A genetic approach has resulted in an extensive framework for the methodical analysis of ovule development. The most recent progress was accomplished in the areas of
primordium formation and integument morphogenesis. Furthermore, systematic screens have identified a number of gametophytic mutations disrupting several distinct steps of embryo sac ontogenesis.
Addresses
Institute of Plant Biology, University of Zürich, Zollikerstr. 107, CH-8008 Zürich, Switzerland; e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:13–17 http://biomednet.com/elecref/1369526600200013 © Elsevier Science Ltd ISSN 1369-5266
Abbreviation
MMC megaspore mother cell
Introduction
Sexual reproduction constitutes an important event in the life cycle of a plant. In seed plants the ovule represents the major female reproductive organ. The ovule carries the egg cell, it is the organ where fertilization occurs, and it eventually develops into the seed which harbors the plant embryo.
For decades, the development of the ovule was only very poorly understood at the genetic and molecular level. This situation is changing and the ovules, particularly of
Arabidopsis, but also of Petuniaand maize, are now under thorough investigation. This is partly due to the exciting biology of the ovule and also due to its suitability to genetic analysis, which makes the ovule an excellent model system to study organogenesis in plants. Mutations in many genes with an important function in ovule development lead to sterility and this trait is easi-ly scorable in regular mutagenesis experiments. The current molecular and genetic work is accompanied by an extensive analysis addressing the evolution of the basal angiosperm ovule [1,2].
A generalized ovule has a simple but nevertheless highly differentiated structure (Figures 1 and 2). It develops from a protrusion of the placental tissue of the gynoecium. Within the apex or nucellus, the megaspore mother cell (MMC) develops, undergoes meiosis, and one of the resulting megaspores gives rise to the multi-cellular hap-loid embryo sac or female gametophyte which includes the egg cell. In addition, usually two integuments originate from a region known as the chalaza. They will grow around the nucellus and eventually become the seed coat. The connection to the placenta is made through a stalk or funiculus. The nucellus, the chalaza with the integuments,
and the funiculus constitute part of the sporophyte and hence consist of diploid tissue.
This review focuses on some of the advances achieved in the past one and a half years. Thus it largely deals with the control of primordium formation, and the identifica-tion and initial genetic characterizaidentifica-tion of gametophytic genes which are important for the development of the embryo sac. By necessity this review is brief; therefore, I would like to point out a number of recent reviews which discuss many aspects of ovule development in much greater detail [3•–6•].
The formation of the ovule primordium
The initial phase of ovule development encompasses aspects such as initiation and outgrowth of a protrusion from the placental tissue, commitment to the ovule fate and pattern formation in the primordium. The molecular basis of ovule specification is best understood in Petunia hybrida where two MADS box genes are known, floral binding protein 7(FBP7) and FBP11, that function in this process, probably in a redundant manner [7,8]. Recently progress was made in the understanding of the mecha-nism controlling the outgrowth of the ovule protrusion. Pattern formation is less well understood; however, some data begin to shed light on certain aspects of this process as well.
Initiation and outgrowth of the ovule protrusion
In Arabidopsis,the AINTEGUMENTA(ANT) gene acts in floral organ initiation, growth and shape in general [9•,10,11,12•]. It encodes a putative transcription factor [10,11] of the EREBP/AP2 domain class [13]. Compared to wild type, plants defective for ANTfunction develop fewer and more distantly spaced ovules indicating that ANT is involved in ovule primordium initiation. The ovules that are produced form a nucellus, chalaza and funiculus, but lack integuments and an embryo sac.
The typical antmutant phenotype is caused even by puta-tive null-alleles (the corresponding proteins would be truncated by about two-thirds) [10,11], suggesting that par-tially redundant factors regulate ovule primordium outgrowth. Are such activities known? The HUELLEN-LOS(HLL) gene [12•] represents one recently described example [14•] of partially redundant regulation. The phe-notype of hll mutants is restricted to the ovule and is similar to the phenotype of antmutants as far as the early block in integument development is concerned. The analysis of hll antdouble mutants revealed the function of
HLLin ovule primordium outgrowth. Plants defective for
HLL and ANT activities bear ovules that are drastically reduced in their longitudinal or proximal–distal axis. Either the funiculus or both the funiculus and the chalaza
The molecular and genetic control of ovule development
fail to form. A nucellus is always present, however. This implies that HLLand ANTcontrol ovule primordium out-growth in a partially redundant fashion.
Patterning the primordium
Pattern formation deals with the spatial control of cell fate. On the basis of morphological criteria, one can recognize a distinct pattern along a proximal–distal axis of the ovule [15] (Figure 1). Distally, the nucellus is characterized by the presence of an MMC, and eventually an embryo sac. Centrally, the chalaza is recognized as the region that initi-ates the two integuments at its flank. Proximally, the funiculus harbors the vascular strand. From an evolutionary point of view, this is a very conserved pattern and is thus a typical feature of a generalized seed plant ovule [16].
What mechanism creates the proximal–distal arrange-ment? We hypothesized that one important aspect is the setup of a corresponding prepattern, with distal, central and proximal pattern elements, during the early prolifera-tive phase of primordium formation [15] (for a detailed discussion see [5•,6•]). Progress on the identification of genes that directly function in the establishment of the pattern elements has been scarce. The present data, how-ever, allow one to infer some conclusions about the patterning process. For example, the BELL1 (BEL1)
expression pattern provides molecular evidence for the existence of the central pattern element [17]. In addition, the region-specific defects of many early-acting ovule genes, for example BEL1, ANT, or INNER NO OUTER
(INO), argue in favor of the presence of such a pattern [12•]. Furthermore, in hll ant double mutants a nucellus is present even in the absence of a chalaza and/or funiculus, which indicates that the distal pattern is independently formed. This finding also raises the possibility that pattern formation takes place progressively, beginning at the distal end of the primordium [14•]. It does not imply, however, that the formation of the distal element is a prerequisite for establishing the central and proximal pattern elements.
The control of integument development
A number of genes have been identified that are important for the growth and morphogenesis of the integuments [4•–6•]. The results indicate that at least two levels of con-trol exist; some genes, such as HLL, ANT or INNER NO OUTER(INO) are required for the initiation of integuments, others, including for example SHORT INTEGUMENT1
(SIN1), SUPERMAN (SUP), STRUBBELIG (SUB) and
TSO1, for their morphogenesis. The functions of members of the first group are likely to precede the activities of mem-bers of the second class and in some instances this is corroborated by genetic analysis [9•]. For example, in corre-sponding double mutant studies it was shown that ant is epistatic to sin1, sup and tso1and inois epistatic to sup.
The analysis of the later aspects of integument morpho-genesis is still in its infancy; however, the results already foreshadow a complex scenario of multiple distinct steps. The genes being implicated in these events appear to be important for cell proliferation and/or cell shape [4•–6•], and the corresponding mutations lead to an altered mor-phology of the integuments, rather than to their absence. As might be expected for genes with a general function in morphogenesis, members of this class usually exhibit pleiotropic activities. One example is TOUSLED (TSL) [18]. Among other functions, TSL appears to control leaf development, and it promotes specific cell divisions within the floral meristem as well as the growth of the ovule integuments [18,19]. TSL is likely to be part of a signal transduction mechanism as this gene encodes a serine/thre-onine kinase [19,20•]. A second example is TSO1, which is also required for flower and ovule development, and which appears to have a role in cell division and/or directed cell expansion during floral organogenesis [21,22].
From megaspore mother cell to mature
embryo sac
Gametogenesis can be subdivided into two phases [3•,5•]. Sporogenesis encompasses processes such as megaspore
Figure 1
A scheme outlining wild-type ovule development in Arabidopsis thalianaand depicting the postulated proximal–distal pattern elements. The dashed line indicates a possible subdivision of the central pattern element [5•,6•]. The central cartoon omits the beginning curvature of the ovule at this stage. The cartoon to the right shows an embryo sac at a stage after fertilization, but where fertilization has not taken place. It
corresponds to Figure 2a. The tetrad within the nucellus, the embryo sac, the integuments and the vascular strand within the funiculus are indicated. Abbreviations: C, central pattern element, ch, chalaza; D, distal pattern element; fu, funiculus; nu, nucellus; P, proximal pattern element.
D C P
n u ch fu Distal
Proximal
mother cell differentiation and meiosis and eventually results in a tetrad of megaspores. In most species, only the proximal or chalazal-most spore will continue develop-ment. Megagametogenesis proceeds from this single spore (monospory) through a syncytial phase, encompassing three mitoses and elaborate nuclear positioning, followed by cellularization, to produce a seven-celled Polygonum -type embryo sac [23] (Figure 2).
Sporogenesis
In many species, an early step in sporogenesis is the sin-gling out of the prospective MMC from a group of cells in the nucellar L2 (the first subepidermal) layer. How is this event regulated? In maize, the mac1gene is central to this process [24] — it appears to suppress the commitment to the gametogenesis pathway in L2 cells other than the prospective MMC [24].
The genetic control of the events following MMC com-mitment is being elucidated as well. Processes, such as establishment of MMC polarity, meiosis and cytokinesis, are affected in a number of mutants including switch1
(swi1) in Arabidopsis[25] (C Horlow, personal communica-tion) and ameiotic 1(am1) in corn [26,27]. Recently, a large group of genes with a function apparently restricted to sporogenesis was identified [12•]. Mutations in this
MEGASPOROGENESIS-DEFECTIVE (MSD) class of genes lead to a block in gametogenesis at or before the mono-nuclear embryo sac stage.
Megagametogenesis
The emerging picture of the genetic and molecular control of female gametophyte ontogenesis is already quite complex. Firstly, embryo sac development appears to be influenced by signals coming from the integuments, as many mutants with a primary defect in integument development also show altered female gametophyte development [10,11,12•,17,28]. Secondly, it is also possible that the sporophytic genome con-trols embryo sac formation through a mechanism not involving the integuments. In Arabidopsis, there exists a large
class of sterile sporophytic mutants, the embryo sac-defective
(emd)mutants [12•], with the corresponding defects appar-ently restricted to gametophyte development prior to fertilization (Figure 2). It may be that some of the EMD-class genes control the establishment of cues in the megaspore mother cell which are required later during embryo sac development. Thirdly, the haploid or gametophytic genome regulates megagametophyte development [29–31].
Female gametophytic mutants
Until recently, very few female gametophytic mutations had been described [3•,5•]. In the last few years however, several laboratories have been successful in isolating and characterizing a number of gametophytic mutations caus-ing aberrant embryo sac ontogenesis ([3•,5•,32•–34•]; M Hülskamp, personal communication).
The successful screens took advantage of two properties of gametophytic mutations. Usually, female gametophytic mutations result in an aberrant or unfunctional embryo sac. As a consequence, 50% of the ovules of a plant heterozy-gous for a female gametophytic mutation will not develop into seeds upon fertilization, and thus the plant exhibits semisterility. Furthermore, the segregation pattern of mark-ers closely linked to the gametophytic mutation deviate from a typical Mendelian 3:1 ratio. The segregation pattern can be analyzed in insertion mutants generated by either T-DNA or transposons, carrying for example a resistance marker, or, in experiments involving multiply-marked chro-mosomes. Additional methods for isolating genes expressed in the gametophyte include enhancer-trap screens [5•,35,36] or screens of cDNA libraries prepared from iso-lated cells of the embryo sac [37,38].
Genes with a function during embryo sac development
include members of the GAMETOPHYTIC FACTOR
(GFA) and FEMALE GAMETOPHYTE (FEM) group of genes [3•,32•], Gf [39•,40], PROLIFERA (PRL) [35],
ANDARTA (ADA), TISTRYA (TYA) and HADAD (HDD) (Figure 2) in Arabidopsis [33•,34•] as well as lethal ovule2
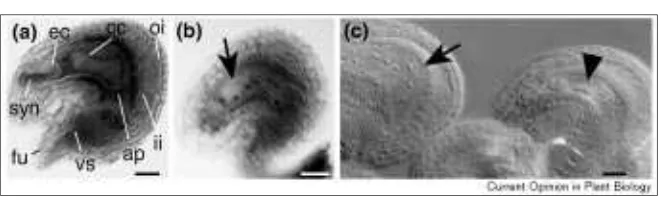
Figure 2
Mid-optical sections through mature, whole-mount ovules from (a) a male-sterile, (b)an emd-class mutant and (c) hdd. (a) A post-fertilization ovule in the absence of fertilization. Under normal circumstances endosperm and embryo development would already be well underway. The salient features are indicated. Within the central cell, the two polar nuclei have fused to yield a large, di-haploid nucleus. By this stage the antipodal cells are already degenerated. (b) The 211E6 mutant [12•]. This sporophytic mutant exhibits a block at the four-nuclear embryo sac stage (stage 3-IV [15] or FG4 [39•]). No cellularization occurred. The sporophytic tissue is apparently normal. (c) The ovules developed within the same
gynoecium of a plant heterozygous for the hddmutation. In the wild-type ovule to the left, fertilization occurred and an eight-nuclear endosperm is present (arrow). In the hdd-mutant ovule to the right, development did not proceed beyond the four-nuclear embryo sac stage (arrow head).
(lo2) and indeterminate gametophyte1 (ig1) in maize [41,42,43•]. Some of these genes also have a function dur-ing male gametophyte development (for example FEM3
or HDD) and could only be identified because the corre-sponding mutations are incompletely penetrant.
The different mutations lead to blocks at various steps dur-ing female gametophyte development. For example, embryo sacs in the fem2or adamutants fail to develop beyond the mono-nuclear embryo sac stage. Genes like HDD, PRLor lo2
appear primarily to control nuclear division, as the corre-sponding mutants show embryo sacs variably arrested at the two-, four or eight-nuclear stage. In the case of PRL this interpretation is corroborated by sequence information, because PRLshows homology to genes from yeast encoding DNA replication initiation factors [35]. Very late aspects of female gametophyte development are affected in several mutants [3•]. For example, in gfa2 mutants the two polar nuclei within the central cell fail to fuse and remain located side by side. In fem4mutants, cellularization appears to be defective, as indicated by the abnormal shapes of the egg cells and synergids, as well as alterations in number, size and shape of the vacuoles of these cells.
Conclusions
Recent years have witnessed a remarkable interest in the molecular and genetic analysis of ovule development. Much of the present challenge lies in the elucidation of the molecular structure of the identified sporophytic and gametophytic genes and the analysis of their genetic interactions. Besides these immediate concerns a number of general issues stand out. What genes specify the iden-tity of the Arabidopsis ovule? Although a number of MADS box genes are candidates [44–46], and APETALA2
(AP2) appears to be involved [47], this significant aspect is not understood. It will also be important to identify the genes that regulate patterning in the early ovule pri-mordium and to understand how pripri-mordium outgrowth and pattern formation are orchestrated. Integument mor-phogenesis is in part characterized by complicated patterns of cell divisions and cell shape changes. As indi-cated by the analysis of TSL, signaling pathways are important in this process. Do genes such as STRUBBE-LIG (SUB) [12•], SHORT INTEGUMENTS (SIN1) [48,49], TSO1 and others also encode components of a signaling mechanism? What connects the signaling machinery to the cytoskeleton and cell wall, which prob-ably play a crucial role in the regulation of cell shape? With respect to gametogenesis, the study of the mecha-nism underlying megasporogenesis will certainly gain more attention. The analysis of gametophytic mutants may be complemented by the study of the interplay between sporophytic and gametophytic factors, which is likely to occur during female gametophyte development.
Acknowledgements
I thank the members of my lab for stimulating discussions and comments on the manuscript. I also thank Martin Hülskamp and Christine Horlow for
allowing me to cite unpublished work, Röbi Dudler for comments on the manuscript and Jay Moore and Ueli Grossniklaus for the hadad mutant picture. I apologize to the colleagues whose work I could not cite directly due to space constrictions. Work in my laboratory is supported by the Swiss National Science Foundation (NF 31-42175.94 and NF 31-53032.97) and by the Kanton of Zürich.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Endress PK: Evolutionary biology of flowers: prospects for the next century.In Evolution and Diversification of Land Plants. Edited by Iwatsuki K, Raven PH. Tokyo: Springer; 1997:99-119.
2. Igersheim A, Endress PK: Gynoecium diversity and systematics of the paleoherbs.Bot J Linn Soc1998, 127:289-370.
3. Drews GN, Lee D, Christensen CA: Genetic analysis of female
• gametophyte development and function.Plant Cell1998, 10:5-17. A very clear and detailed summary about the recent conceptual and experi-mental progress in the genetic analysis of embryo sac development. It includes a survey of known gametophytic mutants.
4. Gasser CS, Broadhvest J, Hauser BA: Genetic analysis of ovule
• development.Annu Rev Plant Physiol Plant Mol Biol1998,
49:1-24.
A detailed review focusing on the development of the sporophytic compo-nent of the ovule. It features an elaborate genetic model for ovule develop-ment (also compare with [6•]) and addresses the evolutionary aspects of the ovule.
5. Grossniklaus U, Schneitz K: The molecular and genetic basis of
• ovule and megagametophyte development.Semin Cell Dev Biol 1998, 9:227-238.
A comprehensive review dealing with the sporophytic as well as the game-tophytic aspects of ovule development.
6. Schneitz K, Balasumbramanian S, Schiefthaler U: Organogenesis in
• plants: the molecular and genetic control of ovule development.
Trends Plant Sci1998, 3:468-472.
This review mostly addresses early ovule development in a detailed manner. It features a genetic model of ovule development (compare with [4•]).
7. Angenent GC, Franken J, Busscher M, van Dijken A, van Went JL, Dons HJM, van Tunen AJ: A novel class of MADS box genes is involved in ovule development in Petunia.Plant Cell1995,
7:1569-1582.
8. Colombo L, Franken J, Koetje E, van Went J, Dons HJM,
Angenent GC, van Tunen AJ: The PetuniaMADS box gene FBP11
determines ovule identity.Plant Cell1995, 7:1859-1868. 9. Baker SC, Robinson-Beers K, Villanueva JM, Gaiser JC, Gasser CS: • Interactions among genes regulating ovule development in
Arabidopsis thaliana.Genetics1997, 145:1109-1124.
An important paper outlining an initial genetic framework of ovule develop-ment on the basis of double-mutant analysis. It further provides evidence that embryo sac development depends on the presence of an inner integument.
10. Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR: AINTEGUMENTA, an APETALA2-like gene of
Arabidopsiswith pleiotropic roles in ovule development and floral organ growth.Plant Cell1996, 8:155-168.
11. Klucher KM, Chow H, Reiser L, Fischer RL: The AINTEGUMENTA
gene of Arabidopsisrequired for ovule and female gametophyte development is related to the floral homeotic gene APETALA2.
Plant Cell1996, 8:137-153.
12. Schneitz K, Hülskamp M, Kopczak SD, Pruitt RE: Dissection of
• sexual organ ontogenesis: a genetic analysis of ovule development in Arabidopsis thaliana.Development1997, 124:1367-1376. A description of a systematic genetic approach to ovule development. The authors present a survey of a large number of ovule mutants, and, on the basis of this analysis, introduce a genetic model of ovule development. They also present indirect evidence for the presence of proximal–distal pattern formation in the ovule primordium.
13. Okamuro JK, Caster B, Villarroel R, van Montagu M, Jofuku KD: The AP2 domain of APETALA2defines a large new family of DNA binding proteins in Arabidopsis.Proc Natl Acad Sci USA1997, 94:7076-7081. 14. Schneitz K, Baker SC, Gasser CS, Redweik A: Pattern formation
• and growth during floral organogenesis: HUELLENLOSand
region of the ovule primordium in Arabidopsis thaliana.
Development1998, 125:2555-2563.
An important paper which directly addresses the genetic control of ovule primordium outgrowth. It shows that at least two genes, HLLand ANT, reg-ulate the outgrowth of the ovule primordium in a partially redundant manner. The data provide further evidence for proximal–distal pattern formation in the ovule primordium and indicate that the distal pattern element forms in an independent fashion. They also raise the possibility that proximal–distal patterning takes place progressively and in a distal–proximal direction.
15. Schneitz K, Hülskamp M, Pruitt RE: Wild-type ovule development in
Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue.Plant J1995, 7:731-749.
16. Esau K: Anatomy of Seed Plants.New York: John Wiley & Sons; 1997. 17. Reiser L, Modrusan Z, L. M, Samach A, Ohad N, Haughn GW, Fischer RL: The BELL1gene encodes a homeodomain protein involved in pattern formation in the Arabidopsisovule primordium.Cell1995,
83:735-742.
18. Roe JL, Nemhauser JL, Zambryski PC: TOUSLEDparticipates in apical tissue formation during gynoecium development in
Arabidopsis.Plant Cell1997, 9:335-353.
19. Roe JL, Sessions RA, Feldmann KA, Zambryski PC: The Tousled
gene in A. thalianaencodes a protein kinase homolog that is required for leaf and flower development.Cell1993, 75:939-950. 20. Roe JL, Durfee T, Zupan JR, Repetti P, McLean BG, Zambryski PC: • TOUSLED is a nuclear serine/threonine protein kinase that
requires a coiled-coil region for oligomerization and catalytic activity.J Biol Chem1997, 272:5838-5845.
The authors provide evidence that TSLencodes a nuclear serine-threonine kinase. Activation of the protein kinase seems to require interaction between TSL molecules.
21. Liu Z, Running M, Meyerowitz EM: TSO1functions in cell division during Arabidopsisflower development.Development1997,
124:665-672.
22. Hauser BA, Villanueva JM, Gasser CS: Arabidopsis TSO1regulates directional processes in cells during floral organogenesis.
Genetics1998, 150:411-423.
23. Maheswari P: An Introduction to the Embryology of Angiosperms. New York: McGraw-Hill; 1950.
24. Sheridan WF, Avalkina NA, Shamrov II, Batygina TB, Golubovskaya IN: The mac1gene: controlling the commitment to the meiotic pathway in maize.Genetics1996, 142:1009-1020.
25. Motamayor JC, Vezon D, Bajon C, Sauvanet A, Grandjean O, Marchand M, Bechtold N, Pelletier G, Horlow C: switch(swi1), an
Arabidopsis thalianamutant effected in the female meiotic switch.Abstract 307, 9th International Conference on Arabidopsis Research, 24-28 June 1998. Madison WI.
26. Golubovskaya IN, Avaklinka N, Sheridan W: Effects of several meiotic mutants on female meiosis in maize.Dev Genet1992, 13:411-424. 27. Golubovskaya I, Avalkina N, Sheridan WF: New insights into the role
of the maize ameiotic1locus.Genetics1997, 147:1339-1350. 28. Reiser L, Fischer RL: The ovule and the embryo sac.Plant Cell
1993, 5:1291-1301.
29. Patterson EB: Translocations as genetic markers.In The Maize Handbook.New York: Springer; 1994:361-363.
30. Vizir IY, Anderson ML, Wilson ZA, Mulligan BJ: Isolation of deficiencies in the Arabidopsisgenome by g-irradiation of pollen.
Genetics1994, 137:1111-1119.
31. Vollbrecht E, Hake S: Deficiency analysis of female gametogenesis in maize.Dev Genet1995, 16:44-63.
32. Feldmann KA, Coury DA, Christianson ML: Exceptional segregation
• of a selectable marker (KanR) in Arabidopsisidentifies genes
important for gametophytic growth and development.Genetics 1997, 147:1411-1422.
The first report of a systematic analysis of segregation ratio distortions in a population of T-DNA-induced mutant lines in Arabidopsis. This work led to the identification of seven gametophytic genes.
33. Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D: • Selection of T-DNA-tagged male and female gametophytic
mutants by segregation distortion in Arabidopsis.Genetics1998,
149:621-631.
A similar study as in [32•]. It includes a phenotypic description of two female gametophytic mutants. In both cases the viable megaspore does not initiate the nuclear division cycles.
34. Moore JM, Vielle Calzada J-P, Gagliano W, Grossniklaus U: Genetic
• characterization of hadad, a mutant disrupting female
gametogenesis in Arabidopsis thaliana.Cold Spring Harbor Symp Quant Biol1997, 62:35-47.
The authors describe the isolation of the gametophytic hadadmutation by a transposon-induced (gene-trap) mutagenesis screen. The hdd mutants exhibit an aberrant segregation ratio and reduced seed set. The embryo sac is predominantly affected and the results indicate that HDDis required for the correct progression through the mitotic division cycles.
35. Springer PS, McCombie R, Sundaresan V, Martienssen RA: Gene trap tagging of PROLIFERA, an essential MCM2-3-5-like gene in
Arabidopsis.Science1995, 268:877-880.
36. Sundaresan V, Springer P, Volpe T, Haward S, Jones J, Dean C, Ma H, Martiensen R: Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements.
Genes Dev1995, 9:1797-1810.
37. Dresselhaus T, Lörz H, Kranz E: Representative cDNA libraries from few plant cells.Plant J1994, 5:605-610.
38. Kranz E, Dresselhaus T: In vitro fertilization with isolated higher plant gametes.Trends Plant Sci1996, 1:82-89.
39. Christensen CA, King EJ, Jordan JR, Drews GN:
• Megagametogenesis in Arabidopsiswild type and the Gfmutant.
Sex Plant Reprod1997, 10:49-64.
An excellent description of embryo sac development in Arabidopsisusing confocal laser scanning microscopy. Analysis of the Gfmutant indicates a very early role for the corresponding gene in wild-type embryo sac ontogenesis.
40. Rédei GP: Non-mendelian megagametogenesis in Arabidopsis.
Genetics1965, 51:857-872.
41. Huang B-Q, Sheridan WF: Embryo sac development in the maize
indeterminate gametophyte1mutant: abnormal nuclear behavior and defective microtubule organization.Plant Cell1996,
8:1391-1407.
42. Kermicle JL: Pleiotropic effects on seed development of the indeterminate gametophyte gene in maize.Amer J Bot1971, 58:1-7. 43. Sheridan WF, Huang B-Q: Nuclear behavior is defective in the
• maize (Zea mays L.) lethal ovule2female gametophyte.Plant J 1997, 11:1029-1041.
A careful analysis of the mutant phenotype using fluorescent microscopy. The results indicate that lethal ovule2is essential for nuclear division, migra-tion and the accompanying tubulin cytoskeleton behavior during maize embryo sac development.
44. Ma H, Yanofsky MF, Meyerowitz EM: AGL1-ALG6, an Arabidopsis
gene family with similarity to floral homeotic and transcription factor genes.Genes Dev1991, 5:484-495.
45. Rounsley SD, Ditta GS, Yanofsky MF: Diverse roles for MADS box genes in Arabidopsisdevelopment.Plant Cell1995,
7:1259-1269.
46. Savidge B, Rounsley SD, Yanofsky M: Temporal relationship between the transcription of two ArabidopsisMADS box genes and the floral organ identity genes.Plant Cell1995, 7:721-733. 47. Modrusan Z, Reiser L, Feldmann KA, Fischer RL, Haughn GW:
Homeotic transformation of ovules into carpel-like structures in
Arabidopsis.Plant Cell1994, 6:333-349.
48. Robinson-Beers K, Pruitt RE, Gasser CS: Ovule development in wild-type Arabidopsisand two female-sterile mutants.Plant Cell 1992, 4:1237-1249.
49. Lang JD, Ray S, Ray A: sin1, a mutation affecting female fertility in