = 220 of the release of C3H7 (M+ - 43) then release 172
NH2COCH3 thus forming the peak m/z = 161. Peak m/z = 161 have thus generated the 173
release of C4H8 peak m/z = 91. Peak m/z = 91 release C2H2 respectively so as to produce the 174
peak m/z = 41. Suggested fragmentation pattern for compound patchouli acetamide shown in 175
Figure 6. 176
177
178
Figure 4. The Mass Spectrum Peak 39 with Retention Time 25,8 minutes. 179
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
6
H N C
O CH2
e
H N C
O CH3 C
O CH3
m/z = 263
m/z = 43
e
H N C
O
CH3
H N C
O
m/z = 248 - CH3
H N CH3
m/z = 220 - CO
- NH2COCH3
m/z = 204
- CH3
H2C
m/z = 189
H N C
O
e
- C3H7 H N C
O
m/z = 220 - NH2COCH3
m/z = 161 - CH2
m/z = 147 - C4H8
m/z= 91
- C2H2
m/z = 67
m/z = 41
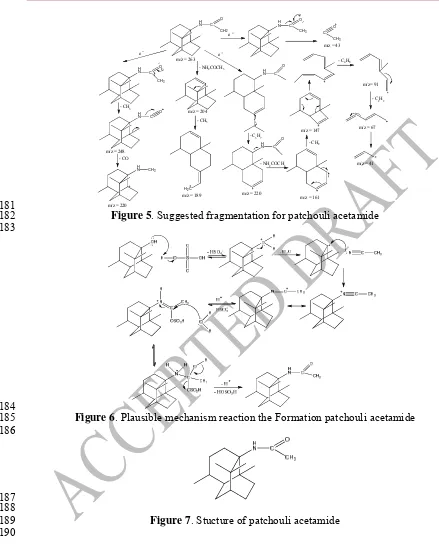
181
Figure 5. Suggested fragmentation for patchouli acetamide 182
183
184
Figure 6. Plausible mechanism reaction the Formation patchouli acetamide 185
186
187 188
Figure 7. Stucture of patchouli acetamide 189
The journal homepage www.jpacr.ub.ac.id
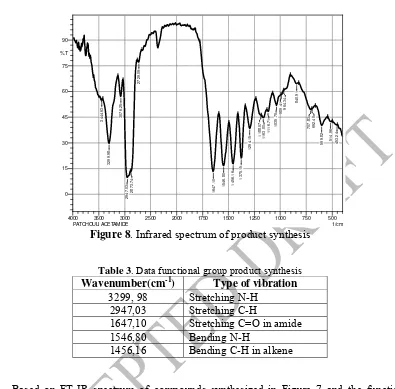
Figure 8. Infrared spectrum of product synthesis 192
193 194
Table 3. Data functional group product synthesis 195
Wavenumber(cm-1) Type of vibration 3299, 98 Stretching N-H
2947,03 Stretching C-H
1647,10 Stretching C=O in amide
1546,80 Bending N-H
1456,16 Bending C-H in alkene 196
197
Based on FT-IR spectrum of compounds synthesized in Figure 7 and the functional 198
groups of data in Table 3, show a strong absorption at wavenumber 3299 cm-1 which is the N-199
H stretching vibration of secondary amide. Uptake was supported by the absorption at 200
wavenumber 1647,10 cm-1 which is the vibration of the amide carbonyl stretching and at 201
wavenumber 1546,80 cm-1 which is an N-H bending vibration. This indicates that the 202
presence of secondary amide compounds contained in the compounds synthesized. Based on 203
the percent results between the mass of the synthesis results compared with the theoretical 204
mass, percent yield of product patchouli acetamide through Ritter reaction is 36,93%. 205
206
Toxicity Test to Artemia salina Leach. 207
208
Table 4. The calculation result data LC50 from BSLT test 209
No. Sampel Graph Equation LC50
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
8
Product synthesis containing patchouli acetamide 36,93% have a higher toxic properties, 211
where the compounds are patchouli acetamide which has a lone pair of N atom and the 212
carbonyl group has two lone pair on O atom. Presence of three lone pairs on these 213
compounds (patchouli acetamide) its active side to form hydrogen bonds with DNA [...]. This 214
binding causes the protein synthesis process is hampered and toxic effect at low doses. 215
Based on research that has been done, it can be concluded that the main products of the 220
synthesis of patchouli alcohol with acetonitrile trough Ritter reaction is N-(4,81,9,9-221
tetramethyl decahydro-6-1-1-yl methanonaphtalene) acetamide or patchouli acetamide, 36,93 222
%. Synthesized compounds have a toxicity level greater than starting material, which is 10,93 223
ppm, so the potential for drug compounds. 224
225
ACKNOWLEDGMENT 226
227
The authors gratefully acknowledge DIKTI for financial support through the Program 228
Kreativitas Mahasiswa 2011 program. 229
of Fitoterapia, 326(06), 224-3. 234
2. Das, K., Gupta, N.K., Vijayabhaskar, S., dan Manjunath, M., 2011, Indian Journal of
235
Novel Drug, 3(2), 104-111. 236
3. Wu, H., Li, B., Wang, X., Jin, M,, dan Wang, G., 2011, Journal Molecules 2011, 16(1), 237
6489-6501, ISSN 1420-3049. 238
4. Sundaresan, Sigh, S.P., Mishra, A.N., Shasany, A.K., Darokar. M.P., Kalra, A., and Naqvi, 239
A.A., 2009, Journal of Essential Oil Research, 21(220). 240
5. Bulan, R., Esterifikasi Patchouli Alkohol Hasil Isolasi dari Minyak Nilam (Patchouli Oil), 241
Jurusan Kimia, Fakultas Matematika dan Ilmu Pengetahuan Alam Universitas Sumatera 242
Utara, Medan, 2004. 243
6. Ma’mun and Maryadhi, 2008, Jurnal Bul. Littro, 19(1), 95 – 99. 244
7. Aisyah, Y., Hastuti, P., Sastrohamidjojo, H., and Hidayat, C., 2008, Komposisi Kimia dan 245
Sifat Antibakteri Minyak Nilam (Pogostemon cablin), Majalah Farmasi Indonesia, 19(3), 246
151-156. 247
8. Hapsari, K.W., Reaksi Esterifikasi Patchouli Alkohol dalam Minyak Nilam (Patchouli oil) 248
dengan Asam Asetat Anhidrid Menggunakan Katalis H2SO4 dan H3PO4, Thesis, 249
Department of Chemistry, Faculty of Mathematics and Natural Sciences Brawijaya 250
University, Malang, 2008. 251
9. Novitasari, R.D., Dehidrasi Patchouli Alkohol dalam Minyak Nilam (Patchouli Oil) 252
Menggunakan Asam Sulfat (H2SO4) dan Asam Fosfat (H3PO4) dengan Variasi Pelarut, 253
Thesis, Department of Chemistry, Faculty of Mathematics and Natural Sciences Brawijaya 254
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
9
10. Rahman, M.F., Retnowati, R., and Suratmo, Derivatisasi α-Pinena: Sintesis Senyawa 256
Organonitrogen dan Kajian Potensinya Sebagai Kandidat Obat, Faculty of Mathematics 257
and Natural Sciences Brawijaya University, Malang, 2011. 258
11. Rollas, S., and Küçükgüzel, G., 2007, Molecules,1910-1939. 259
12. Czako, B and Kurti, L., 2005, Strategic Application of Named Reaction in Organic 260
Synthesis, Elsevier Academic Press, London. 261
13. Tamat, S.R., Wikanta, T., and Maulina, L.S., 2007, Jurnal Ilmu Kefarmasian Indonesia, 262
5(1), 31-36. 263