Summary Thinning of forest stands is widely carried out to minimize the slowing of growth of individual stems that fol-lows from increasing competition among trees as they become bigger. After thinning, there is an increase in the growth rate of remaining trees because of an increase in the availability of resources per tree. Often, there is also an increase in foliar efficiency (biomass increase/foliage amount). On sites where mineral nutrient supply is limiting, fertilizers may be applied, often in association with thinning, to boost productivity. Growth responses to fertilizer application depend on an ade-quate supply of other resources, but also involve nonlinear interactions among mineral nutrients and between nutrients and other growth-limiting environmental factors. The effects of thinning and fertilizing on the carbon gain and growth responses of Pinus radiata D. Don to availability of resources (light, mineral nutrients and water) and to changes in the canopy are discussed.
Keywords: foliar efficiency, light, mineral nutrients, productiv-ity, water.
Introduction
Thinning of forest stands is carried out to minimize the slowing of growth of individual stems that follows from increasing competition among trees as they increase in size. Fertilizers are often applied in association with thinning, where mineral nu-trient availability limits productivity. Responses to thinning and fertilizing vary in magnitude among stands even of the same species. Mensurational data obtained from field trials is valuable for predicting responses of stands of a particular age and species under particular edaphic and climatic conditions, but cannot be applied generally. Thus, empirical fertilizer and thinning trials, which contribute little to understanding of the processes involved in growth responses, have frequently been undertaken to allow prediction of the responses of specific stand types to particular silvicultural treatments. Such trials have led to a large accumulation of information but little advance in understanding about the mechanisms that underlie silvicultural treatment responses. Without such understanding, accurate predictions about stand responses to management actions cannot be made in many circumstances.
Thinning and fertilizing both have potential for increasing
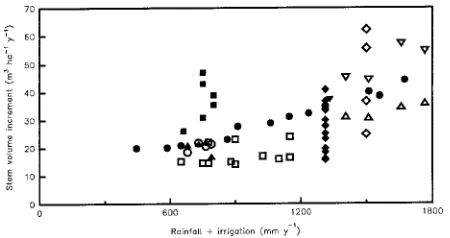
stand productivity. Thinning potentially reduces limitations by all resources, whereas fertilizing alters only nutrient supply. Fertilizing has the same effect on the productivity of both trees and stands, whereas thinning has different effects on produc-tivity at the tree and stand scales. A mechanistic explanation of these responses requires an understanding of the process of carbon gain and assimilate allocation. The scarcity of informa-tion about belowground responses often limits the scope for mechanistic interpretations of silvicultural treatment effects. In Pinus radiata D. Don, stem biomass increase, stem growth and net primary production (NPP) are closely correlated over a wide range of conditions (Sheriff and Rook 1990, McMurtrie and Landsberg 1992). Moreover, there are more published data on stem growth than productivity. Consequently, in this analy-sis of how environmental factors and silvicultural treatments affect P. radiata I have used stem growth as a surrogate for productivity. Many of the data used are from monoculture stands at Eyrewell, Kaingaroa, Puruki and Woodhill forests, New Zealand, and at the Biology of Forest Growth Experiment (BFG), ACT, Australia, which have large differences in above-ground productivity (Figure 1).
The focus of this review is on stand responses to thinning and fertilizing as determined by resource availability, interac-tions between resources, and foliage. Specifically, I consider: (1) the effects of resource availability on productivity and stem growth; (2) the relative importance of foliage mass and foliar efficiency; and (3) the allocation of assimilate to different processes and end uses.
Effects of resource availability on productivity
Resource availability and biomass productivity of individual trees are closely but nonlinearly related. The best combination of site and individual tree productivity occurs at an intermedi-ate stocking, the magnitude of which depends on tree size and resource availability. At high stocking, productivity of indi-viduals is small because of competition for resources, but site productivity is high. At low stocking, inter-tree competition is small so individual productivity is high, but inherent limita-tions to physiological activity and the maximum size individu-als can attain limit site utilization, so that site biomass productivity is low (e.g., Woollons and Whyte 1989, Whyte and Woollons 1990).
Responses of carbon gain and growth of
Pinus radiata
stands to
thinning and fertilizing
D. W. SHERIFF
Plantation Forest Research Center, Division of Forestry and Forest Products, CSIRO, P.O. Box 946, Mount Gambier, SA 5290, Australia
Received March 2, 1995
Light
The relationship between aboveground biomass production and intercepted light can be represented as a linear regression:
P = a + bI, (1)
where P is aboveground biomass productivity, I is intercepted PPFD, b is biomass production per unit of intercepted PPFD, and a is a negative quantity related to losses of carbon from the aboveground portion. Parameter a is composed of two compo-nents: (1) photosynthate respired aboveground, and (2) the proportion of assimilate allocated belowground. Differences in I, due to differences in LAI, will have a greater effect on P than on a, so for a size and age class, light use efficiency (LUE, equivalent to the parameter b) of aboveground biomass pro-duction increases with light interception.
Although aboveground biomass production of conifer stands is often linearly related to light interception (e.g., Sten-berg et al. 1995), carbon gain per unit intercepted light is reduced by various constraints, which change the values of parameters a and b in Equation 1. For example, following thinning, LUE (biomass gain/light absorbed) of Eucalyptus regnans J.F. Muell increased and stand biomass production was unchanged in the absence of weeds, whereas in the pres-ence of weed competition LUE was unchanged and stand biomass production was reduced (West and Osler 1995).
Water deficits
There is a negative correlation between water deficits in the plant or in the air surrounding conifers and foliar carbon assimilation (ACO2) (e.g., Bennett and Rook 1978, Whitehead
et al. 1983, Sheriff 1995, Sheriff and Mattay 1995, Teskey et al. 1995). Sheriff and Whitehead (1984) found ACO2 of con-tainer-grown P. radiata began to decline at a foliar water potential (Ψ) of −1.6 to −1.8 J g−1, and reached zero at a Ψ of
−2.0 to −2.4 J g−1, over a narrower range than other measured conifers (e.g., Teskey et al. 1995). On the other hand, Sheriff (1995) found ACO2 of field-grown P. radiata changes as a result of stomatal and nonstomatal effects (e.g., Bunce 1977), so that it is constant at a soil water potential (Ψs) above about −0.6 J g−1, and is greater than zero at a Ψ
s of −3.0 J g−1.
The negative relationship between leaf to air vapor pressure difference (D) and ACO2 results from a negative effect of D on gs, and sometimes also from a direct effect of D on ACO2 (Sheriff 1995).
Lowering of ACO2 by a reduction in gs will increase assimi-latory transpiration efficiency (ATE = assimilation/transpira-tion), but when there is a direct effect of water deficits on ACO2, ATE will decline (Sheriff 1995).
Water use
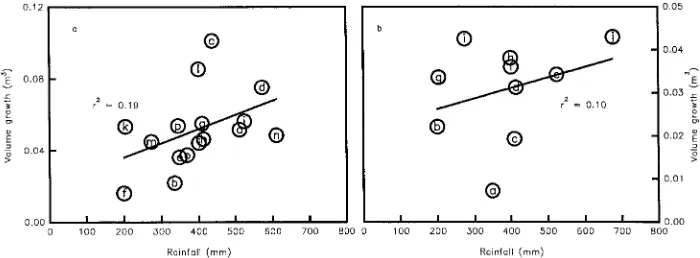
Biomass productivity and transpirational water loss are gener-ally positively associated (e.g., Leith 1976, Ritchie 1983) be-cause both processes are driven by diffusion and climatic factors, especially radiation and water. Greater availability of water increases aboveground productivity of water-limited stands (Figure 1). Figure 2 provides examples of interrelation-ships between water supply and yield based on data analyzed by Nambiar (1994) from 10--15-year-old Pinus radiata stands across a range of sites and on data from Puruki and Woodhill (comparisons between data sets are only valid for sets of either MAI or CAI data). At one site (denoted by solid circles), where only water availability was varied, water supply and yield changed together (Snowdon and Waring 1991). A linear re-gression for this relationship produced an r2 of 0.97. A linear
Figure 1. Relationships between aboveground biomass production and foliar biomass at several sites. The lines (fitted with functions of the form: Productivity = a(1 −e−kfb); where a and k are parameters and fb
is foliar biomass) denote: a = Puruki site (ages 2--12, minimum water or nutrient limitations, Beets and Pollock 1987); b = Woodhill site (ages 7--10.5, with fertilizer, Beets and Madgwick 1988); c = same as b, but without fertilizer; d = Kaingaroa site (ages 2--22, with thinning at 8 years, Madgwick et al. 1977); e = Eyrewell site (ages 7--11 years, with and without thinning and fertilizer, Mead et al. 1984); f = Rotorua site (ages 5--13, close-spaced stands, Madgwick and Oliver 1985); g = BFG site (with irrigation + nutrients (IL and IF), Snowdon and Benson 1992); h = same as g, but with irrigation only; and i = same as h, but unirrigated (C and F).
regression calculated for the CAI data in Figure 2 produced:
V = 8.5 + 0.019 W (r2 = 0.35) (2)
where V is the annual volume stem increment, and W is the annual water input. Thus, 65% of the variance in the data can be ascribed to factors other than differences in water supply. At the water-limited BFG site, additional water alone increased stem growth less than irrigation + nutrients, whereas nutrients alone had little effect. Variations in productivity and stem growth at the well-watered, fertile Puruki site are attributable to different thinning and pruning regimes. A reliable indication of how much climatic factors other than precipitation affect increment at any sites in Figure 2 is not possible because, apart from the Woodhill data, they were collected in a single year (e.g., Snowdon and Waring 1991) or had matching data from different treatments for each of several years (e.g., BFG data) when there were large, varying responses to treatment. How-ever, r2 values calculated from data collected over several years on individual P. radiata trees (MacDougal 1938) indicate that 80--90% of the variation in stem growth can be attributed to factors other than variation in rainfall (Figure 3).
There are strong interactions between fertilizer and water in water-limited environments. At the water-limited BFG site, fertilization increased diameter growth and estimated NPP of irrigated trees by 42 and 18%, respectively, whereas corre-sponding values for unirrigated trees were only 14 and 5%, respectively (McMurtrie et al. 1990a). Mineralization and ab-sorption are higher in wetter soils and when periods of higher soil water are longer (e.g., Snowdon and Waring 1990). There-fore, fertilization may not alter productivity unless accompa-nied by thinning if the response to fertilization is constrained by a low soil water content (e.g., Butcher 1977, Donald 1987, Turner and Lambert 1987, Snowdon and Waring 1990). How-ever, fertilizing a thinned, nutrient-limited stand may not ap-preciably increase water use in the short to medium term if WUE is increased (Sheriff et al. 1986, Squire et al. 1987). Fertilization will improve tree water status and so increase growth when improved nutrition causes greater water uptake (e.g., Myers 1988) by enhancing root hydraulic conductance (Minshall, 1975).
Nutrients
Increased mineral nutrient availability can increase
productiv-ity if the nutrient in question is limiting carbon gain, provided that the greater supply causes an increase in nutrient uptake. Carbon gain has been widely linked to the nitrogen status of individual leaves and canopies (Field and Mooney 1983, Evans 1989, Field 1991, Teskey et al. 1995). In conifers there may be a strong positive association between foliar ACO2 and [N] (both per foliage area or mass) (e.g., Nambiar et al. 1984, Sheriff and Mattay 1995), but at times there is little (e.g., Teskey et al. 1994) or no (e.g., Sheriff 1995) association. Sometimes the relationship may be present only when other limitations are small, for example at BFG ACO2 and foliar [N] were interrelated only when foliar conductance was greater than 75 mmol m−2 s−1 (Thompson and Wheeler 1992).
The observation that site productivity is often influenced more by plant nitrogen content than by light interception or foliage mass led Ågren (1985) to formulate the nitrogen ductivity concept, namely that ‘‘the amount of biomass pro-duced is directly related to the amount of nitrogen in that biomass.’’ This relationship is often strong because nitrogen is often a growth-limiting nutrient (Ågren 1985). Nitrogen con-tributes positively to carbon gain by increasing both LUE and LAI, i.e., light interception (e.g., Linder and Rook 1984). Although relationships between tissue nitrogen content and biomass productivity are generally good at a particular site, the relationships vary among sites because of differences in other factors that affect productivity (Figure 4).
Positive associations between ACO2 and many of the other elements required for growth have been reported (Terry and Rao 1991). Of these, effects of phosphorus on ACO2 (e.g., Brown 1981, Conroy et al. 1986, Sheriff et al. 1986, Black 1988, Reich and Schoettle 1988, Conroy et al. 1990, Rousseau and Reid 1990, Sheriff 1995, Teskey et al. 1995) and on growth (Raupach et al. 1975) are the most studied in conifers as well as in other tree species (e.g., Fei et al. 1990, Kirschbaum and Tompkins 1990, Cromer et al. 1993).
Responses of NPP and yield to variation in supply of a nutrient depend on potential limitations by other factors, in-cluding other nutrients, immobilization in the soil and interac-tions among nutrients (e.g., MacLeod 1969, Dey and Rao 1989). Interactions among nutrients may involve interactions in nutrient uptake where, for example, addition of a limiting nutrient can stimulate root growth, and therefore increase uptake of other mineral nutrients (Hopmans and Clerehan 1991). In trees, the best known interaction in nutrient
utiliza-Figure 3. Stem volume increase of two P. radiata trees in California.
tion is the N × P interaction (Figure 5). Thus, fertilization may only increase productivity if both N and P are applied (Donald 1987), or it may reduce productivity if nitrogen is added to P-deficient soils (Hunter et al. 1986). Surfaces in Figure 5 indicate stem growth is limited by the nutrient in shortest supply relative to the amount needed. Thus, to use foliar nutrient content to predict growth we need information on a range of foliar elements (Ingestad 1962). Failure to recognize limitations by non-nutrient factors can also lead to unpre-dictable responses of productivity to fertilization and tissue nutrient concentrations (e.g., Hunter and Hoy 1983).
Sink--source interactions
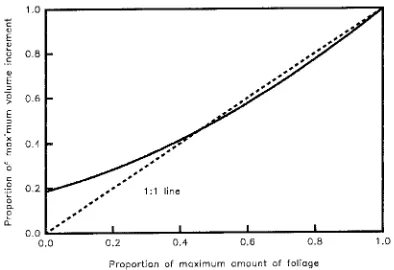
Sink activity, for example growth rate, and source size, the amount of assimilatory tissue, can both regulate source activity (Sweet and Wareing 1966, Luxmoore et al. 1995, Teskey 1995). Because current assimilate is important for growth during the main growing season (when growth is high), growth
is reduced when foliage is removed at this time (Rook and Whyte 1976). Replotting Rook and Whyte’s (1976) data as the proportions of maximum foliage and stem volume increment produces a 1/1 agreement over a wide range of values (Fig-ure 6); but a close relationship is not found for low growth rates (Rook and Whyte 1976). These interrelationships complicate analysis of mechanistic connections between growth and as-similation.
Respiration
Daily integrals of plant respiration and carbon assimilation are often linearly related, but several factors, for example light intercepted over the previous few days (Hansen and Jensen 1977), affect the magnitude of this relationship. More specifi-cally, growth respiration is usually proportional to ACO2 and maintenance respiration is usually proportional to protein turn-over (Amthor 1984). The latter probably explains the positive relationship between maintenance respiration and protein or N content (Kawahara et al. 1976, Sprugel et al. 1995), although in conifers a better relationship has been found between respi-ration and intercepted light (Sprugel et al. 1995). However, these simple relationships break down when conditions vary; for example different genotypes, species, or N/protein ratios (Greenwood and Barnes 1978, Greenwood et al. 1978, Crop-per and Gholz 1991).
Foliage quantity and efficiency
Productivity depends on the quantity of foliage, and on foliar efficiency (FE = (increase in biomass)/(foliage quantity)). Fo-liar efficiency can be calculated per leaf area (FEla) or per foliage mass (FElb). Thus, FE is partly derived from light interception per foliage quantity and partly from the efficiency of converting intercepted light into biomass. Foliar efficiency is almost always related to aboveground productivity, so a component of FE can result from the proportional allocation of assimilate aboveground versus belowground. Thus, although it is often used in discussion, FE is defined by several parts of the system and does not explain the system or how it operates. Light interception is nonlinearly related to LAI (e.g., Gower et
Figure 4. Aboveground biomass production in relation to aboveground nitrogen content of fertilized (d) and unfertilized (s) P. radiata
seedlings. Numbers next to data points indicate the width of the weed-free strip along the rows of plants. The relationships are: Fertil-ized: Biomass increase = 2.37 + 9.79N, r2 = 1.00; and Unfertilized:
Biomass increase = 17.52 + 3.83N, r2 = 1.00. Adapted from data of
Woods et al. (1992).
Figure 5. Interacting effects of foliar phosphorus and foliar nitrogen on biomass production of Araucaria cunninghamii. Adapted from data
of Richards and Bevedge (1969).
Figure 6. Relationship between the amount of foliage on a P. radiata
al. 1995), which explains at least in part the observed increases in FEla following thinning, because at high LAI, thinning changes LAI more than light interception.
At the well-watered, fertile Puruki site, where biomass pro-duction was little constrained by water or nutrients, thinning increased both FE (FEla = 1.9 Mg ha−1 year−1, FElb = 1.6 Mg ha−1 year−1) and LUE (1.6 Mg ha−1 per GJ m−2) (Figures 7a and 7b). The increase in LUE may have resulted from pruning of shaded foliage, from greater partitioning of biomass above-ground or from less tissue desiccation, which can be apprecia-ble even on well-watered sites. For example, during a wet midwinter period in southeastern South Australia, Ψ of P. ra-diata on sunny days was −0.4 (predawn) to −1.25 J g−1 (mini-mum) (Sheriff, unpublished observations), which could reduce growth considerably (e.g., Rook et al. 1977).
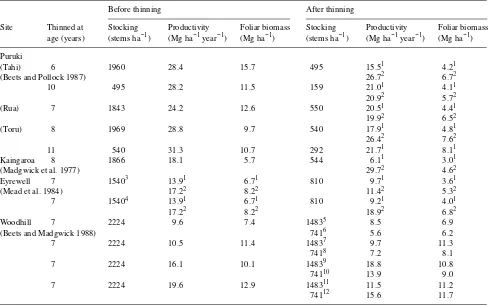
Where productivity is not seriously limited by resource availability before thinning, site productivity is generally re-duced by thinning. However, even under these conditions, greater resource availability will enable productivity to recover to near prethinning values in the second year after thinning (Table 1). Productivity of individual trees usually increases rapidly after thinning because foliar biomass increases and has a higher FE (Table 2). The contribution of FE to productivity
Figure 7. Effects of thinning, indicated by vertical dotted lines, at Puruki on (a) FEla and (b) LUEi. Adapted from data of Beets and
Pollock (1987), Grace et al. (1987) and Whitehead (1986).
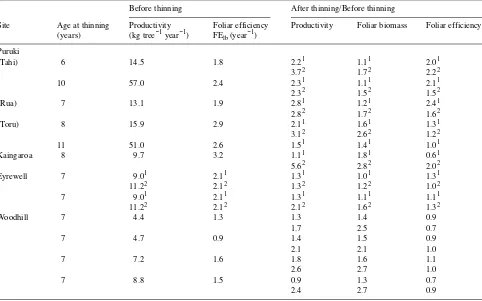
Table 1. Effects of thinning P. radiata on aboveground biomass production and foliar biomass. Except where indicated in the footnotes,
comparisons are between pre-and post-thinning values. Where possible, values are given for the first and second years after thinning, which are indicated by the superscript numerals 1 and 2, respectively.
Before thinning After thinning
Site Thinned at Stocking Productivity Foliar biomass Stocking Productivity Foliar biomass age (years) (stems ha−1) (Mg ha−1 year−1) (Mg ha−1) (stems ha−1) (Mg ha−1 year−1) (Mg ha−1) Puruki
(Tahi) 6 1960 28.4 15.7 495 15.51 4.21
(Beets and Pollock 1987) 26.72 6.72
10 495 28.2 11.5 159 21.01 4.11
20.92 5.72
(Rua) 7 1843 24.2 12.6 550 20.51 4.41
19.92 6.52
(Toru) 8 1969 28.8 9.7 540 17.91 4.81
26.42 7.62
11 540 31.3 10.7 292 21.71 8.11
Kaingaroa 8 1866 18.1 5.7 544 6.11 3.01
(Madgwick et al. 1977) 29.72 4.62
Eyrewell 7 15403 13.91 6.71 810 9.71 3.61 (Mead et al. 1984) 17.22 8.22 11.42 5.32 7 15404 13.91 6.71 810 9.21 4.01
17.22 8.22 18.92 6.82
Woodhill 7 2224 9.6 7.4 14835 8.5 6.9
(Beets and Madgwick 1988) 7416 5.6 6.2
7 2224 10.5 11.4 14837 9.7 11.3
7418 7.2 8.1
7 2224 16.1 10.1 14839 18.8 10.8
74110 13.9 9.0
7 2224 19.6 12.9 148311 11.5 11.2
74112 15.6 11.7
3 The same year’s data, between unthinned and thinned stands; 4 the same year’s data, between unthinned and thinned, nitrogen fertilized stands; 5,6 the same year’s data, between thinned and unthinned stands with no added nutrients; 7,8 the same year’s data, between thinned and unthinned
is greatest in the first year after thinning, before the canopy has expanded greatly. Before thinning, potential for carbon gain explains 93% of the variance in aboveground productivity at the sites in Table 2 (foliage biomass contributes 62% and FElb contributes 31%). One to two years after thinning, these values were 88, 26 and 62%, respectively, indicating that FElb is an important factor driving aboveground productivity after thin-ning, but the data do not indicate causes or mechanisms. Data from other pine species indicate an important part of the thinning response is explained by greater carbon gain per unit foliage (e.g., Donner and Running 1986, Ginn et al. 1991). This contributes both to the general growth response and to crown expansion as remaining trees reoccupy the site. Crown expansion enhances the increase in productivity that results from more foliage.
At BFG, adding nutrients to a nutrient-limited stand well supplied with water increased foliage mass and FE, such that they contributed an estimated 70 and 30% to the greater pro-ductivity of the fertilized stand (McMurtrie et al. 1990a). Generally, FElb increased as soil resources increased: control, fertilized, irrigated, irrigated and fertilized, and irrigated with nutrient solution treatments had FElb values of 2.01, 2.34, 2.33, 2.75, and 2.82 Mg ha−1 year−1 per Mg ha−1, respectively (Snowdon and Benson 1992).
Stand characteristics defined by FElb and foliar biomass before thinning explain 90% of the variance in data for the same characteristics after thinning. Thus the factors driving
prethinning productivity are strongly related to the factors that drive postthinning productivity.
Effects on biomass partitioning below- and aboveground Most studies have indicated that greater availability of soil nutrients or water causes the proportion of assimilate allocated to belowground biomass to fall. Thus, greater aboveground productivity as a result of fertilization, irrigation and thinning can be caused by changes in assimilate allocation. Many stud-ies of this effect have been on annual crops, but the principles are generally applicable, even though controls on allocation may be species specific (e.g., Gower et al. 1995). Thus, al-though greater assimilate allocation to aboveground biomass can contribute to greater aboveground productivity following fertilization, irrigation or thinning, the magnitude of this con-tribution is species specific. For example, fine root turnover is a major sink for assimilate after canopy closure in conifers, but effects of changed soil nutrient availability on assimilate allo-cation to fine roots vary in size and direction in different studies (e.g., Gower et al. 1995).
In P. radiata there seems to be a close negative link between biomass partitioning to fine roots and to stems (Santantonio 1989), but variation in nutrient supply has produced contradic-tory reports of changes in assimilate allocation to roots. Thomas and Mead (1992) observed a 9 and 37% greater biomass partitioning belowground and to fine roots,
respec-Table 2. Effects of thinning P. radiata on aboveground biomass production of trees and foliar efficiency at four sites. Details of treatments are in
Table 1.
Before thinning After thinning/Before thinning
Site Age at thinning Productivity Foliar efficiency Productivity Foliar biomass Foliar efficiency (years) (kg tree−1 year−1) FElb (year−1)
Puruki
(Tahi) 6 14.5 1.8 2.21 1.11 2.01
3.72 1.72 2.22
10 57.0 2.4 2.31 1.11 2.11
2.32 1.52 1.52
(Rua) 7 13.1 1.9 2.81 1.21 2.41
2.82 1.72 1.62
(Toru) 8 15.9 2.9 2.11 1.61 1.31
3.12 2.62 1.22
11 51.0 2.6 1.51 1.41 1.01
Kaingaroa 8 9.7 3.2 1.11 1.81 0.61
5.62 2.82 2.02
Eyrewell 7 9.01 2.11 1.31 1.01 1.31
11.22 2.12 1.32 1.22 1.02
7 9.01 2.11 1.31 1.11 1.11
11.22 2.12 2.12 1.62 1.32
Woodhill 7 4.4 1.3 1.3 1.4 0.9
1.7 2.5 0.7
7 4.7 0.9 1.4 1.5 0.9
2.1 2.1 1.0
7 7.2 1.6 1.8 1.6 1.1
2.6 2.7 1.0
7 8.8 1.5 0.9 1.3 0.7
tively, in N-fertilized trees, which is qualitatively the same as found by Snowdon and Waring (1985) and Smith et al. (1994), whereas Nambiar (1980) found a smaller carbon allocation to roots of fertilized than unfertilized trees and Squire et al. (1978) observed a small, but similar effect on mycorrhizal roots. Barker (1973) found no clear relationships between biomass production with variying nutrient status and partition-ing belowground. Differences between modeled net carbon gain and aboveground biomass production in P. radiata indi-cate a 50% reduction in allocation of assimilate to roots in response to an increase in nutrient supply (McMurtrie and Landsberg 1992). Similar results have been obtained for other species (Santantonio 1989). Reasons for these contradictions are unclear, but could result from genetic variation in root/shoot ratios or efficiencies of nutrient uptake and utiliza-tion (Theodorou and Bowen 1993), or responses to nutriutiliza-tion (Snowdon and Waring 1985), or to ontogenic changes in par-titioning (e.g., Madgwick 1981).
Thinning probably increases carbon partitioning below-ground in the short term (Santantonio 1989). At Puruki, thin-ning increased carbon allocation to fine roots by 33%. However, fine roots were a minor component (4.6%) of biomass production immediately after thinning (Santantonio and Santantonio 1987a, 1987b), whereas maintenance of grafted roots after thinning requires carbon from an initially smaller canopy (Will 1966).
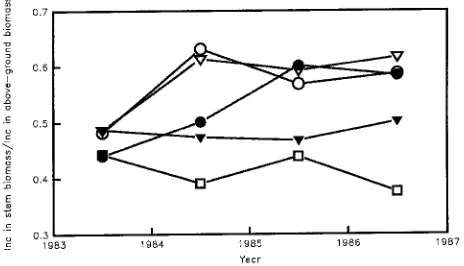
Partitioning of aboveground biomass to stem
Thinning increased partitioning of aboveground biomass to stem (Ps) at Puruki and (beginning one year after thinning) at Kaingaroa, it lowered Ps at Eyrewell, and had little effect on Ps at Woodhill (Figures 8a--d). Aboveground biomass production and Ps were negatively related. This was significant both be-fore thinning and in the second year after thinning, with r2 values of 0.36 and 0.59. There was a positive correlation between aboveground biomass production before and after thinning. There was also a positive correlation between Ps before thinning and one year or two years after thinning (r2 = 0.62 P < 0.001, r2 = 0.63 P < 0.003). Reduced competition for
resources allowed individual trees to grow more rapidly, but did not affect stand characteristics. However, a large increase in supply of soil resources can alter stand characteristics. At BFG, which is a nutrient- and water-limited site, Ps was greater with more water and nutrients (Figure 9). Simulations indicate the proportion of assimilate allocated to stem biomass in high nutrient stands at BFG was almost twice that in low nutrient stands (McMurtrie et al. 1990b). Fertilizing, especially with nitrogen, generally increases the proportion of biomass in the crown and reduces that in stems (e.g., Smith et al. 1994).
Conclusions
To improve silvicultural management we need to develop techniques for making reliable, quantitative predictions of stand responses. Of particular importance is an understanding of the mechanisms and interactions involved, including the way in which productivity responds to interactions between resource and non-resource factors, and of how FE is implicated
Figure 8. Effects of thinning and fertilizing on stem growth as a fraction of the increase in above-ground dry matter. Vertical dotted lines indicate times of thinning at Puruki and Kaingaroa. Treatments at Eyrewell were unthinned con-trol (s), unthinned fertilized (d), thinned unfertilized (,) and thinned fertilized (.). At
Wood-hill all treatments were thinned with an unfertilized control (s), lupins (d), fertilizer (,) and
fer-tilizer + lupins (.). Replotted from data of Beets and Pollock (1987), Madgwick et al. (1977), Mead et al. (1984) and Beets and Madgwick (1988), respectively.
in this. Increased FE in response to thinning appears to result from greater carbon gain per unit foliage, whereas responses of FE to fertilizing may be caused by changes in carbon partitioning and carbon gain. We need to understand factors that control both carbon gain and carbon partitioning if we are to predict stand responses reliably.
Acknowledgments
I thank Joe Landsberg and Peter Snowdon for their helpful and thoughtful comments on this paper.
References
Ågren, G.I. 1985. Theory for growth of plants derived from the nitrogen productivity concept. Physiol. Plant. 64:17--28.
Amthor, J.S. 1984. The role of maintenance respiration in plant growth. Plant, Cell Environ. 7:561--569.
Barker, P.R. 1973. Nutrition of Pinus radiata D. Don with particular
reference to fine root production and phosphorus-zinc relationships. Ph.D. Diss. Australian National Univ., Canberra, 277 p.
Beets, P.N. and R.K. Brownlie. 1987. Puruki experimental catchment: site, climate, forest management, and research. N.Z. J. For. Sci. 17:137--160.
Beets, P.N. and H.A.I. Madgwick. 1988. Above-ground dry matter and nutrient content of Pinus radiata as affected by lupin, fertiliser,
thinning, and stand age. N.Z. J. For. Sci. 18:43--64.
Beets, P.N. and D.S. Pollock. 1987. Accumulation and partitioning of dry matter in Pinus radiata as related to stand age and thinning.
N.Z. J. For. Sci. 17:246--271.
Bennett, K.J. and D.A. Rook 1978. Stomatal and mesophyll resis-tances of two clones of Pinus radiata D. Don known to differ in
transpiration and survival rate. Aust. J. Plant Physiol. 5:231--238. Black, C.H. 1988. Interaction of phosphorus fertilizer form and soil
medium on Douglas-fir seedling phosphorus content, growth and photosynthesis. Plant Soil 106:191--199.
Brown, S. 1981. A comparison of the structure, primary productivity, and transpiration of cypress ecosystems in Florida. Ecol. Mono-graphs 51:403--427.
Bunce, J.A. 1977. Nonstomatal inhibition of photosynthesis at low water potentials in intact leaves of species from a variety of habitats. Plant Physiol. 59:348--350.
Butcher, T.B. 1977. Impact of moisture relationships on the manage-ment of Pinus pinaster Ait. plantations in Western Australia. For.
Ecol. Manage. 1:97--107.
Conroy, J., E.W. Barlow and D.I. Bevege. 1986. Response of Pinus radiata seedlings to carbon dioxide enrichment at different levels of water and phosphorus: growth, morphology and anatomy. Ann. Bot. 57:165--177.
Conroy, J.P., P.J. Milham, D.I. Bevege and E.W.R. Barlow. 1990. Management of water and nutrient relations to increase forest growth. For. Ecol. Manage. 30:175--188.
Cromer, R.N., P.E. Kriedemann, P.J. Sands and L.G. Stewart. 1993. Leaf growth and photosynthetic response to nitrogen and phospho-rus in seedling trees of Gmelina arborea. Aust. J. Plant Physiol.
20:83--98.
Cropper, W.P. and H.L. Gholz. 1991. In situ needle and fine root
respiration in mature slash pine (Pinus elliottii) trees. Can. J. For. Res. 21:1589--1595.
Dey, S.K. and C.H.N. Rao. 1989. Influences of nutrient deficiency on photosynthesis and productivity in early rice varieties. Oryza 26:317--319.
Donner, B.L. and S.W. Running. 1986. Water stress response after thinning Pinus contorta stands in Montana. For. Sci. 32:614--625.
Donald, D.G.M. 1987. The application of fertiliser to pines following second thinning. S. Afr. For. J. 142:13--16.
Evans, J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9--19.
Fei, H.M., D.L. Godbold, S.S. Wang and A. Huttermann. 1990. Gas exchange in Populus maximowiczii in relation to potassium and
phosphorus nutrition. J. Plant Physiol. 135:675--679.
Field, C. and H.A. Mooney. 1983. The photosynthesis--nitrogen rela-tionship in wild plants. In Proc. Sixth Maria Moors Cabot
Sympo-sium. Ed. J. Givnish. Cambridge Univ. Press, Cambridge, pp 25--55.
Field, C. 1991. Ecological scaling of carbon gain to stress and resource availability. In Responses of Plants to Multiple Stresses. Academic
Press, San Diego, pp 35--65.
Ginn, S.E., J.R. Seiler, B.H. Cazell and R.E. Kreh. 1991. Physiological and growth responses of eight-year-old loblolly pine stands to thinning. For. Sci. 37:1030--1040.
Gower, S.T., J.G. Isebrands and D.W. Sheriff. 1995. Carbon allocation and accumulation in conifers. In Resource Physiology of Conifers
Acquisition, Allocation and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 217--254.
Grace, J.C., P.G. Jarvis and J.M. Norman. 1987. Modelling the inter-ception of solar radiant energy in intensively managed stands. N.Z. J. For. Sci. 17:193--209.
Greenwood, D.J. and A. Barnes. 1978. A theoretical model for the decline in protein content of plants during growth. J. Agric. Sci. 91:461--466.
Greenwood, D.J., A. Barnes and T.J. Cleaver. 1978. Measurement and prediction of the changes in protein contents of field crops during growth. J. Agric. Sci. 91:467--477.
Hansen, G.K. and C.R. Jensen. 1977. Growth and maintenance respi-ration in whole plants, tops and roots of Lolium multiflorum.
Physiol. Plant. 39:155--164.
Hopmans, P. and S. Clerehan. 1991. Growth and uptake of N, P, K and B by Pinus radiata D. Don in response to application of borax. Plant
Soil 131:115--127.
Hunter, I.R. and G.F. Hoy. 1983. Growth and nutrition of Pinus radiata
on a recent coastal sand as affected by nitrogen fertilizer. N.Z. J. For. Sci. 13:3--13.
Hunter, I.R., J.D. Graham, J.M. Prince and G.M. Nicholson. 1986. What site factors determine the 4-year basal area response of Pinus radiata to nitrogen fertilizer. N.Z. J. For. Sci. 16:30--40.
Ingestad, T. 1962. Macro element nutrition of pine, spruce and birch seedlings in nutrient solutions. Med. Stat. Skogsfor. 51:1--150. Jackson, D.S., E.A. Jackson and H.H. Gifford. 1983. Soil water in
deep Pinaki sands: some interactions with thinned and fertilised
Pinus radiata. N.Z. J. For. Sci. 13:183--196.
Kawahara, T., K. Hatiya, I. Takeuti and A. Sato. 1976. Relationship between respiration rate and nitrogen concentration of trees. Jpn. J. Ecol. 26:165--170.
Kirschbaum, M.U.F., and D. Tompkins. 1990. Photosynthesis and phosphorus nutrition in Eucalyptus grandis seedlings. Aust. J. Plant
Physiol. 17:527--535.
Leith, H. 1976. The use of correlation models to predict primary productivity from precipitation or evapotranspiration. In Water and
Linder, S. and D.A. Rook. 1984. Effects of mineral nutrition on carbon dioxide exchange and partitioning in trees. In Nutrition of
Planta-tion Forests. Eds. G.D. Bowen and E.K.S. Nambiar. Academic Press, London, pp 211--236.
Luxmoore, R.J., R., Oren, D.W. Sheriff and R.B. Thomas. 1995. Source-sink-storage relationships of conifers. In Resource
Physiol-ogy of Conifers Acquisition, Allocation and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 179--216. MacDougal, D.T. 1938. Tree growth. Chronica Botanica Company,
Leiden. 240 p.
MacLeod, L.B. 1969. Effects of N, P, and K and their interactions on the yield and kernel weight of barley in hydroponic culture. Agron. J. 61:26--29.
Madgwick, H.A.I. 1981. Above-ground dry-matter content of a young close-spaced Pinus radiata stand. N.Z. J. For. Sci. 11:203--209.
Madgwick, H.A.I., D.S. Jackson and P.J. Knight. 1977. Above-ground dry matter, energy, and nutrient contents of trees in an age series of
Pinus radiata plantations. N.Z. J. For. Sci. 7:445--468.
Madgwick, H.A.I. and G.R. Oliver. 1985. Dry matter content and production of close-spaced Pinus radiata. N.Z. J. For. Sci.
15:135--141
McMurtrie, R.E., M.L. Benson, S. Linder, S.W. Running, T. Talsma, W.J.B. Crane and B.J. Myers. 1990a. Water/nutrition interactions
affecting the productivity of stands of Pinus radiata. For. Ecol.
Manage. 30:415--423.
McMurtrie, R.E., D.A. Rook and F.M. Kelliher. 1990b. Modelling the
yield of Pinus radiata on a site limited by water and nitrogen. For.
Ecol. Manage. 30:381--413.
McMurtrie, R.E. and J.J. Landsberg. 1992. Using a simulation model to evaluate the effects of water and nutrients on the growth and carbon partitioning of Pinus radiata. For. Ecol. Manage.
52:243--260.
Mead, D.J., D. Draper and H.A.I. Madgwick. 1984. Dry matter pro-duction of a young stand of Pinus radiata: some effects of nitrogen
fertiliser and thinning. N.Z. J. For. Sci. 14:97--108.
Minshall, W.H. 1975. Stimulation of transpiration by nitrogenous materials. Can. J. Bot. 53:1259--1265.
Myers, B.J. 1988. Water stress integral----a link between short-term stress and long-term growth. Tree Physiol. 4:315--323.
Nambiar, E.K.S. 1980. Root configuration and root regeneration in
Pinus radiata seedlings. N.Z. J. For. Sci. 10:249--263.
Nambiar, E.K.S., R.O. Squire, R. Sands and G.M. Will. 1984. Manipu-lation of water and nutrients in plantations of fast growing species.
In IUFRO Symposium on Site and Productivity of Fast Growing
Plantations Vol 1. Eds. D.C. Grey, A.P.G. Schonau, C.J. Schulz and A. Van Laar. IUFRO, Pretoria/Pietermaritzburg, pp 489--506. Nambiar, E.K.S. 1994. Relationships between water and nutrients in
Australian forests: application to wood production and quality. Plant Soil 168--169:427--435.
Raupach, M., A.R.P. Clarke, B.F. Gibson, and K.M. Cellier. 1975. Cultivation and fertilizer effects on the growth and foliage nutrient concentrations of P. radiata, P. pinaster and P. caribaea on three
soil types at Anglesea (Victoria). CSIRO Division of Soils Tech. Paper 25. CSIRO, Melbourne, 20 p.
Reich, P.B., and A.W. Schoettle. 1988. Role of phosphorus and nitro-gen in photosynthetic and whole plant carbon gain and nutrient use efficiency in eastern white pine. Oecologia 77:25--33.
Richards, B.N. an D.I. Bevedge. 1969. Critical foliage concentrations of nitrogen and phosphorus as a guide to the nutrient status of
Araucaria underplanted to Pinus. Plant and Soil 31:328--336.
Ritchie, J.T. 1983. Efficient water use in crop production: discussion on the generality of relations between biomass production and evapotranspiration. In Limitations to Efficient Water Use in Crop
Production. Eds. H.M. Taylor, W.R. Jordan and T.R. Sinclair. American Soc. of Agronomy, Wisconsin, pp 29--44.
Rook, D.A. and A.G.D. Whyte. 1976. Partial defoliation and growth of 5-year-old radiata pine. N.Z. J. For. Sci. 6:40--56.
Rook, D.A., R.H. Swanson and A.M. Cranswick. 1977. Reaction of radiata pine to drought. In Proc. Soil and Plant Water Symposium. DSIR, Wellington, pp 55--68.
Rousseau, J.V.D. and C.P.P. Reid. 1990. Effects of phosphorus and ectomycorrhizas on the carbon balance of loblolly pine seedlings. For. Sci. 36:101--112.
Santantonio, D. 1989. Dry-matter partitioning and fine-root produc-tion in forests----new approaches to a difficult problem. In Biomass
Production by Fast-Growing Trees. Eds. J.S. Pereira and J.J. Landsberg. Kluwer Academic Publishers, London, pp 57--72. Santantonio, D. and E. Santantonio. 1987a. Seasonal changes in live
and dead fine roots during two successive years in a thinned planta-tion of Pinus radiata in New Zealand. N.Z. J. For. Sci. 17:315--328.
Santantonio, D. and Santantonio, E. 1987b. Effect of thinning on production and mortality of fine root in a Pinus radiata plantation
on a fertile site in New Zealand. Can. J. For. Res. 17:919--928. Sheriff, D.W. 1995. Gas exchange of field-grown Pinus radiata
rela-tionships with foliar nutrition and water potential, and with climatic variables Aust. J. Plant Physiol. 22:1015--1026.
Sheriff, D.W. and J.P. Mattay. 1995. Simultaneous effects of foliar nitrogen, temperature, and humidity on gas exchange of Pinus radiata. Aust. J. Plant Physiol. 22:615--626.
Sheriff, D.W. and D. Rook. 1990. Wood density and above-ground growth in high and low wood density clones of Pinus radiata D. Don. Aust. J. Plant Physiol. 17:615--628.
Sheriff, D.W. and D. Whitehead. 1984. Photosynthesis and wood structure in Pinus radiata D. Don during dehydration and
immedi-ately after rewatering. Plant, Cell Environ. 7:53--62.
Sheriff, D.W., E.K.S. Nambiar and D.N. Fife. 1986. Relationships between nutrient status, carbon assimilation and water use effi-ciency in Pinus radiata (D. Don) needles. Tree Physiol. 2:73--88.
Smith, C.T., W.J. Dyck, P.N. Beets, P.D. Hodgkiss and A.T. Lowe. 1994. Nutrition and productivity of Pinus radiata following harvest
disturbance and fertilization of coastal sand dunes. For. Ecol. Man-age. 66:5--38.
Snowdon, P. and M.L. Benson. 1992. Effects of combinations of irrigation and fertilisation on the growth and above-ground biomass production of Pinus radiata. For. Ecol. Manage. 52:87--116.
Snowdon, P. and H.D. Waring. 1985. Responses of some genotypes of
Pinus radiata to clover and fertilization. Aust. For. Res.
15:125--134.
Snowdon, P. and H.D. Waring. 1990. Growth responses by Pinus radiata to combinations of superphosphate, urea and thinning type.
For. Ecol. Manage. 30:313--325.
Snowdon, P. and H.D. Waring. 1991. Effects of irrigation and artificial drought on the growth and health of Pinus radiata near Canberra, A.C.T. Aust. For. 54:124--186.
Sprugel, D.G., M.G. Ryan, J.R. Brooks, K.A. Vogt, and T.A. Martin. 1995. Respiration from the organ level to the stand. In Resource
Physiology of Conifers Acquisition, Allocation and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 255--299.
Squire, R.O., G.C. Marks and F.G. Craig. 1978. Root development in a Pinus radiata D. Don plantation in relation to site index,
Squire, R.O., P.M. Attiwill and T.F. Neales. 1987. Effects of changes of available water and nutrients on growth, root development and water use in Pinus radiata seedlings. Aust. For. Res. 17:99--111.
Stenberg, P., E.H. DeLucia, A.W. Schoettle and H. Smolander. 1995. Photosynthetic light capture and processing from cell to canopy. In
Resource Physiology of Conifers. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 3--38.
Sweet, G.B. and P.F. Wareing. 1966. Role of plant growth in regulating photosynthesis. Nature 210:77--79.
Terry, N. and I.M. Rao. 1991. Nutrients and photosynthesis: iron and phosphorus as case studies. In Plant growth: interactions with
nutrition and environment. Eds. J.R. Porter and D.W. Lawlor. Cam-bridge University Press, CamCam-bridge, pp 55--74.
Teskey, R.O., H.L. Gholz and W.P. Cropper, Jr. 1994. Influence of climate and fertilization on net photosynthesis of mature slash pine. Tree Physiol. 14:1215--1227.
Teskey, R.O., D.W. Sheriff, D.Y. Hollinger and I.M. Thomas. 1995. External and internal factors regulating photosynthesis. In Resource
Physiology of Conifers: Acquisition, Allocation and Utilization. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 105--140.
Theodorou, C. and G.D. Bowen. 1993. Root morphology, growth and uptake of phosphorus and nitrogen of Pinus radiata families in
different soils. For. Ecol. Manage. 56:43--56.
Thomas, R.C. and D.J. Mead. 1992. Uptake of nitrogen by Pinus radiata and retention within the soil after applying 15N-labelled
urea at different frequencies. 1. Growth response and nitrogen budgets. For. Ecol. Manage. 53:131--151.
Thompson, W.A. and A.M. Wheeler. 1992. Photosynthesis by mature needles of field-grown Pinus radiata. For. Ecol. Manage.
52:225--242.
Turner, J. and M.J. Lambert. 1987. Forest water usage and interactions with nutrition of Pinus radiata. Oecol. Plant. 8:37--43.
West, P.W. and G.H.R. Osler. 1995. Growth response to thinning and its relation to site resources in Eucalyptus regnans. Can. J. For. Res.
25:69--80.
Whitehead, D. 1986. Dry matter production and transpiration by Pinus radiata stands in relation to canopy architecture. In Crown and
Canopy Structure in Relation to Productivity. Eds. T. Fujimori and D. Whitehead. Forestry and Forest Products Research Institute, Ibaraki, Japan, pp 243--262.
Whitehead, D., D.W. Sheriff and D.H. Greer. 1983. The relationship between stomatal conductance, transpiration rate and tracheid structure in Pinus radiata clones grown at different water vapour
saturation deficits. Plant, Cell Environ. 6:703--710.
Whyte, A.G.D. and R.C. Woollons. 1990. Modelling stand growth of radiata pine thinned to varying densities. Can. J. For. Res. 20:1069--1076.
Will, G.M. 1966. Root growth and dry-matter production in a high-producing stand of Pinus radiata. Research Note No. 44. N.Z.
Forest Research Institute, Rotorua. 15 p.
Woods, P.V., E.K.S. Nambiar and P.J. Smethurst. 1992. Effect of annual weeds on water and nitrogen availability to Pinus radiata
trees in a young plantation. For. Ecol. Manag. 48:145--163. Woollons, R.C. and A.G.D. Whyte. 1989. Analysis of growth and yield