THE ISOLATION OF XANTHONES FROM TRUNK LATEX OF
Garcinia mangostana
Linn.
AND THEIR ANTIMICROBIAL ACTIVITIES
Vivi Anggia, Amri Bakhtiar, and Dayar Arbain
* Faculty of Pharmacy/Sumatran Biota Laboratory, Andalas University,Kampus Limau Manis, Padang, 25163, West Sumatera, Indonesia
Received October 6, 2014; Accepted March 1, 2015
ABSTRACT
ɑ-Mangostin (1), β-mangostin (2) and gartanin (3) have been isolated from the trunk latex of Garcinia mangostana Linn. and investigated for their antimicrobial activities against Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Salmonella typhosa, Staphylococcus epidermidis, Streptococcus mutans and Vibrio cholerae.
The significant antibacterial activity showed by α-mangostin (1) against Bacillus subtilis, Enterococcus faecalis. Staphylococcus epidermidis and Vibrio cholerae, but all compounds showed no activity to inhibit the growth of Micrococcus luteus and Streptococcus mutans.
Keywords:Garcinia mangostana Linn.; trunk latex;ɑ-mangostin; β-mangostin; gartanin
ABSTRAK
Telah diisolasi ɑ-mangostin (1), β-mangostin (2) dan gartanin (3) dari getah batang tumbuhan manggis (Garcinia mangostana Linn.) dan telah dilakukan uji antibakteri terhadap bakteri Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Salmonella typhosa, Staphylococcus epidermidis, Streptococcus mutans dan Vibrio
cholerae. α-Mangostin (1) menunjukan aktivitas antibakteri yang signifikan terhadap bakteri Bacillus subtilis, Enterococcus faecalis, Salmonella typhosa, Staphylococcus epidermidis dan Vibrio cholerae, tetapi semua senyawa tidak aktif menghambat pertumbuhan bakteri Micrococcus luteusdanStreptococcus mutans.
Kata Kunci:Garcinia mangostanaLinn.; getah manggis; ɑ-mangostin; β-mangostin; gartanin
INTRODUCTION
Garcinia mangostana Linn. (mangosteen), family Guttiferae has been cultivated for centuries in tropical areas of the world. The tree is presumed to have originated from Southeast Asia or Indonesia and has largely remained indigenous to Malay Peninsula, Myanmar, Thailand, Cambodia, Vietnam and the Moluccas [1]. It has also been known to be of good medicinal value and is traditionally used in folk medicines for treatment of abdominal pain, diarrhea, dysentery, infected wounds, suppuration, chronic ulcer, leucorrhoea and gonorrhea [2].
Various studies from different part of G. mangostana have been carried out. From young fruit peel Suksamrarn et al. reported the presence of 1,6-dihydroxy-7-methoxy-8-(3-methylbut-2-enyl)6’6,6’-dimethyl pyrano(2’,3’:3,2)xanthone, demethylcalabaxanthone, 8-desoxygartanin, gartanin, ɑ-mangostin, β-mangostin, γ-mangostin, garcinone B, garcinone C, garcinone D, garcinone E, mangostenone C, mangostenone D, mangostenone E, mangostanol, 11-hydroxy-1-isomangostin, mangostinone, thwaitesixanthone and mangostanin [2]. From mature fruit peel Jung et al. found
8-hydroxycudraxanthone G and mangostingone [7-methoxy-2-(3-methyl-2-butenyl)-8-(3-methyl-2-oxo-3-bu tenyl)-1,3,6-trihydroxyxanthone and other 12 known’s xanthones [3]. From stem bark, Dharmaratne et al. obtained ɑ-mangostin, β-mangostin,γ-mangostin, from root bark found ɑ-mangostin, β-mangostin, 3-hydroxy-4-geranyl-5-methoxybiphenyl andβ-sitosterol and from young fruit latex found ɑ-mangostin, β-mangostin, γ-mangostin, methoxy-β-mangostin and garcinon E [4]. From seeds and fruit pulp Suksamrarn et al. found 1,7-dihydroxy-2-(3-methylbut-2-enyl)-3-methoxyxanthone, 2-isoprenyl-1,7-dihydroxy-3-methoxyxanthone, 1,7-dihydroxy-8-(3-methylbut-2-enyl)-6’, 6’-dimethylpyrano (2’,3’:3,2)-xanthone and trapezifolixanthone [5]. Beside that, several compounds have been found in other parts of G. mangostana and mainly individual xanthones were reported to have a great variety of pharmacological activities including antioxidant, antifungal, antibacterial, cytotoxic, antiinflammation, antihistamine, anti-HIV and other activities [6].
Several antibacterial testing on these xanthones against some pathogenic bacteria such as
So, in our present work we tried to isolate the major compounds of the trunk latex of G. mangostana. Interestingly, in our work we found that the major compounds isolated from trunk latex of G. mangostana
gave significant antibacterial activity against Bacillus subtilis, Enterococcus faecalis, Staphylococcus epidermidisandVibrio cholerae.
EXPERIMENTAL SECTION
Materials
Plant material: 100 g dry trunk latex of
G. mangostana was collected at Kubang Landai, Batu Sangkar, West Sumatera on August 2013.
Testing microbes: Bacillus subtilis ATCC 6633, Enterococcus faecalisATCC 29212, Micrococcus luteus
ATCC 10240, Salmonella typhosa NCTC 786, Staphylococcus epidermidis ATCC 12228, Streptococcus mutansATCC 25175 and Vibrio cholerae
Inaba were provided by Indonesian Food and Drug Administration (BPOM) Padang and Pekanbaru offices and Microbiology Laboratory of Medical Faculty of Andalas University and were pre-cultured before being used.
All solvents were distilled before being used, nutrient agar (NA) (Merck), paper disc (Whatman), dimethyl sulfoxide (DMSO) (Merck) and chloramphenicol (Sigma). TLC was carried out using silica gel 60F254
(Merck) and visualized under UV light (254 nm). Column chromatography was performed on silica gel 60 (0.063-0.200 nm) (Merck).
Instrumentation
Melting points were measured on a Sybron Thermolyne Melting Point Apparatus MP-12615 and are uncorrected. FT-IR Spectra was on Perkin Elmer FT-IR Frontier. UV Spectra was recorded on Shimadzu Spectrometer UV-Vis Pharmaspec 1700. 1H and
13
C-NMR spectra were recorded with an Agilent DD2 system (Agilent Technologies, Santa Clara, CA, USA) operating at 500 (1H) and 125 (13C) MHz using residual and deuterated solvents as reference standards. High-resolution mass spectra were obtained on ESI-TOF
latex was practically soluble in EtOAc except for small parts of wood and bark. This was dissolved in EtOAc then precipitated by addition of hexane to give yellow amorphous solid, then recrystallized from ethanol-water to give pale yellow plates ofɑ-mangostin (1) (2.5 g).
All uncrystallized fractions (73 g) were combined and pre-adsorbed on SiO2 then column
chromatographed on the same adsorbent and eluted with step gradient polarity solvents started from hexane, hexane-dichloromethane, dichloromethane-EtOAc, EtOAc-MeOH and MeOH to give ten subfractions (GM 1-10). Fraction GM1-2 (23 g) was recrystallized from EtoAc-Hexane then EtOH-Water to give ɑ-mangostin (1) (5.98 g) then all of mother liquor of this fraction were combined, rechromatographed as above with isocratic solvent hexane-EtOAc (4:1). Fractions eluted from the column were monitored on TLC and fractions that gave similar TLC pattern were combined to give more of ɑ-mangostin (440 mg) and six subfractions (GM 11-17). Other crystalline subfractions were combined and recrystallized from EtOAc-Hexane then EtOH-water to give yellowish needles. Based on its spectroscopic data this compound identified was as β-manggostin (2) (11.1 mg).
Subfraction GM 13 was rechromatographed on SiO2eluted with isocratic solvents hexane-EtOAc (9:1).
Major fractions that showed similar behavior on TLC were combined then recrystallized from dichloromethane-hexane to give yellow needles of gartanin (3) (16 mg).
ɑ-mangostin (1).Pale yellow plates; MP: 180-181 °C. IR (KBr) (cm-1): 3418, 3239, 2962, 1639, 1373, 1238, 1169, 1050, 946, 839. UV λmax MeOH nm (log ε): 315 (4.23), 243 (4.41), 204 (4.44). 1H-NMR (Table 1) and
13
C-NMR (Table 2). TOF MS-ESm/z: 409.1647 (M-1).
β-mangostin (2). Yellowish needles; MP: 166-168 °C. IR (KBr) (cm-1): 3384, 2933, 1645, 1600, 1482, 1456, 1381, 1281, 1148, 1169, 939, 882. UV λmax MeOH nm
(log ε): 347(4.48), 315(4.87), 258 (4.98), 244(5.05), 203 (5.12).1H-NMR (Table 1) and 13C-NMR (Table 2). TOF MS-ESm/z: 423.1801 (M-1).
Fig 1.Structures of compounds1-3
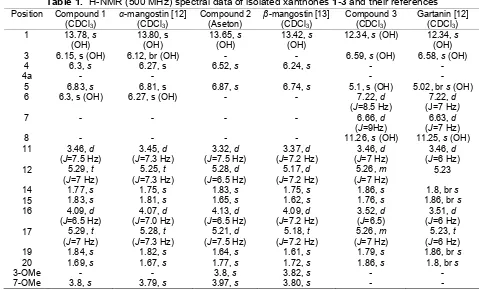
Table 1.1H-NMR (500 MHz) spectral data of isolated xanthones1-3and their references Position Compound 1
Before being used each bacterium was grown separately in nutrient agar (NA) (Merck) and incubated at 37 °C for 24 h. These cultures were used for antimicrobial assay by modified agar disc diffusion method of Kirby and Bauer [11]. Single colony of the respective testing bacterium was transferred into NA medium and incubated for 24 h. Culture suspension in sterile NaCl 0.9% with 25% transmittant were respectively swabbed onto agar medium. Each compound (1-3) was prepared to the concentration of 2, 1, 0.5, 0.25, 0.125, 0.0625 mg/mL in DMSO. Each 10 µL of the above solution was dropped onto the paper disc
(Whatman, 5 mm diameter) and carefully put onto culture media and the test was done in triplicate. Control disc contained chloramphenicol 30 µg/paper disc was similarly prepared in DMSO. Each plate was incubated at 37 °C for 24 h. Inhibition zones (including the diameter of disc) were measured and recorded and the inhibition results were reported as means of the triplo data.
RESULT AND DISCUSSION
TLC profile of the trunk latex G. mangostana
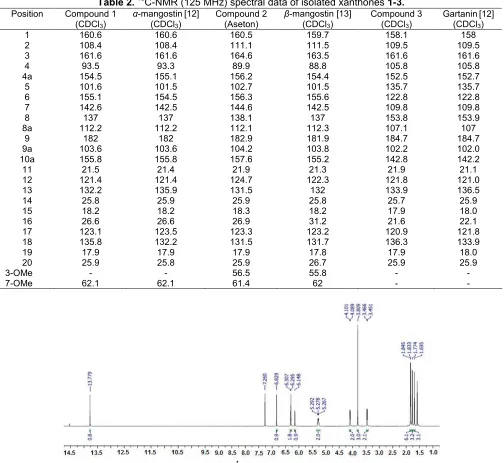
7 142.6 142.5 144.6 142.5 109.8 109.8
8 137 137 138.1 137 153.8 153.9
8a 112.2 112.2 112.1 112.3 107.1 107
9 182 182 182.9 181.9 184.7 184.7
9a 103.6 103.6 104.2 103.8 102.2 102.0
10a 155.8 155.8 157.6 155.2 142.8 142.2
11 21.5 21.4 21.9 21.3 21.9 21.1
12 121.4 121.4 124.7 122.3 121.8 121.0
13 132.2 135.9 131.5 132 133.9 136.5
14 25.8 25.9 25.9 25.8 25.7 25.9
15 18.2 18.2 18.3 18.2 17.9 18.0
16 26.6 26.6 26.9 31.2 21.6 22.1
17 123.1 123.5 123.3 123.2 120.9 121.8
18 135.8 132.2 131.5 131.7 136.3 133.9
19 17.9 17.9 17.9 17.8 17.9 18.0
20 25.9 25.8 25.9 26.7 25.9 25.9
3-OMe - - 56.5 55.8 -
-7-OMe 62.1 62.1 61.4 62 -
-Fig 2.Spectrum1H NMR of compound 1
fraction of the trunk latex gave the two minor less polar compounds in a very small quantity compared to that of ɑ-mangostin. Attempt to isolate another more polar minor compound was unsuccessful because the amount was too small to isolate. Identification of the isolated compounds was done by spectroscopic method particularly1H and13C-NMR.
The characteristics of xanthone molecules of isolated ɑ-mangostin (1), β-mangostin (2) and gartanin (3) were readily showed by the presence of xanthone chromophore ultraviolet absorption at (UV λmax (log ε): 350 nm (shoulder), 315 nm (4.23); 347 nm (4.48), 315 nm (4.87) and 351 nm (4.14), 319 nm (4.19),
respectively. Infrared spectra also showed characteristics of xanthones hydrogen bond of hydroxyl protons attached to either C1 or C8(cm
-1
); 3418, 3418 and 3600 and 3400 to carbonyl function of C9
respectively. The highly deshielded signals of hydroxyl protons attached to C1 due to hydrogen bond to
carbonyl oxygen of C9 of above compounds were
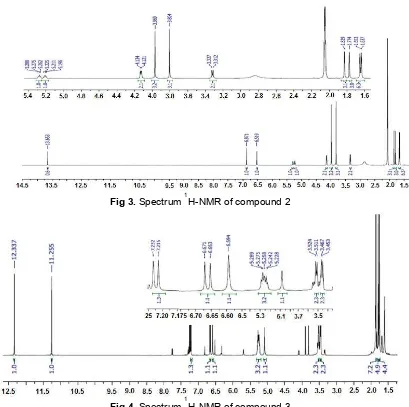
Fig 3.Spectrum1H-NMR of compound 2
Fig 4.Spectrum1H-NMR of compound 3
comparison of its TLC profile with that of available dichloromethane fraction of methanolic extract of fruit pericarp of G. mangostana which was wellknown for its major constituents a-mangostin. Based on the comparison of its spectorscopic data to those of reported data in literature particularly 1H and 13C (Table 1 and Table 2) compound (1) was identified as well-known ɑ-mangostin [12].
The 1H-NMR spectrum of compound (2) looked very similar to that of ɑ-mangostin (1), except that instead of having one methoxyl group it had two at (d, ppm, multplicity) 56.30 (3H, s) and 61.4 (3H, s). Other
1
H-NMR signals such as 4 methyl signals of prenyl group (d, ppm, multiplicity) : C14(25.9, 3H, s), C15(18.3, 3H, s),
C19(17.9, 3H, s) and C20 (25.9, 3H, s), as well as its 2
aromatic protons attached to C4 (6.52, 1H, s) C5 (6.87,
1H, s), all very similar to that of ɑ-mangostin (1). In addition, all 13C signals as well as its molecular ion (M-1) at m/z 423.18 which agreed with empirical formula
C26H28O6 and compared to reported data in the
literature particularly its1H and13C-NMR spectra (Table 1 and Table 2) it was concluded that compound (2) was identified as knownβ-mangostin [13].
The 1H-NMR of compound (3) showed different
1
H-NMR pattern compared to the above two compounds. Only the presence of two aromatic protons was observed (d, ppm, multiplicity, coupling constant); 7.22 (d, 1H, J 7 Hz) and 6.63 (d, 1H, J 7 Hz) which coupled to each other with coupling constant 7 Hz, indicating the presence ofortho-coupling of protons H6
and H7. The presence of two prenyl functions were also
Table 4. Inhibition of isolated xanthones against E.
Table 6. Inhibition of isolated xanthones against S. thyposa
Table 7. Inhibition of isolated xanthones against V. cholerae
Traditionally the fruit pericarps ofG.mangostanain West Sumatra is used for various purposes particularly for skin infections, wound healing as well as throat and gastrointestinal infection. As part of our continuing search for antimicrobial compounds from Sumatran medicinal plants [14], we decided to check the antimicrobial activity of those isolated compounds using different variety of standard testing pathogenic bacteria as in Table 7.
The decoction of pericarps of G. mangostanawas reported to be active against Escherichia coli, Vibrio cholerae and Salmonella typhi, the crude water extract was active against Streptococcus faecalis, Vibrio cholerae[5]. CrudeG. mangostanaextract also showed inhibition toward the growth ofPropionibacterium acnes
and Staphylococcus epidermidis. Some antibacterial activity of ɑ-mangostin towards E. faecalis, B. subtilis
and S. typhosa using different strains of the above microbes has also been reported before [15]. However, since the main components of the pericarps of G. mangostana is ɑ-mangostin and following the above important traditional use of G. mangostana, we decided to continue the study of its trunk latex chemical constituents to explore another source ofɑ-mangostin as well to check their antimicrobial activity by using available different standard pathogenic testing microbes.
Our antibacterial experiments results showed that ɑ-mangostin (1), β-mangostin (2) and gartanin (3) inhibited the growth of V. choleraesignificantly, while ɑ
-mangostin (1), β-mangostin (2) were also active inhibited the growth pathogenic gastrointestinal bacteriaB. subtilis, E. faecalisandV. cholerae.
The above results showed that the traditional use of the decoction of fruit pericarps ofG. mangostana to treat gastrointestinal infections caused byB. subtilis, E. faecalisand V. cholerae can now be understood. This preliminary result also indicated that ɑ- and β -mangostin which were also the main component of xanthones in the pericarps of G. mangostana showed inhibition toward the growthS. epidermidisthat caused acne. Based on the above results, it seems that the antibacterial activity of xanthones of G. mangostana
might be worth investigated further.
CONCLUSION
The isolated compounds from trunk latex of G. mangostana were identified as ɑ-mangostin, β -mangostin and gartanin. ɑ-Mangostin gave significant activity to inhibit the growth ofB. subtilis, E. faecalis,S. typhosa, S. epidermidis and V. cholerae. While gartanin gave significant activities against V. cholerae
and moderate activities against B. subtilis, S. typhosa
and S. epidermidis. β-mangostin gave significant activities against B. subtilis, E. faecalis and V. cholera
and moderate activities against S. epidermidis, but there were no inhibition toward M. luteus and S. mutans.
ACKNOWLEDGEMENT
Laboratory of Medical Faculty of Andalas University for providing testing microbes. We thank Prof. Dr. Yana Maolana Syah from Institut Teknologi Bandung for obtaining NMR and MS Spectroscopic data.
REFERENCES
1. Akao, Y., Nakagawa, Y., Iinuma, M., and Nozawa, Y., 2008,Int. J. Mol. Sci.,9(3), 355–370.
2. Suksamrarn, S., Komutiban, O., Ratananukul, P., Chimnoi, N., Lartpornmatulee, N., and Suksamrarn, A., 2006,Chem. Pharm. Bull., 54(3), 301–305. 3. Jung, H-A., Su, B-N., Keller, W.J., Mehta, R.G., and
Kinghorn, A.D., 2006, J. Agric. Food. Chem., 54, 2077–2082.
4. Dharmaratne, H.R.W., Piyasena, K.G.N.P., and Tennakoon, S.B., 2005, Nat. Prod. Res., 19(3), 239–243.
5. Suksamrarn, S., Suwannapoch, N., Phakhodee, W., Thanuhiranlert, J., Ratananukul, P., Chimnoi, N., and Suksamrarn, A., 2003, Chem. Pharm. Bull., 51(7), 857–859.
6. Obolskiy, D., Pischel, I., Siriwatanametanon, N., and Heinrich, M., 2009, Phytother. Res., 23(8), 1047–1065.
7. Mahabusarakam, W., Wiriyachtra, P., and Pongpaichit, S., 1986, J. Sci. Soc. Thailand, 12, 239–242.
8. Sakagami, Y., Iinuma, M., Piyasena, K.G.N.P., and Dharmaratne, H.R.W., 2005,Phytomedicine,12(3), 203–208.
9. Iinuma, M., Tosa, H., Tanaka, T., Asai, F., Kobayasi, Y., Shimano, R., and Miyauchi, K., 1996,
J. Pharm. Pharmacol,48(8), 861–865.
10. Sundaram, B.M., Gopalakhrishnan, C., Subramanian, S., Shankaranarayanan, D., and Kameswaran L., 1983,Planta Med., 48(1), 59–60. 11. Chen, I-N., Chang, C-C., Ng, C-C., Wang, C-Y.
Shyu, Y-T., and Chang, T-L., 2008, Plant Foods Hum. Nutr., 63(1), 15–20.
12. Ragasa, C.Y., Crisostomo, C.J.J., Garcia, K.D.C., and Shen, C-C., 2010,Philippine Sci.,47, 63–75. 13. Al-Massarani, S.M., El Gamal, A.A., Al-Musayeib,
N.M., Mothana, R.A., Basudan, O.A., Al-Rehaily, A.J., Farag, M., Assaf, M.H., El Tahir, K.H., and Maes, L., 2013,Molecules,18(9), 10599–10608. 14. Arbain, D., 2012, Nat. Prod. Commun., 7(6),
799–806.