The strategies of plant virus gene expression: models of economy
Gabrie`le Drugeon *, Silvio Urcuqui-Inchima, Malgosia Milner
1, Gress Kadare´,
Rosaura P.C. Valle
2, Ariane Voyatzakis, Anne-Lise Haenni, Jan Schirawski
Institut Jacques Monod,2Place Jussieu-Tour43,75251Paris Cedex05,France Received 27 May 1999; received in revised form 22 June 1999; accepted 24 June 1999
Abstract
Given the small size of their genome, the genetic information of viruses is extremely compact, and non-coding regions are very limited as compared to those of prokaryotic and eukaryotic cell systems. Viruses utilize cell components at all levels of the replication cycle for their own benefit, not the least being the translation machinery. They have also evolved a number of highly sophisticated strategies to produce and regulate the production of the proteins required for their propagation. In addition, these proteins are often multifunctional, encoding several essential virus-specific proteins. At the level of transcription, these strategies include splicing, the production of subgenomic RNAs from virus templates and cap-snatching. At the level of translation, regulation exists at all steps: initiation, elongation and termination. Furthermore, viruses frequently resort to co- and/or post-translational cleavage of a polyprotein precursor to yield the mature proteins. © 1999 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Regulation of protein synthesis; Plant viruses; Post-transcriptional regulation; Gene expression
www.elsevier.com/locate/plantsci
1. Introduction
For over a century — that is, ever since their discovery — viruses have been a constant source of new and amazing information and have been
determinant in helping us unravel mechanisms used not only by the viruses themselves, but also by cell systems. Some of these mechanisms appear to be largely the prerogative of viruses, whereas others often also occur in cells. The study of these mechanisms has been facilitated in the case of viruses because of the small size of their genome, and consequently of the relative ease with which viral genes can be isolated as compared to cell genes (genera of viruses are based on Ref. [1]). Thus, it is among viruses that many heretofore unexpected mechanisms were first described (rev. in Ref. [2]).
The small size of viral genomes explains the extreme compactness of their genetic information. Viral genes are often not even separated by inter-genic regions but frequently overlap, and whatever non-coding regions exist, they are generally of
prime importance to regulate replication/
transcrip-tion of the genome, and translatranscrip-tion of the viral proteins. Indeed, few sequences in the genome are devoid of known function.
Abbre6iations: CP, coat protein; g, genomic; IRES, internal ribo-some entry site; MP movement protein; ORF, open reading frame; RdRp, RNA-dependent RNA polymerase; sg, subgenomic; TGB, triple gene block; UTR, untranslated region; VPg, virus protein genome-linked; BSMV, Barley stripe mosaic hordeivirus; BYDV-PAV, Barley yellow dwarf luteovirus PAV isolate; CaMV, Cauliflower mosaic caulimovirus; crTMV, crucifer-infecting TMV; PVX, Potato potexvirus X; RTBV, Rice tungro bacilliform bad-navirus; SBWMV, Soil-borne wheat mosaic furovirus; STNV, Satel-lite tobacco necrosis necrovirus; TMV, Tobacco mosaic tobamovirus; WDV, Wheat dwarf geminivirus.
* Corresponding author. Tel.: +33-1-44274035; fax: + 33-1-44273580.
E-mail address:[email protected] (G. Drugeon)
1Permanent address: Institute of Biochemistry and Biophysics,
Polish Academy of Sciences, ul. Pawinskiego 5a, 02-106 Warszawa, Poland.
2Present address: Milagen, Inc., 1387 Marina Way South,
Rich-mond, CA 94804, USA.
The diversity in shape and structure of viruses is immense (rev. in Ref. [1]). Viruses can be envel-oped or non-envelenvel-oped. Their genome can be com-posed of single or double stranded DNA or RNA; it can be monopartite (plant and animal viruses) or multipartite (plant viruses, rarely animal viruses). In viruses with a single stranded RNA, the genome can be of positive or of negative polarity, or it can be ambisense.
To date, the vast majority of plant viruses — the topic of the present review — that have been investigated contain an RNA genome. Conse-quently, it is among such viruses that many strate-gies of expression were first described and have been the most thoroughly examined. Plant viral genomes are small, generally ranging from 4 to 15 kb or kbp. They code for four to 12 proteins. These include proteins involved in RNA
replica-tion/transcription, as for example the
RNA-depen-dent RNA polymerase (RdRp) among RNA viruses or the reverse transcriptase activity in Caulimoviruses, transport of the infectious agent from cell to cell by the movement protein (MP), encapsidation of the viral genome by the coat protein (CP), vector transmission of the virus by a protein frequently referred to as the helper compo-nent, proteolytic maturation of viral precursor polyproteins by viral proteinases, and transactiva-tors that facilitate translation of downstream open reading frames (ORFs). As mentioned above, syn-thesis of the viral proteins is ensured by the host translation machinery.
Recently, other reviews have dealt with the syn-thesis and function of plant virus proteins [3 – 6]. The aim of the present review is to offer an overview of the strategies of expression as found in plant viruses. For the sake of convenience, four levels are distinguished at which regulation of gene expression can occur. These are at the level of the genome segments, transcription and translation, and at the post-translational level.
2. Regulation of gene expression at the level of the genome segments
In the case of multipartite genomes, each genome segment can contain one or more ORF. Although still hypothetical because sparsely inves-tigated, one can postulate that regulation of syn-thesis of the proteins deriving from distinct
genome segments could be cell-type specific, and could depend on such features as the nature of the
leader sequence (the 5% untranslated region, UTR)
or the nature of the initiation codon and its nucle-otide environment.
3. Regulation of gene expression at the transcription level
Regulation at the level of transcription can oc-cur by splicing of viral mRNAs thereby generating new ORFs, the production of subgenomic (sg) RNAs (i.e. mRNAs) produced from the genomic (g) RNA template and consisting of viral genomes
truncated to varying degrees in their 5%region, the
ambisense strategy in which both viral and viral complementary gRNAs code for viral proteins, and cap-snatching, a mechanism by which the
virus ‘snatches’ the 5% end of host mRNAs to
prime synthesis of the viral mRNAs. Editing of viral RNAs is observed only in the animal
Paramyxo6iridae (rev. in Ref. [7]). It results in the introduction of generally one or two nucleotides (most frequently G residues) in a population of newly synthesized viral RNAs probably as a result of ‘stuttering’ of the RdRp at the level of a run of G’s. However, since no plant viruses belonging to this genus have been described to date, editing will not be discussed here.
3.1. Splicing
Splicing of mRNAs is encountered in DNA-containing plant viruses. It has been described among certain single strand DNA-containing
Gemini6iridae, and among the double strand
DNA-containing Caulimoviruses and
Bad-naviruses. Splicing is required for replication of Wheat dwarf geminivirus (WDV; [8]) and for
in-fectivity of Cauliflower mosaic caulimovirus
(CaMV; [9]). In Rice tungro bacilliform bad-navirus (RTBV) it is required for the expression of the otherwise silent ORF IV [10].
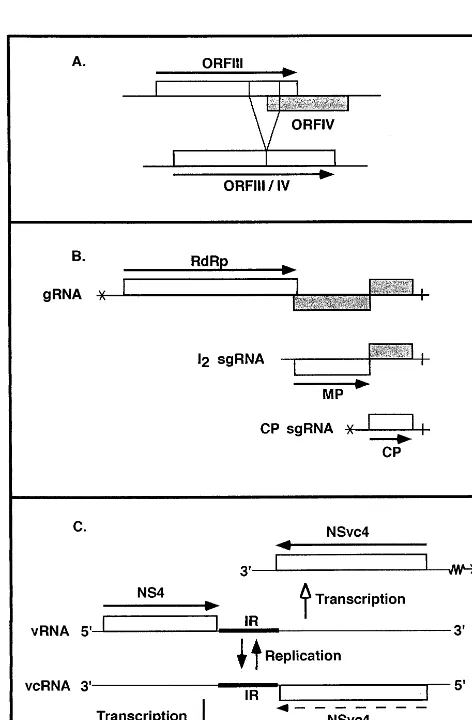
Splicing in the monocot Gemini6iridae is
illus-trated in Fig. 1A for WDV. TheGemini6iridaethat
a fusion product of ORF III and IV that presents maximum homology to the corresponding
contin-uous ORF of dicot Gemini6iridae. The sequences
surrounding the intron fit the consensus sequences of splice donor and acceptor sites, and are
con-served among monocot Gemini6iridae, as is also a
lariat sequence positioned upstream of the accep-tor site. Both unspliced and spliced forms of the corresponding mRNA are detected in infected plants, even though the fused protein is sufficient
for DNA replication of the virus in vivo [8]. It thus seems quite likely that expression of ORF III from unspliced RNA as well as the fusion protein resulting from processed RNA are important for
the monocot infecting Gemini6iridae, i.e. in host
specificity [8].
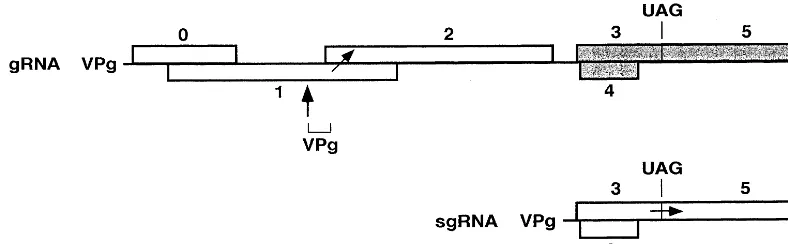
3.2. Subgenomic RNAs
In addition to gRNAs, a large number of viruses, whether of positive or negative polarity, also produce one or more sgRNA species that derive from the genome by internal initiation of RNA synthesis on the complementary gRNA strand; sgRNAs are not required for replication. They allow expression of cistrons which occupy internal positions in the genome. When several
genes are present in the 3%region of the gRNA, as
in the case of Tobacco mosaic tobamovirus
(TMV) illustrated in Fig. 1B, a family of 3%
colin-ear sgRNAs is frequently produced, such that
each gene to be expressed is located at the 5%end
of one sgRNA, a position required for optimal expression in eukaryotes. The sgRNAs are or are not encapsidated, depending on whether they har-bor the encapsidation site.
In general, sgRNAs are the mRNAs for the 3%
proximal genes on polycistronic viral RNAs, and
are identical in sequence to the 3% end of the
gRNAs. Barley stripe mosaic hordeivirus (BSMV) is an exception. All three segments terminate by a tRNA-like structure and contain a poly(A) stretch
about 200 nucleotides upstream from the 3%end. It
has been reported that the sgRNAs deriving from
RNAb have the expected tRNA-like structure at
their 3% end, whereas surprisingly, the sgRNA
derived from RNAg terminates at its 3%end by the
poly(A) stretch [11].
3.3. Ambisense and cap-snatching
A particular case of sgRNA synthesis is that encountered in viruses which resort to the am-bisense strategy. Since among the plant viruses this strategy is accompanied by cap-snatching, these two aspects are discussed together. The seg-ments of the RNA genome of Tospoviruses (rev. in Ref. [12]) and Tenuiviruses (rev. in Ref. [13]) are either of negative polarity or are ambisense.
The viral RNA contains an ORF in its 5% region,
whereas another ORF is located in the 5%region of
the viral complementary RNA. The two ORFs are separated by an intergenic region. This is illus-trated for RNA4 of Tenuiviruses in Fig. 1C. Viruses using this strategy have evolved a unique mechanism to produce the sgRNAs which direct the synthesis of their proteins. Contrary to initia-tion by classical RdRps, initiainitia-tion of synthesis of the mRNAs by a mechanism known as cap-snatching is primer-dependent, the primers
deriv-ing from the 5% end of host mRNAs. Thus, the
viral mRNAs are capped as opposed to the viral and viral complementary RNAs that are not capped. Nevertheless, as shown in vitro for Tenuiviruses, the uncapped viral RNAs can serve directly as templates in translation, and translation follows the scanning mode of initiation [14]. The mechanism whereby cap-snatching occurs among plant viruses is unknown.
4. Regulation of gene expression at the translation level
Regulation at the level of translation can occur at all steps: initiation, elongation and termination.
4.1. Initiation
4.1.1. Factors influencing initiation
Both cis- and trans-acting elements concur to
allow efficient initiation of translation of plant
viruses. The cis-acting elements are discussed in
Section 4.1.1.1, Section 4.1.1.2, Section 4.1.1.3,
Section 4.1.1.4 and Section 4.1.1.5 and the trans
-acting elements in Section 4.1.1.6 and Section 4.1.1.7.
4.1.1.1. Nature of the 5% end. Initiation of protein
synthesis from monocistronic mRNAs presumably generally occurs by the classical scanning
mecha-nism, starting from the capped 5% end of the
mRNA. In several plant virus groups, the 5% end
bears a viral-coded protein (virus protein genome-linked, VPg) in place of a cap structure. The VPg is covalently linked to the RNA. This is the case in
plant RNA viruses (Como6iridae, Poty6iridae,
Lu-teoviruses, Sobemoviruses) that belong to the pi-corna-like supergroup (rev. in Ref. [15]). By
analogy to the animal Picorna6iridae, attempts
have been made to determine whether in the VPg-containing plant viral RNAs, initiation of protein
synthesis occurs at the level of an ‘internal ribo-some entry site’ (IRES). The data indicate that in certain cases initiation possibly occurs on an
inter-nal site in the 5% leader sequence even though this
sequence in plant viruses is considerably shorter and presumably less structured than in animal viruses; in other cases, the data have been contro-versial (rev. in Ref. [16]) . Further investigations based on the use of the dicistronic mRNA assay (rev. in Ref. [16]) should allow conclusive results to be reached. Yet, in other plant RNA viruses such as the Necroviruses and the members of
subgroup I of the Luteoviruses, the 5%terminus of
the genome bears neither a cap structure nor a VPg [17] (rev. in Ref. [18]) . The mechanism of initiation of protein synthesis in these virus groups is discussed below.
4.1.1.2.Nature of the leader sequence. Efficiency of
translation can also depend on whether the 5%
UTR is poorly or highly structured, and if struc-tured, where this structure resides (rev. in Ref. [19]).
4.1.1.3. Initiation codon. Except for two instances,
an AUG codon serves as the natural initiation codon among plant viruses. ORF I of RTBV, the first large ORF following the long leader sequence with its various short ORFs, possesses an AUU initiation codon [20]. This AUU codon seems to be important for virus viability. The other case is found in Soil-borne wheat mosaic furovirus (SB-WMV) which contains a bipartite RNA genome. RNA2 contains the ORF for the 19-K CP. How-ever, in vitro studies with site-directed mutants have revealed that in addition to the 19-K protein, a 25-K CP-related protein is produced that is initiated at a CUG codon upstream of the AUG which initiates the 19-K protein [21]. Conservation of the CUG codon in several SBWMV isolates suggest that the 25-K protein plays a role in the virus life cycle.
4.1.1.4.Context surrounding the initiation codon. A
favorable context surrounding the initiation codon increases the efficiency of translation. This seems to apply to mRNAs with AUG as initiator, as well as to those with a non-AUG initiator. A purine at
−3 and a G at +4 (A of AUG is +1) constitute
4.1.1.5. Influence of the 3% region. As mentioned
above, the 5% terminus of the RNA can possess a
cap, a VPg, or it can be devoid of either. Similarly,
the 3% end can bear a poly(A) tail, a tRNA-like
structure, or neither. An increasing number of
cases is being reported in which the 3%UTR of an
mRNA plays a crucial role in initiation of transla-tion of that mRNA [3]. The RNA of Tobacco etch
potyvirus contains a 5% VPg and a 3% poly(A) tail.
It has been shown that functional interaction
be-tween the 5%leader and the poly(A) tail stimulates
translation of the mRNA, possibly by way of the poly(A) binding protein [23].
On the other hand, the Tobamovirus genome
carries a cap at its 5% end, and a tRNA-like
structure preceded by an upstream pseudoknot
domain at its 3% end. Here, a region within the
pseudoknot domain appears to functionally re-place the poly(A) tail and to enhance efficiency of
translation, in concert with the cap at the 5% end
[24]. A similar effect has been described in the case of Brome mosaic bromovirus [25]. However, only a very minor enhancing effect of the pseudoknot upstream of the tRNA-like structure on efficiency of translation was observed with Turnip yellow mosaic tymovirus [25].
Finally, there is still another scenario in which
interaction between the 5%leader and a 3%region is
required for translation. This is illustrated by the RNA genome of Satellite tobacco necrosis ne-crovirus (STNV; [26,27] and of the PAV isolate of Barley yellow dwarf luteovirus (BYDV-PAV;
[28,29]) that have neither cap nor VPg at their 5%
end, and neither poly(A) nor tRNA-like structure
at their 3% end.
Efficient translation in vitro of STNV RNA
which contains a 3%UTR of over 600 nucleotides,
requires both the 5% leader and a translational
enhancer located within the 3% UTR, just
down-stream of the ORF [26,27], and which forms an extended stem-loop structure. Possible
base-pair-ing interactions between the 5% leader and the 3%
enhancer have been proposed based on comple-mentarity between nucleotides within these re-gions. Removal of the translational enhancer dramatically reduces translation, but translation efficiency can be restored by capping the RNA. Mutations that destroy the translation capacity of the enhancer increase the amount of eIF4F re-quired for translation.
In BYDV-PAV, a 3% translational enhancer
which is located in the intergenic region between ORFs 5 and 6 (the two distal ORFs on the viral
genome), is required for translation of the 5%
-prox-imal ORF in vivo and in vitro [30]. The enhancer decreases the concentration of eIF4F needed for maximum translation of uncapped RNA, and ap-pears to mimic the cap in translation initiation. As
in STNV, it is likely that the requirement of a 3%
translational enhancer for efficient translation of
the 5%ORF of BYDV-PAV is related to the lack of
either a cap or a VPg at the 5% end of the genome
[29].
No protein has been described that might be required for such translation regulation in the case of STNV or BYDV-PAV.
4.1.1.6. Viral encoded transacti6ators. These are
viral-encoded proteins that specifically enhance translation of a downstream ORF in a bicistronic or a polycistronic mRNA, probably by stimulating reinitiation of translation. Transactivation activity has been demonstrated in the case of several Caulimoviruses; the most thoroughly investigated transactivator is that of CaMV [4].
4.1.1.7. Cellular initiation factors. These factors
play a crucial role in the efficiency of translation that can be enhanced in viral mRNAs in which
one of the cis-elements in the RNA is lacking or
mutated. This is for instance the case of the STNV RNA as mentioned above. The influence of these factors will not be discussed further since they have been extensively reviewed recently [31].
4.1.2. Leaky scanning
Even more complex is regulation in the case of polycistronic mRNAs, and such mRNAs are ex-tremely frequent among viruses. Leaky scanning is the general mechanism used by viruses in such circumstances. This mechanism is most certainly
facilitated if the AUG codon of the 5%-proximal
ORF is in a less favorable context than the AUG of the second ORF. Here, various genome organi-zations can be found.
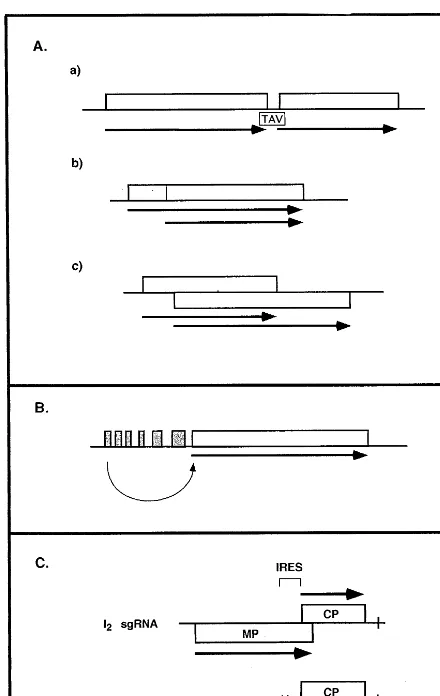
The two ORFs can be consecutive on the mRNA (Fig. 2Aa), and in this case, termination of
translation of the 5%-proximal ORF is presumably
Fig. 2. Regulation of gene expression at the level of initiation of translation. (A) Leaky scanning/reinitiation. (a) Consecu-tive ORFs; the transactivator (TAV) facilitates reinitiation of the second ORF. (b) In-frame initiation. (c) Overlapping ORFs. (B) Shunting. Curved arrow indicates ‘jumping’ of ribosomes to major ORF. (C) Internal ribosome entry. Exam-ple of the crTMV sgRNAs. The position of the internal ribosome entry site (IRES) on the I2 RNA within the MP
ORF of crTMV is indicated. Not to scale. All other indica-tions are as in Fig. 1.
encountered strategy among plant viruses. How-ever, it is encountered in the well-studied situation of the MP of Cowpea mosaic comovirus [33]. It is
also observed in vivo in the second ORF of RNAb
of BSMV. Two proteins are produced from two in-frame AUG codons.
By far the most frequent examples of leaky scanning concern overlapping ORFs that are in different reading frames (Fig. 2Ac). The overlap-ping regions can in certain cases be extremely long. A large number of RNA containing plant viruses resort to this strategy. A well documented example is that of Peanut clump furovirus RNA2 [34]. Here the AUG codon of the second ORF overlaps the UGA termination codon of the first ORF which codes for the CP. It has been shown that the second ORF is initiated in vitro by con-text-dependent leaky scanning. A similar mecha-nism probably accounts for the synthesis of the two overlapping proteins contained in sgRNA1 of BYDV [35] and Potato leafroll luteovirus [36]: in both cases, the shorter ORF is nested within the longer CP ORF. Triple gene blocks (TGB) are a group of three proteins whose genes generally overlap on the genome. They are encountered in
Potexviruses, Carlaviruses, Furoviruses and
Hordeiviruses, and are believed to be important for virus movement through the plant. In BSMV
RNAb [37] and in Potato potexvirus X (PVX)
[38], two 3% coterminal sgRNAs are required for
the production of the three TGB proteins: the first ORF of the TGB is translated from a functionally monocistronic sgRNA, whereas the second and third ORF are translated from a functionally bi-cistronic sgRNA. In BSMV and in PVX, the initiation codon of the second ORF of the TGB is in a less favorable context for initiation than is that of the third ORF, and it has been demon-strated that leaky scanning is responsible for translation of the third ORF [37].
4.1.3. Shunting
In certain cases, a shunt mechanism allows the scanning ribosome to ‘ignore’ certain regions within the leader sequence of an mRNA that can contain short ORFs, jumping, so to speak, to a downstream, longer ORF (Fig. 2B). This mecha-nism transfers the ribosome from a donor to an acceptor site on the mRNA, without involvement of mRNA scanning between these two sites. Shunting may be favored by the presence of exten-translation of the upstream ORF. Although not
very common among plant viruses, it has been clearly demonstrated in the case of CaMV [4]. Translation of downstream ORFs is facilitated by the action of the viral transactivator, the product of ORF VI.
sive secondary structures within the leader se-quence. This mechanism is believed to account for the synthesis of ORF I in the CaMV [39], and in the RTBV [20] transcripts.
4.1.4. Internal ribosome entry
Interesting cases have recently been reported on the expression of a downstream ORF (the CP in the cases described) in a dicistronic mRNA. In PVX, a sgRNA contains two non-overlapping
ORFs, the 5% proximal ORF coding for an 8-K
protein, and the distal ORF coding for the CP. This sgRNA is responsible for the synthesis of both proteins in vitro and in vivo [40]. Although one cannot exclude the possibility that expression of the CP could result from termination of 8-K synthesis followed by reinitiation, the most likely mechanism proposed to account for expression of the CP, is by internal ribosome entry. In a
cruci-fer-infecting TMV (crTMV), the I2 sgRNA
con-tains the information for the MP and the CP (Fig. 2C), the ORF of the latter overlapping the MP ORF [41]. In PVX as in crTMV, the dicistronic mRNA assay as well as other assays has revealed that the region upstream of the CP ORF is pre-sumably responsible for initiation of CP synthesis by an internal ribosomal entry mechanism. Since the studies with crTMV were performed in vitro, they do not exclude the possibility that in vivo a traditional mode of CP expression via the CP mRNA also operates to produce the CP. In agree-ment with this possibility is the demonstration that
two sgRNA species corresponding in size to the I2
and the CP sgRNA are detected in plants infected with crTMV.
4.2. Elongation
Viruses also regulate synthesis of their proteins at the level of elongation, and this is achieved by way of frameshifting (rev. in Refs. [5,42,43]) . This strategy leads to the synthesis of two proteins, a frame and a transframe protein, that are identical in their N-terminal region up to the point of frameshift, but differ in their C-terminal region. The level of synthesis of the frame protein always exceeds that of the transframe protein. Schemati-cally, frameshift appears to result from movement of the ribosome by one nucleotide on the mRNA,
either in the 5% or in the 3% direction, leading to a
−1 or a +1 frameshift event, respectively.
A number of signals in the RNA are required
for −1 frameshift. These are (1) a heptanucleotide
(‘slippery’) sequence where frameshift occurs, (2) a hairpin structure following the heptanucleotide that in many instances can form a pseudoknot with downstream RNA sequences, and (3) a spacer region of four to nine nucleotides between the heptanucleotide sequence and the hairpin structure. Mutations or removal of any of these
elements abolishes frameshift. Cases of −1
frameshift are frequent among plant RNA viruses, and have been reported for Carlaviruses, Di-anthoviruses, Enamoviruses, Luteoviruses and Sobemoviruses, where they are required for syn-thesis of the RdRp.
A +1 frameshift event requires a slippery run
of bases and a rare or ‘hungry’ codon on the ribosomal A site (rev. in Ref. [44]) . This mecha-nism has been postulated but as yet not demon-strated in the case of the Closteroviruses (rev. in Ref. [5]).
4.3. Termination
At the level of termination, regulation can occur by readthrough. An in-frame termination codon in an mRNA normally dictates termination of trans-lation. However, ever more examples are being reported in which termination codons are occa-sionally recognized by tRNAs known as suppres-sor tRNAs (rev. in Refs. [5,42]). This phenomenon referred to as suppression of termination, repre-sents a means of regulating synthesis of specific proteins, and is frequently encountered in plant viruses. It has been postulated or demonstrated
among the Carmo6iridae, Enamoviruses,
Furoviruses, Luteoviruses, Tobamoviruses, To-braviruses, Tombusviruses and Necroviruses, and also in an as yet unclassified virus, Oat chlorotic stunt virus. Readthrough produces two proteins, a stopped and a readthrough protein, that are iden-tical over the total length of the stopped protein. As in frameshift, the level of stopped protein
produced largely exceeds the level of the
readthrough protein. Among plant viruses,
Until fairly recently, only suppressible UAG and UGA codons had been described. However suppression at the level of a UAA codon has been proposed for Beet soil-borne furovirus [45] and for Beet virus Q, a furo-like virus [46]. In addition to the termination codon, other elements on the
mRNA are required in cis for efficient
readthrough. In the case of TMV RNA, the na-ture of the two codons following the suppressible UAG codon are crucial for efficient readthrough [47,48]. The requirements in BYDV are totally different. Here, two elements, both located down-stream of the suppressible UAG codon
terminat-ing theCPgene are mandatory for readthrough in
vitro and in vivo [49]. The proximal element is located six to 15 nucleotides downstream of the UAG, and is composed of 16 repeats of the se-quence CCN NNN (N: any nucleotide); deletion
of the 5% proximal third of these repeats
dramati-cally reduces readthrough. The distal element is about 60 nucleotides long and is located nearly 700 nucleotides downstream of the UAG codon, in the readthrough ORF. Deletions within this element also strongly impair readthrough. The distal element is well conserved among Lute-oviruses and in Pea enation mosaic enamovirus, lending weight to the possibility that a similar role may also be ascribed to the corresponding region in these viruses. It will be interesting to establish whether the proximal and distal regions interact by long-distance base-pairing. As opposed to the situation observed in animal viruses in which a hairpin and even a pseudoknot structure down-stream of the suppressible termination codon are frequently required for readthrough, among plant viruses there has to date been no report that regions downstream of the suppressible termina-tion codon could adopt secondary structures im-portant for efficient readthrough.
tRNAs have been isolated from various plant tissues that act as suppressor tRNAs and misread
termination codons in trans[5]. These are two Tyr
accepting tRNAs for the UAG codon in TMV RNA, as well as a Trp and a Cys accepting tRNA for the UGA codon in the Tobacco rattle to-bravirus RNA. To date, no suppressor tRNA has been isolated that specifically recognizes suppress-ible UAA codons. Nevertheless, the fact that mu-tating the suppressible UAG codon in TMV RNA to a UAA codon leads to virion formation in plants, suggests that a tRNA is present in the host that can recognize UAA or UAG containing TMV RNA [50].
5. Regulation of gene expression at the post-translational level
Proteolytic processing of precursor polyproteins is a crucial process among many viruses [51]. It results in the production of mature as well as intermediate-sized viral proteins. The activity of a processed protein may be different from its activ-ity when in a precursor form. Cleavage which is triggered by viral-encoded proteinases, can occur
in cis and/or in trans; it can be a co- and/or
post-translational event. The viral proteinases can be grouped on the basis of their relatedness to cellular proteinases. Hence, DNA containing viruses possess pepsin-like proteinases, whereas the RNA containing viruses possess either
chy-motrypsin-like (Como6iridae, Poty6iridae) or
pa-pain-like (Poty6iridae, Tymoviruses) proteinases.
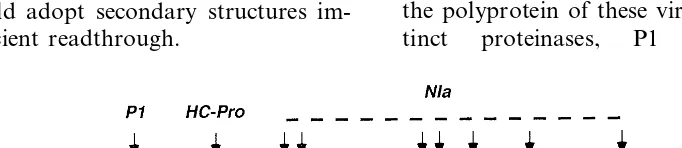
Fig. 3 provides a schematic representation of the genome organization of Potyviruses. Processing of the polyprotein of these viruses requires three
dis-tinct proteinases, P1 a chymotrypsin-like
Fig. 4. ORFs of Grapevine fleck virus. The ORFs 1, 2 and 3 are in different reading frames. The function of ORFs 3 and 5 is unknown. Other indications are as in Fig. 1.
from two distinct ORFs (Bromo6iridae), or result
from frameshift or from readthrough
(Furoviruses, Necroviruses, Tobamoviruses, To-braviruses, Tombusviruses). This illustrates the fascinating flexibility of protein expression among different viruses.
Probably the highest level of sophistication with respect to the use of multiple strategies by a single virus, is to be found among the Luteoviruses (Fig. 6). The ingenuity of the Luteovirus genome of
B6000 nucleotides is extreme: it resorts to
over-lapping ORFs, frameshift, readthrough, the pro-duction of a sgRNA, and proteolytic processing to
release its VPg for attachment to the 5%end of the
genome. In addition, as outlined above, for at least one Luteovirus, BYDV-PAV whose genome proteinase and HC-Pro a papain-like proteinase
which cleave themselves at their C-terminus, and NIa a chymotrypsin-like proteinase which is re-sponsible for all the other cleavages in the
polyprotein, and hence operates incisand intrans.
6. Conclusions
As emphasized here, the information content of the viral genome is extremely compact and viruses use an astounding wealth of different strategies to express their proteins. Ever since their discovery viruses have fascinated researchers and they con-tinue to do so. A recent example is the discovery of Grapevine fleck virus (discussed in Ref. [52]), which represents a true lesson in economy. This virus is related to Tymoviruses and to Mar-afiviruses. Its monopartite RNA genome of about 8800 nucleotides contains five ORFs (Fig. 4). What is remarkable in the genome of this virus, is that over a range of 1000 nucleotides, there are three overlapping ORFs. The shortest of these
ORFs potentially encodes a protein of 37-K. It
is difficult to imagine a more economical
organization.
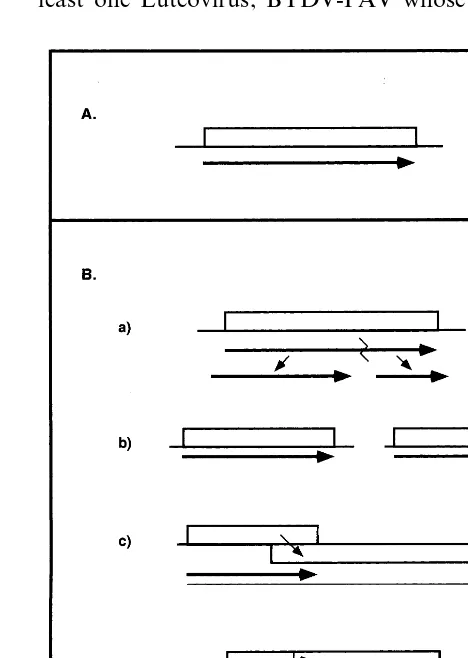
A look at the different strategies employed by RNA viruses to synthesize the RdRp complex testifies to their enormous variability. As men-tioned above, all RNA viruses synthesize an RdRp complex. This comprises the RdRp itself charac-terized by a conserved Gly-Asp-Asp (GDD) motif, and in virtually all cases also by an NTP binding-RNA helicase motif (rev. in Ref. [53]) in the same or in another protein. Yet, they resort to very different strategies to produce this enzyme com-plex (Fig. 5), depending on the virus group to which they belong. The enzyme complex can be
composed of a single protein (Carlaviruses,
Hordeiviruses, Potexviruses, Trichoviruses), or it can be composed of two proteins that either are produced by processing (Tymoviruses), originate
Fig. 6. Strategies of expression used by Luteoviruses. Numbers correspond to the ORFs. The position of the N-terminal cleavage of the VPg is provided by a vertical arrow, and the approximate size of the VPg is shown below the ORF. The position of the suppressible UAG codon is shown. To scale. Other indications are as in Figs. 1 and 4; see text for details.
lacks a VPg, an element within the 3%region of the
mRNA facilitates initiation of translation by
mim-icking a 5% cap structure.
In this review, we have tried to highlight a few salient features that characterize the expression of plant viruses and to illustrate the diversity of the mechanisms employed by these pathogens. Unde-niably, much of what we know about macromolec-ular structures and molecmacromolec-ular biology we owe to the study of viruses. There is no doubt that many exciting discoveries are still to be made.
Acknowledgements
We are grateful to Andrzej Palucha for his suggestions and for helpful discussions. S.U.-I. is grateful to Colciencias (Colombia) and the French ‘Ministe`re des Affaires Etrange`res’ for fellowships, and thanks Claude Vuillaume (CIRAD, Guade-loupe) for his constant encouragement. M.M. benefited from a grant from the Twin-Association between the Institut Jacques Monod and the Cen-tre de Ge´ne´tique Mole´culaire (France), and the Institute of Biochemistry and Biophysics (Poland). This work was performed with the financial sup-port in the form of a stipendium to J.S. within the frame of the ‘Gemeinsames Hochschulsonderpro-gramm III von Bund und La¨ndern u¨ber den DAAD’, Germany. The Institut Jacques Monod is an ‘Institut Mixte, CNRS-Universite´s Paris 6 & 7’.
References
[1] F.A. Murphy, C.M. Fauquet, D.H.L. Bishop, S.A. Ghabrial, A.W. Jarvis, G.P. Martelli, M.A. Mayo, M.D. Summers, Virus Taxonomy, Springer, Wien, New York, 1995.
[2] F. Bernardi, A.-L. Haenni, Viruses: exquisite models for cell strategies, Biochimie 80 (1998) 1035 – 1041.
[3] D.R. Gallie, Translational control of cellular and viral mRNAs, Plant Mol. Biol. 32 (1996) 145 – 158.
[4] J. Fu¨tterer, T. Hohn, Translation in plants — rules and exceptions, Plant Mol. Biol. 32 (1996) 159 – 189. [5] I.G. Maia, K. Se´ron, A.-L. Haenni, F. Bernardi, Gene
expression from viral RNA genomes, Plant Mol. Biol. 32 (1996) 367 – 391.
[6] F. He´ricourt, I. Jupin, A.-L. Haenni, The genome of plant RNA viruses, in: C.L. Mandahar (Ed.), The Molecular Biology of Plant Viruses, Kluwer, Norwell, USA, 1999, pp. 1 – 28.
[7] R. Cattaneo, Different types of messenger RNA editing, Annu. Rev. Genet. 25 (1991) 71 – 88.
[8] H.-J. Schalk, V. Matzeit, B. Schiller, J. Schell, B. Gronenborn, Wheat dwarf virus, a geminivirus of grami-naceous plants needs splicing for replication, EMBO J. 8 (1989) 359 – 364.
[9] Z. Kiss-La´szlo´, S. Blanc, T. Hohn, Splicing of cauliflower mosaic virus 35S RNA is essential for viral infectivity, EMBO J. 14 (1995) 3552 – 3562.
[10] J. Fu¨tterer, I. Potrykus, M.P. Valles Brau, I. Dasgupta, R. Hull, T. Hohn, Splicing in a plant pararetrovirus, Virology 198 (1994) 663 – 670.
[11] J. Stanley, R. Hanau, A.O. Jackson, Sequence compari-son of the 3% ends of a subgenomic RNA and the genomic RNAs of barley stripe mosaic virus, Virology 139 (1984) 375 – 383.
[12] C.S. Schmaljohn, Bunyaviridae: the viruses and their replication, in: B.N. Fields, D.M. Knipe, P.M. Howley, et al. (Eds.), Fundamental Virology, Lippincott-Raven, Philadelphia, PA, 1996, pp. 649 – 673.
[13] B.-C. Ramirez, A.-L. Haenni, Molecular biology of tenuiviruses, a remarkable group of plant viruses, J. Gen. Virol. 75 (1994) 467 – 475.
[14] B.-C. Ramirez, G. Macaya, L.A. Calvert, A.-L. Haenni, Rice hoja blanca virus genome characterization and ex-pression in vitro, J. Gen. Virol. 73 (1992) 1457 – 1464. [15] R. Goldbach, R. Eggen, C. de Jager, A. van Kammen,
[16] R.J. Jackson, S.L. Hunt, J.E. Reynolds, A. Kaminski, Cap-dependent and cap-independent translation: opera-tional distinctions and mechanistic interpretations, Curr. Top. Microbiol. Immunol. 203 (1995) 1 – 29.
[17] R.H.A. Coutts, J.E. Rigden, A.R. Slabas, G.P. Lomonossoff, P.J. Wise, The complete nucleotide se-quence of tobacco necrosis virus strain D, J. Gen. Virol. 72 (1991) 1521 – 1529.
[18] M.A. Mayo, V. Ziegler-Graff, Molecular biology of lute-oviruses, Adv. Virus Res. 46 (1996) 413 – 460.
[19] M. Kozak, Determinants of translational fidelity and efficiency in vertebrate mRNAs, Biochimie 76 (1994) 815 – 821.
[20] J. Fu¨tterer, I. Potrykus, Y. Bao, L. Li, T.M. Burns, R. Hull, T. Hohn, Position-dependent ATT initiation dur-ing plant pararetrovirus rice tungro bacilliform virus translation, J. Virol. 70 (1996) 2999 – 3010.
[21] Y. Shirako, Non-AUG translation initiation in a plant RNA virus: a 40-amino-acid extension is added to the N terminus of the soil-borne wheat mosaic virus capsid protein, J. Virol. 72 (1998) 1677 – 1682.
[22] H.A. Lu¨tcke, K.C. Chow, F.S. Mickel, K.A. Moss, H.F. Kern, G.A. Scheele, Selection of AUG initiation codons differs in plants and animals, EMBO J. 6 (1987) 43 – 48. [23] D.R. Gallie, R.L. Tanguay, V. Leathers, The tobacco etch viral 5% leader and poly(A) tail are functionally synergistic regulators of translation, Gene 165 (1995) 233 – 238.
[24] V. Leathers, R. Tanguay, M. Kobayashi, D.R. Gallie, A phylogenetically conserved sequence within the viral 3% untranslated RNA pseudoknots regulates translation, Mol. Cell. Biol. 13 (1993) 5331 – 5347.
[25] D.R. Gallie, M. Kobayashi, The role of the 3% -untrans-lated region of non-polyadeny-untrans-lated plant viral mRNAs in regulating translational efficiency, Gene 142 (1994) 159 – 165.
[26] X. Danthinne, J. Seurinck, F. Meulewaeter, M. van Montagu, M. Cornelissen, The 3%untranslated region of satellite tobacco necrosis virus RNA stimulates transla-tion in vitro, Mol. Cell. Biol. 13 (1993) 3340 – 3349. [27] R.T. Timmer, L.A. Benkowski, D. Schodin, S.R. Lax,
A.M. Metz, J.M. Ravel, K.S. Browning, The 5% and 3% untranslated regions of satellite tobacco necrosis virus RNA affect translational efficiency and dependence on a 5%cap structure, J. Biol. Chem. 268 (1993) 9504 – 9510. [28] M. Shams-bakhsh, R.H. Symons, Barley yellow dwarf
virus-PAV RNA does not have a VPg, Arch. Virol. 142 (1997) 2529 – 2535.
[29] E. Allen, S. Wang, W.A. Miller, Barley yellow dwarf virus RNA requires a cap-independent translation se-quence because it lacks a 5% cap, Virology 253 (1999) 139 – 144.
[30] S. Wang, K.S. Browning, W.A. Miller, A viral sequence in the 3%-untranslated region mimics a 5%cap in facilitat-ing translation of uncapped mRNA, EMBO J. 16 (1997) 4107 – 4116.
[31] K.S. Browning, The plant translational apparatus, Plant Mol. Biol. 32 (1996) 107 – 144.
[32] I.T.D. Petty, A.O. Jackson, Two forms of the major barley stripe mosaic virus nonstructural protein are syn-thesizedin6i6ofrom alternative initiation codons, Virol-ogy 177 (1990) 829 – 832.
[33] J. Verver, O. Le Gall, A. van Kammen, J. Wellink, The sequence between nucleotides 161 and 512 of cowpea mosaic virus MRNA is able to support internal initiation of translation in vitro, J. Gen. Virol. 72 (1991) 2339 – 2345.
[34] E. Herzog, H. Guilley, C. Fritsch, Translation of the second gene of peanut clump virus RNA 2 occurs by leaky scanning in vitro, Virology 208 (1995) 215 – 225. [35] S.P. Dinesh-Kumar, V. Brault, W.A. Miller, Precise
mapping and in vitro translation of a trifunctional subge-nomic RNA of barley yellow dwarf virus, Virology 187 (1992) 711 – 722.
[36] E. Tacke, D. Pru¨fer, F. Salamini, W. Rohde, Characteri-zation of a potato leafroll luteovirus subgenomic RNA: differential expression by internal translation initiation and UAG suppression, J. Gen. Virol. 71 (1990) 2265 – 2272.
[37] H. Zhou, A.O. Jackson, Expression of the barley stripe mosaic virus RNAb ‘triple gene block’, Virology 216 (1996) 367 – 379.
[38] J. Verchot, S.M. Angell, D. Baulcombe, In vivo transla-tion of the triple gene block of potato virus X requires two subgenomic mRNAs, J. Virol. 72 (1998) 8316 – 8320. [39] J. Fu¨tterer, Z. Kiss-La´szlo´, T. Hohn, Nonlinear ribo-some migration on cauliflower mosaic virus 35S RNA, Cell 73 (1993) 789 – 802.
[40] K.L. Hefferon, H. Khalilian, H. Xu, M.G. AbouHaidar, Expression of the coat protein of potato virus X from a dicistronic mRNA in transgenic potato plants, J. Gen. Virol. 78 (1997) 3051 – 3059.
[41] P.A. Ivanov, O.V. Karpova, M.V. Skulachev, O.L. Tomashevskaya, N.P. Rodionova, Yu.L. Dorokhov, J.G. Atabekov, A tobamovirus genome that contains an internal ribosome entry site functional in vitro, Virology 232 (1997) 32 – 43.
[42] W. Rohde, A. Gramstat, J. Schmitz, E. Tacke, D. Pru¨fer, Plant viruses as model systems for the study of non-canonical translation mechanisms in higher plants, J. Gen. Virol. 75 (1994) 2141 – 2149.
[43] I. Brierley, Ribosomal frameshifting on viral RNAs, J. Gen. Virol. 76 (1995) 1885 – 1892.
[44] D.F. Voytas, J.D. Boeke, Yeast retrotransposons and tRNAs, Trends Genet. 9 (1993) 421 – 426.
[45] R. Koenig, S. Loss, Beet soil-borne virus RNA 1: genetic analysis enabled by a starting sequence generated with primers to highly conserved helicase-encoding domains, J. Gen. Virol. 78 (1997) 3161 – 3165.
[46] R. Koenig, C.W.A. Pleij, C. Beier, U. Commandeur, Genome properties of beet virus Q, a new furo-like virus from sugarbeet, determined from unpurified virus, J. Gen. Virol. 79 (1998) 2027 – 2036.
[47] J.M. Skuzeski, L.M. Nichols, R.F. Gesteland, J.F. Atkins, The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons, J. Mol. Biol. 218 (1991) 365 – 373.
[49] C.M. Brown, S.P. Dinesh-Kumar, W.A. Miller, Local and distant sequences are required for efficient readthrough of the barley yellow dwarf virus PAV coat protein gene stop codon, J. Virol. 70 (1996) 5884 – 5892. [50] M. Ishikawa, T. Meshi, F. Motoyoshi, N. Takamatsu, Y. Okada, In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus, Nucleic Acids Res. 14 (1986) 8291 – 8305.
[51] W.G. Dougherty, B.L. Semler, Expression of virus-en-coded proteinases: functional and structural similarities with cellular enzymes, Microbiol. Rev. 57 (1993) 781 – 822.
[52] B. Walter, Virus et viroses de la vigne: diagnostic et me´thodes de lutte, Virologie 2 (1998) 435 – 444.
[53] G. Kadare´, A.-L. Haenni, Virus-encoded RNA helicases, J. Virol. 71 (1997) 2583 – 2590.