L
Journal of Experimental Marine Biology and Ecology 247 (2000) 51–65
www.elsevier.nl / locate / jembe
Comparison of the metabolism of Acartia clausi and
A
. tonsa: influence of temperature and salinity
*
Raymond Gaudy , Guillermo Cervetto, Marc Pagano
´
Centre d’Oceanologie de Marseille, Station Marine d’Endoume, rue de la Batterie des Lions, 13007 Marseille, France
Received 30 September 1999; received in revised form 16 December 1999; accepted 20 December 1999
Abstract
In the Marseilles region (French Mediterranean coast), A. clausi is one of the most abundant copepod species of the Gulf of Fos while A. tonsa constitutes the almost exclusive copepod species of the Berre lagoon, a neighbouring semi-closed brackish area communicating with the gulf. As different ecophysiological capabilities to stand the various temperature, salinity and food conditions could explain why these two species do not coexist in the same environment, comparative experiments were performed on metabolism and feeding. The respiration and ammonia excretion of the two species were measured in different combinations of temperature (10, 15 and 208C) and salinity (15, 25 and 35‰). For each temperature, at the salinity of 35‰, respiration rates were less in A. clausi than in A. tonsa, the contrary being observed at the lowest salinity. At any temperature ammonia excretion was greater at the intermediate salinity in A. tonsa and least in A. clausi. In Acartia tonsa, Q10 of respiration and excretion were minimum at the lowest salinity, while in A. clausi they were unaffected by salinity variation. The O:N atomic ratio (from respiration and ammonia excretion rates) was significantly more elevated in A. clausi (mean 21.2; range 13.6–28.7) than in A. tonsa (mean 11.3; range 4.2–25) suggesting a more proteinic oriented metabolism in the later. Feeding experiments where Dunaliella tertiolecta was used as food showed that both species obtained the same specific daily food ration at marine (.30‰) or lagoon (,16‰) salinity. The relationships between ingestion and food concentration in the two species were not significantly different. These different results are compared to other ecophysiological information concerning these Acartia species (survival tolerances, osmotic regulation, feeding behaviour) and are discussed in relation with the characteristics of their niches in the studied region. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Acartia; Respiration; Excretion; Ingestion
*Corresponding author. Tel.: 133-49-104-1644; fax: 133-49-104-1635.
E-mail address: [email protected] (R. Gaudy)
1. Introduction
The pelagic copepods Acartia clausi and A. tonsa characterize many coastal or estuarine environments where they can reach high population densities. In Atlantic temperate regions, they sometimes co-exist at the same time (Lee and McAlice, 1979) but, most frequently, A. tonsa dominates during summer and A. clausi during winter (Deevey, 1948; Conover, 1956; Heinle, 1966; Jeffries, 1967), which contributes to reduce food competition.
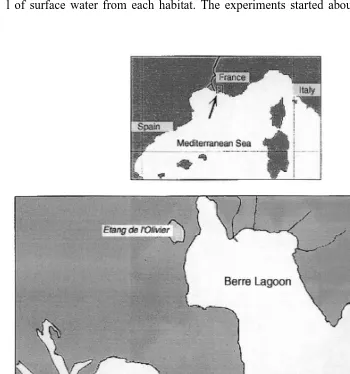
In the Marseilles region (French Mediterranean coast), the zooplankton of the Gulf of Fos is dominated by A. clausi (Blanc et al., 1975; Benon et al., 1976), while in the adjacent eutrophic brackish lagoon, the Etang de Berre, it is mainly composed of Acartia
tonsa (Gaudy, 1989). These two areas are connected by a channel where currents allow
the transportation of the pelagic fauna in both directions (Gaudy, 1986). The local populations of A. clausi and A. tonsa are challenged by highly variable conditions of temperature, salinity or food conditions. During a previous study on the distribution of these two Acartia species we observed that each Acartia population remained largely dominant in its respective habitat during most of the year, but did not maintain themselves in the adjacent pelagic ecosystem despite water transfer through the channel (Cervetto, 1995). Among the possible factors which could explain this separation, the strong salinity gradient existing between the Gulf of Fos (average salinity 35‰) and the Etang de Berre (average salinity 15‰) was considered. Experimental studies on the immediate and short term mortality of the two species in relation to salinity variations (Cervetto et al., 1995, 1999) demonstrated that adult and copepodites of both species displayed a large degree of tolerance to rapid salinity change. Tester and Turner (1991) demonstrated that nauplii of A. tonsa had low tolerance to salinity particularly above 25‰. As the long term maintenance of animal populations depends on the success of their reproduction, it is advantageous that the part of energy devoted to ovogenesis would be as large as possible. Considering the energy balance equation, as adult copepods do not moult, energy available for reproduction is directly related to food acquisition and inversely to metabolic expenditures (Kiørboe et al., 1985). Thus it is important to know to what extent metabolism is affected by differences in the temperature and salinity conditions of marine and brackish habitats. According to
¨
Paffenhofer and Stearns (1988), the restriction of Acartia tonsa to nearshore or estuarine waters would be due to the impossibility to complete its food ration at the lower food concentration met in the open sea. In the literature, many works concern the ecophysiol-ogy of A. clausi or A. tonsa, but few are devoted to a direct comparison of the physiology of the two species in view of explaining their difference of distribution (Conover, 1956; Anraku, 1964) and no data is available for the more salty and less productive waters of the Mediterranean region.
The aim of this paper is to analyze the variations of respiration and excretion rates in
2. Methods
Samples were collected on several occasions in October 1994 from two stations located in the Berre lagoon and in the Eastern part of the Gulf of Fos, respectively, in front of each outlet of the channel connecting these two areas (Fig. 1). Zooplankton was sampled by several oblique hauls with a WP2 net (UNESCO, 1968), screened through a coarse mesh silk (350mm) then transported to the laboratory in cold boxes filled with 30 l of surface water from each habitat. The experiments started about 6–8 h later.
2.1. Respiration and ammonia excretion
Six successive experiments were carried out. In each of them, several sets of equal numbers of adult females were prepared. This number varied from 10 to 50 in Acartia
clausi and 100 to 200 in Acartia tonsa according to the abundance of the species in the
samples. Animals were incubated for 24 h in 125-ml flasks filled with 0.45-mm filtered water adjusted at three different salinities (15, 25 and 35‰). The flasks were maintained in the dark, at three different temperatures (10, 15 and 208C). For each temperature– salinity combination, test flasks (three with Acartia clausi, three with A. tonsa) and one control flasks (without copepods) were prepared. As non significant difference appeared between the results of the six successive experiments (ANOVA test, P,0.05) for a given temperature–salinity combination, the data were pooled for statistical treatment, giving a maximum of 18 data points (range 12–18) per species, for each of the nine temperature–salinity combinations. At the end of the incubation period, the water oxygen concentration of each flask was measured with an YSI model 57 oxymeter compensated for salinity variation. Samples of 25 ml were withdrawn from the different flasks for ammonia concentration measurement according to the Koroleff (1969) colorimetric method. Respiration and excretion rates were calculated from differences in oxygen and ammonia concentration between controls and test flasks, taking into account the number of individuals in each flask and the incubation time. They were expressed as specific rates, using an average dry weight of 10mg for A. clausi and 5 mg for A. tonsa (Cervetto, 1995).
2.2. Feeding experiments
In each experiment, 40–70 Acartia clausi females (from the Gulf of Fos) and 50–200
Acartia tonsa females (from the Berre lagoon) were incubated in 300-ml flasks for 24 h.
Under each experimental condition, three to four test flasks (with copepods) and two to three control flasks (without copepods) were prepared and placed on a vertically rotating wheel (1 rpm), to avoid particles settlement. The experiments were run in the dark, at 188C. The food medium consisted in a non-axenic culture of Dunaliella tertiolecta. The characteristics of the incubation water differed according to the type of experiment as explained below.
Five experiments were completed to study the effect of salinity. The water from each sampling station was filtered on Millipore 0.45 mm and enriched with a part of the
Dunaliella culture adjusted to obtain a similar food concentration in the successive
experiments (range 0.9–1.14 ppm for experiments 1–4, except in the last experiment where the concentration were lower (0.41–0.43 ppm). The salinity ranged from 13 to 15‰ for the Berre brackish station and 30 to 40‰ for the Fos marine station, according to the dates of sampling. In the first experiment, Acartia tonsa, the only species present at that time, was incubated in low and high salinity water. In the four following experiments, crossed incubations were prepared with A. clausi and A. tonsa, each species being incubated in low salinity or high salinity water.
16–20‰ salinity (an intermediate value between sea and lagoon conditions) to obtain four different concentrations of food medium.
A Coulter Counter-type multisizer (256 channels) equipped with a 70-mm aperture tube was used to determine particles concentrations. Daily ingestion rates were calculated from the difference in particles concentration (in volume units) between control and experimental flasks, taking into account the incubation time and the biomass of incubated copepods. In the successive experiments, as less than 50% of phytoplankton was cleared (24–44%), no food limitation occurred in the incubation flasks.
3. Results
3.1. Respiration
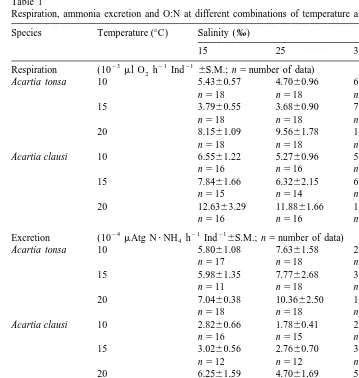
Respiration rates were maximum at the highest temperature tested (208C) but the effect of temperature varied according to species and salinities (Table 1). In Acartia
clausi, respiration increased regularly with temperature. In A. tonsa this was observed only at the salinity of 35‰, while at 15 and 25‰, respiration was least at the intermediate temperature. Q10 values derived from 10 and 208C rates were similar in both species at each salinity condition (Table 2). The salinity influence at a given temperature was marked only in A. tonsa, in which the respiration rate obtained at the highest salinity largely exceeded those obtained at 15 and 25‰. In A. clausi, for each temperature condition, respiration rates were slightly higher at the lowest salinity and lower at the upper salinity, a tendency which is opposite to the results obtained in A.
tonsa.
The ANOVA performed on total data showed that temperature and salinity had significant effects on respiration rate in A. tonsa only (Table 3).
3.2. Excretion
In both species, ammonia excretion rates showed a small range of variation between 10 and 158C but increased markedly at the highest temperature tested (Table 1).
The salinity variation had no marked effect on the ammonia excretion rates in A.
clausi, at any temperature. In A. tonsa, the excretion rates were relatively homogeneous at low and medium salinity for a given temperature but decreased (10 and 158C) or increased (208C) at 35‰.
The excretory Q10 of A. tonsa was more than three times higher at 35‰ than at the other salinities (Table 2). This contrasted with the relative stability of Q10 values observed at the different salinities in A. clausi.
The ANOVA performed on total data shows that, in both species, temperature had a significant effect on ammonia excretion, contrary to salinity (Table 3).
3.3. O:N ratio
Table 1
Respiration, ammonia excretion and O:N at different combinations of temperature and salinity Species Temperature (8C) Salinity (‰)
15 25 35
23 21 21
Respiration (10 ml O h2 Ind 6S.M.; n5number of data)
Acartia tonsa 10 5.4360.57 4.7060.96 6.3861.56
n518 n518 n518
15 3.7960.55 3.6860.90 7.5761.80
n518 n518 n518
20 8.1561.09 9.5661.78 14.1862.63
n518 n518 n518
Acartia clausi 10 6.5561.22 5.2760.96 5.8161.78
n516 n516 n514
15 7.8461.66 6.3262.15 6.3162.20
n515 n514 n514
20 12.6363.29 11.8861.66 11.3162.07
n516 n516 n515
24 21 21
Excretion (10 mAtg N?NH h4 Ind 6S.M.; n5number of data)
Acartia tonsa 10 5.8061.08 7.6361.58 2.2760.72
n517 n518 n513
15 5.9861.35 7.7762.68 3.0160.80
n511 n518 n516
20 7.0460.38 10.3662.50 10.8862.41
n518 n518 n515
Acartia clausi 10 2.8260.66 1.7860.41 2.4860.62
n516 n515 n513
15 3.0260.56 2.7660.70 3.4360.64
n512 n512 n517
20 6.2561.59 4.7061.69 5.6561.74
n515 n516 n514 O:N (atomic ratio, from respiration and ammonia excretion)
Acartia tonsa 10 8.36 5.5 25.09
15 5.66 4.23 22.45
20 10.34 8.24 11.64
Acartia clausi 10 20.74 26.43 20.92
15 28.69 20.45 24.28
20 18.04 13.58 17.87
Table 2
Values of Q10(calculated between 10 and 208C) of respiration and excretion at different salinities
Function Species Salinity (‰)
15 25 35
Respiration Acartia tonsa 1.5 2.03 2.22
Acartia clausi 1.93 2.25 1.94
Excretion Acartia tonsa 1.21 1.36 4.79
Table 3
Results of ANOVA on temperature and salinity effects on respiration and excretion rates
Species Source of Sum of df Mean F P value
variation squares square
Respiration
a 26
Acartia tonsa Temperature 0.0245 2 0.0122 15.09 1.04 10
Salinity 0.0076 2 0.0038 4.68 0.011
Interaction 0.0017 4 0.0004 0.53 0.713
Within 0.1244 153 0.0008
Total 0.1583 161
Acartia clausi Temperature 0.0402 2 0.0201 2.32 0.101
Salinity 0.0054 2 0.0027 0.31 0.731
Interaction 0.0667 4 0.0166 1.92 0.108
Within 1.3254 153 0.0086
Total 1.4379 161
Excretion
26 27
Acartia tonsa Temperature 1.65 10 2 8.29 10 6.08 0.003
27 27
Salinity 6.68 10 2 3.34 10 2.45 0.089
27 27
Interaction 8.66 10 4 2.17 10 1.58 0.179
25 27
Within 2.09 10 153 1.36 10
25
Total 2.41 10 161
26 27 25
Acartia clausi Temperature 3.63 10 2 1.82 10 12.59 1.22 10
27 27
Salinity 2.26 10 2 1.13 10 0.78 0.46
27 27
Interaction 3.19 10 4 7.97 10 0.55 0.69
25 27
Within 1.65 10 108 1.44 10
25
Total 1.98 10 116
a
Significant values of F are in bold.
ammonia excretion at each temperature–salinity combination. The values ranged from 13.6 to 28.69 in Acartia clausi and from 4.2 to 25.09 in A. tonsa (Table 1). Except the high values recorded at 35‰ (for 10 and 158C), O:N ratios were considerably lower in
A. tonsa than in A. clausi. Considering total data, the difference between species was highly significant (F532.6, P,0.00002).
3.4. Food ingestion
The specific ingestion rates calculated in the salinity experiments are reported on Table 4. The results of experiments 2–5 (in which both species were present) were pooled in two groups of data corresponding to low salinity (,16‰) and high salinity (.30‰) conditions. ANOVA performed on these grouped data indicated that specific daily ingestion did not differ significantly according to species or salinity.
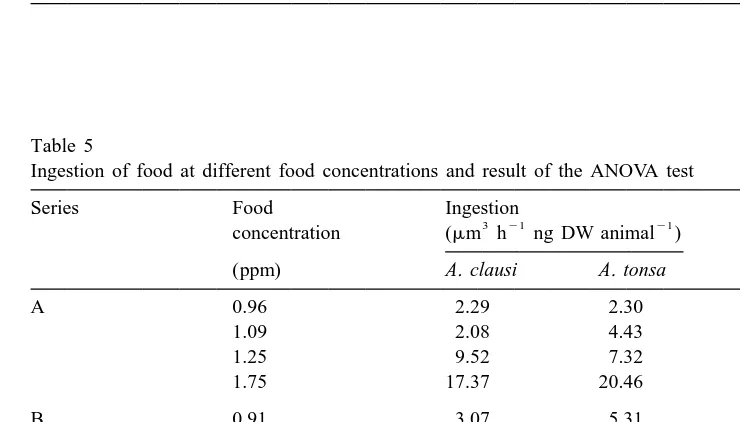
In the two food density experiments, ingestion increased with food concentration in both species, but the ANOVA showed that the effect of concentration upon ingestion was significant only for series A (Table 5).
Table 4
Effect of the salinity on food ingestion and result of the ANOVA
Exp. no. Salinity Concentration Ingestion
3 21 21
range (ppm) (mm h ng DW animal )
A. clausi A. tonsa
1 ,16‰ 1.05 nd 6.18
2 1.14 6.81 6.29
3 1.01 3.29 3.63
4 1.03 9.07 2.10
5 0.43 4.80 3.37
1 .30‰ 1.21 nd 10.80
2 1.02 6.97 7.05
3 1.03 4.10 4.71
4 0.89 6.66 6.54
5 0.41 1.48 2.39
ANOVA
Source of variation F P value
Salinity 0.01 0.95
Species 0.62 0.45
Interaction 1.24 0.29
Table 5
Ingestion of food at different food concentrations and result of the ANOVA test
Series Food Ingestion
3 21 21
concentration (mm h ng DW animal )
(ppm) A. clausi A. tonsa
A 0.96 2.29 2.30
1.09 2.08 4.43
1.25 9.52 7.32
1.75 17.37 20.46
B 0.91 3.07 5.31
1.23 4.85 5.24
1.42 7.02 10.07
1.50 5.90 11.70
Source of variation F P value
ANOVA Series A Concentration 10.41 0.04*
Species 4.44 0.12 ns
ANOVA Series B
Concentration 4.43 0.13 ns
Species 6.51 0.08 ns
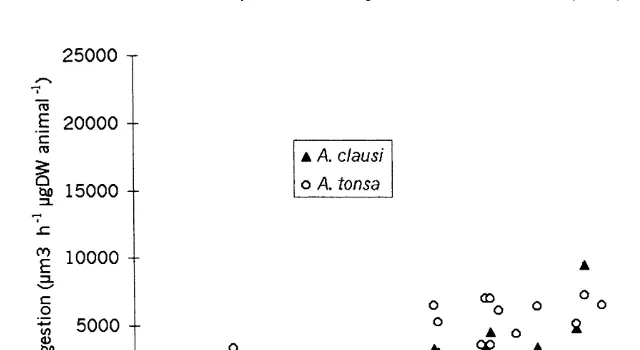
Fig. 2. Relationship between the food ingestion (in volume units) and the particles concentration in Acartia
clausi and Acartia tonsa.
to saturation of ingestion in the limits of our experimental concentrations (Fig. 2). The curves were linearized using a log 10 transformation of the ingestion values. The correlation coefficients (r) between log10 ingestion and concentration were 0.72 in A.
3 21
clausi and 0.80 in A. tonsa. Regression equations between ingestion I (log10mm h ng 21
DW animal ) and food concentration C (ppm) were I50.57 log10C14.07 in A.
clausi and I50.59 log10C13.78 in A tonsa. The slopes and the intercepts of the two regressions were not significantly different at P,0.05 (ANCOVA test).
4. Discussion
4.1. Effect of temperature
Low Q10 values which characterize eurythermic species (Rao and Bullock, 1954; Precht, 1958) are advantageous for populations submitted to rapid temperature fluctua-tions as those occurring in shallow coastal environments. They correspond to homeo-static mechanism to maintain the physiological activity at low temperatures and to save energy at high temperatures (Hiromi et al., 1988). In estuarine species such as the copepod Eurytemora, metabolism is also characterized by low Q10 values (Gyllenberg and Lundqvist, 1979; Roddie et al., 1984; Pagano and Gaudy, 1986). The extreme case of regulation is found in the littoral copepod Oithona davisae which has a respiratory Q10 close to 1 (independence from temperature variations) within a large temperature range (5–308C), Hiromi et al. (1988).
thermal sensitivity of both species in the studied region, near Marseilles could explain their continuous distribution year around (Cervetto, 1995), at temperatures ranging between 8 and 268C. Along the Atlantic North American or North European coasts, A.
tonsa was restricted to the hot season while A. clausi was present throughout the year in a large range of temperature conditions (Deevey, 1948; Conover, 1956; Heinle, 1966; Lee and McAlice, 1979; Brylinski, 1981). Contrary to the similarity of the respiratory response of the two species in front of thermal variations, marked interspecific differences were found in their excretory Q10 values which were higher at low and medium salinities than at 35‰ in A. clausi, the contrary being observed in A. tonsa. In addition the range of excretory Q10 obtained at the different salinities tested was more extended in Acartia tonsa (1.21–4.79) than in A. clausi (2.21–3.64) showing that the relationship between excretion rate and temperature was more dependent on salinity conditions in the first species. This suggests that any transportation of A. tonsa outside its habitat would result in much increased metabolism expenses related to short term temperature variation.
4.2. Effect of salinity
Highest respiration rates were found at low salinity in the marine species A. clausi and at high salinity in the estuarine species A. tonsa. Kinne (1964) showed that the increase of respiration when salinity conditions diverged from the normal habitat of species was related to the need of supplementary energy for osmoregulation. This type of metabolic responses have been observed in many estuarine organisms such as the crab
Eriocheir or the fish Fundulus heteroclitus (Vernberg and Vernberg, 1972) or the
copepods Eurytemora (Gyllenberg and Lundqvist, 1979; Pagano and Gaudy, 1986). In
Acartia tonsa, Lance (1965) also demonstrated that respiratory rates increased by a
(35‰). The excretion observed at the intermediate salinity is the highest in the local population of A. tonsa and the lowest in Farmer and Reeve’s experiments. This could be the consequence of the choice of our lowest experimental salinity (15‰), which represents an average value for the Berre lagoon but not the minimum salinity which can seasonally approach values close to 5‰. If we consider that the average salinity of 15‰ probably represents the best adaptation value for the local population of A. tonsa, the increase of ammonia excretion at the salinity of 25‰ would correspond to the cost of osmotic regulations, in accordance with Farmer and Reeve (1978) explanation. The decrease observed at the highest salinity tested (a value rarely reached in the lagoon) could be a stress effect suggesting that osmotic regulation under marine salinity conditions could be difficult to maintain for the local population of A. tonsa.
Despite the large salinity differences existing between the habitats of the two species, their food ingestion was similar at low or high salinities. This result is expected for A.
tonsa, an estuarine species for which its euryhalinity is well documented (Lance, 1963,
1964; Jeffries, 1967) but seems surprising for A. clausi which is generally found in coastal marine waters. Nevertheless A. clausi may also inhabit areas characterized by a very large salinity range such as Ebrie lagoon: 0–30‰ or Amvarakicos Gulf: 7–36‰ (Gaudy et al., 1989). The salinity conditions of the northern part of the Gulf of Fos, which is frequently influenced by low salinity water coming from the neighbouring Rhone river mouth and / or from the Etang de Berre itself (Blanc et al., 1975; Benon et al., 1976), could have favoured the formation of a local physiological race of A. clausi well adapted to the occurrence of low salinity water. This is supported by Cervetto et al. (1995) experiments which demonstrated that A. clausi originated from the Gulf of Fos could survive to a very large extent of salinity (from 1 up to 65‰) and that its optimal adaptation (survival) range was between 24 and 30‰.
4.3. Effect of food concentration
Many previous experimental studies showed that ingestion and food concentration are directly related, a process classically observed in pelagic copepods (Frost, 1972, Lam and Frost, 1976) in experiments using natural food concentrations. This was the case in our experiments where the concentration range corresponded to a mean level for the Etang de Berre and the Gulf of Fos sampling stations during the time course of this study (Cervetto, 1995). In our experimental conditions (maximum particle density of 1.85 ppm), Acartia clausi and A. tonsa presented the same response to food con-centration increase. Under natural food conditions of the Berre Lagoon, A. tonsa ingestion curve does not display any tendency to saturation until at very high particle concentrations (20–40 ppm) (Gaudy, 1989; Gaudy et al., 1996). In A. clausi, the curve of food ingestion shows a tendency to saturation only at food concentrations exceeding greatly the natural range (Gaudy, 1974). As the efficiency of both species to ingest food at different natural concentrations was comparable, food abundance does not appear as a decisive factor for the distribution of each of the Acartia species in the considered area.
experimental conditions. This hypothesis was contradicted by Tester and Turner (1991) who showed that in several regions inhabited by A. tonsa along the American coasts the
¨ concentration of food was relatively low, under the minimum values used in Paffenhofer and Stearns experiments.
4.4. Food quality and O:N ratio
The highest ammonia excretion rate of Acartia tonsa results in O:N ratio values considerably lower than in A. clausi. Low O:N values (catabolism of proteins) characterize carnivorous oriented feeders while high O:N values (use of carbohydrates and lipids) would correspond to plant utilization (Conover and Corner, 1968; Le Borgne, 1986). The ability of A. tonsa to feed upon animal material is well documented (Conover, 1956; Anraku and Omori, 1963; Anraku, 1964; Lonsdale et al., 1979; Gifford and Dagg, 1988; White and Roman, 1992). Large sized protozoa such as ciliates are often preferred to plant food (Stoecker and Egloff, 1978; Robertson, 1983; Sheldon et al., 1986; Wiadnyana and Rassoulzadegan, 1989; Jonnsson and Tisellius, 1990). At the sampling stations, the average C:N ratio of the particles was 12.9 in the Gulf of Fos and 8.6 in the Berre lagoon, a difference due to the higher proportion of nitrogen in the lagoon seston. Microzooplankton was found to constitute an appreciate proportion of the available food in the Berre lagoon (Gaudy et al., 1996). Thus, the local A. tonsa population was probably used to feed upon this type of animal food. Variations in O:N ratio with temperature and salinity integrate the differential aptitude of each species for osmoregulation by ammonia mobilization (see before). In Acartia clausi, the range of O:N values obtained under the different temperature and salinity combinations is relatively narrow (22.7–39.9) compared to values recorded in A. tonsa (3.6–29.2). This could indicate that, in the considered area, A. clausi metabolism regulation is less sensitive to temperature and salinity variations than in A. tonsa.
4.5. Incidence of temperature, salinity and food conditions upon species distribution
The tolerance of the two species to salinity variations is also very broad but A. tonsa shows more differences in excretion rates obtained at different salinity than A. clausi, probably because it needs to mobilize more ammonia for osmotic regulation. Previous survival experiments on adults and copepodites in strong salinity gradients simulating the conditions sustained by the fauna transiting between the lagoon and the sea showed that both species easily tolerated salinity decreases but that A. tonsa was more sensitive to salinity increase than A. clausi (Cervetto, 1995; Cervetto et al., 1995, 1999). Then comparatively high salinities in the Gulf of Fos could be a barrier for the extension of
Acartia tonsa in coastal marine water. Tester and Turner (1991) showed the importance
of salinity factor for the survival of A. tonsa nauplii, which are more sensitive than adults.
¨
According to Paffenhofer and Stearns (1988), the low concentrations of appropriate food in sea water comparatively to estuaries could explain the failure of A. tonsa to develop in coastal marine water, because its inability to filter sufficient food at concentrations ,0.25 ppm. Reeve and Walter (1977) showed that its capacity to graze
21
can be reduced to zero in concentrations less than 1mg Chl a l . As the mean particle and Chl a concentrations in the Gulf of Fos were largely higher (Cervetto, 1995), food would not be an exclusion factor for A. tonsa entering the gulf. Our data also indicate that no difference was noted in the relationship between ingestion and food con-centration of the two species, suggesting an equal ability to ingest food at any concentration.
Considering the qualitative aspect of food, the strong ammonia excretion of A. tonsa (low O:N ratio) indicates that its feeding regime was more oriented toward proteinic food than in A. clausi. Compared to lagoonal water, the lower abundance of prey of convenient size in sea water such as ciliates could be unfavourable for A. tonsa maintenance in the Gulf of Fos. On the contrary, the elevated O:N ratio observed in A.
clausi suggests a good aptitude to use plant food. Considering its very high chlorophyll
concentration (Gaudy, 1989), the Berre lagoon could potentially constitute a favourable environment for this species.
Therefore, the absence of A. clausi population in the lagoon could result from other different processes such as predation by the dense resident A. tonsa population or, according to Lutz et al. (1992), a failure of hatching of their eggs due to the anoxic conditions such as those prevailing in the bottom layer of the southern part of the lagoon, where the marine fauna is introduced.
The failure of A. tonsa to develop in shelf water would probably be due to the joint effect of different processes. Among them were identified the qualitative nature of food (less proteinic material in marine seston), and the salinity factor, as suggested by the higher sensitivity of metabolism to temperature variations in marine salinity and by the noxious effect of increasing salinity upon the survivorship of populations entering the shelf water from the lagoon. [RW]
References
Anraku, M., Omori, M., 1963. Preliminary survey of the relationship between the feeding habit and the structure of the mouth-parts of marine copepods. Limnol. Oceanogr. 8, 116–126.
Benon, P., Blanc, F., Bourgade, B., Charpy, L., Kantin, R., Kerambrun, P., Leveau, M., Romano, J.C., Sautriot, D., 1976. Golfe de Fos. Impact de la pollution. Bull. Obs. Mer, Fondation Ricard 3 (suppl. 1), 1–13. Blanc, F., Leveau, M., Kerambrun, P., 1975. Eutrophie et pollution: structure et fonctionnement du
sous-´ `
ecosysteme planctonique. In: Proc. 10th Eur. Marine Biology Symp., Ostende, Vol. 2, pp. 61–83. Brylinski, J.M., 1981. Report on the presence of Acartia tonsa Dana (Copepoda) in the harbour of Dunkirk
(France) and its geographical distribution in Europe. J. Plankton Res. 3, 255–260.
´ ´ `
Cervetto, G., 1995. Comparaison de la repartition spatio-temporelle et de l’ecophysiologie de deux especes de
´ ´ ´ ˆ
copepodes calanoides congeneriques (Acartia tonsa et Acartia clausi ) en milieu cotier et lagunaire (Golfe
` ´
de Fos, Etang de Berre). These Doctoral, Universite Aix-Marseille II, 225 pp.
´ ´
Cervetto, G., Pagano, M., Gaudy, R., 1995. Adaptation aux variations de la salinite chez le copepode Acartia
clausi. J. Rech. Oceanogr. 20, 42–49.
Cervetto, G., Gaudy, R., Pagano, M., 1999. Influence of salinity on distribution of Acartia tonsa (copepoda calanoida). J. Exp. Mar. Biol. Ecol. 235, 33–45.
Conover, R.J., 1956. Oceanography of Long Island Sound, 1952–1954. VI. Biology of Acartia clausi and A.
tonsa. Bull. Bingham Oceanogr. Coll. 15, 156–233.
Conover, R.J., Corner, E.D.S., 1968. Respiration and nitrogen excretion by some marine zooplankton in relation to their life cycles. J. Mar. Biol. Assoc. UK 48, 49–75.
Deevey, B.G., 1948. The zooplankton of Tisbury Great Pond. Bull. Bingham Oceanogr. Coll. 15, 156–233. Farmer, L., Reeve, M.R., 1978. Role of the amino acid pool of the copepod Acartia tonsa in adjustment to
salinity change. Mar. Biol. 48, 311–316.
Frost, B.W., 1972. Effects of size and concentration on the feeding behavior of the marine planktonic copepod
Calanus pacificus. Limnol. Oceanogr. 17, 807–815.
Gaudy, R., 1974. Feeding four species of pelagic copepods under experimental conditions. Mar. Biol. 25, 125–141.
´ ˆ
Gaudy, R., 1986. Le chenal de Caronte, voie de transit pour les faunes pelagiques marines et saumatres. Rapp. ´
Comm. Int. Explor. Sci. Mer Medit. 30, 197–198.
Gaudy, R., 1989. The role of zooplankton in the nitrogen cycle of a Mediterranean brackish lagoon. Scientia Marina 53, 609–618.
Gaudy, R., Moraitou Apostolopoulou, M., Pagano, M., Saint Jean, L., Verriopoulos, G., 1989. Salinity as a decisive factor in the length of cephalothorax of Acartia clausi from three different areas (Greece and Ivory Coast). Rapp. Proc. Verb. Comm. Int. Explor. Sci. Mer Medit. 31 (2), 233.
Gaudy, R., Pagano, M., Cervetto, G., Saint-Jean, L., Verriopoulos, G., Beker, B., 1996. Short term variations in feeding and metabolism of Acartia tonsa (pelagic copepod) in the Berre lagoon (France). Oceanol. Acta 19, 635–644.
Gifford, D.J., Dagg, M.J., 1988. Feeding of the estuarine copepod Acartia tonsa Dana, carnivory vs. herbivory in natural microplankton assemblages. Bull. Mar. Sci. 43, 458–468.
Gyllenberg, G., Lundqvist, G., 1979. The effects of temperature and salinity on the oxygen consumption of
Eurytemora hirundoides (Crustacea, Copepoda). Ann. Zool. Fennici 16, 205–208.
¨
Heinle, D.R., 1966. Production of a calanoıd copepod, Acartia tonsa, in the Patuxent river estuary. Chesapeake Sci. 7 (2), 59–74.
Hiromi, J., Nagata, T., Kadota, S., 1988. Respiration of the small planktonic copepod Oithona davisae at different temperatures. Bull. Plankton Soc. Jpn. 35, 143–148.
Jeffries, H.P., 1967. Saturation of estuarine zooplankton by cogeneric associates. In: Lauff, G.M. (Ed.), Estuaries, Am. Assoc. Adv. Sci, Washington, DC, pp. 500–508, Publ. No. 83.
Jonnsson, P.R., Tisellius, P., 1990. Feeding behavior, prey detection and capture efficiency of the copepod
Acartia tonsa feeding on planktonic ciliates. Mar. Ecol. Prog. Ser. 601, 35–44.
Kinne, O., 1964. Salinity and temperature combinations. Oceanogr. Mar. Biol. Annu. Rev. 2, 281–339. Kiørboe, T., Mohlenberg, F., Hamburger, K., 1985. Bioenergetics of the planktonic copepod Acartia tonsa,
relation between feeding, egg production and respiration, and composition of specific dynamic action. Mar. Ecol. Prog. Ser. 26, 85–97.
Lam, R.K., Frost, B.W., 1976. Model of copepod filtering response to changes in size and concentration of food. Limnol. Oceanogr. 21, 490–500.
Lance, J., 1963. The salinity tolerance of some estuarine plankton copepods. Limnol. Oceanogr. 8, 440–449. Lance, J., 1964. Feeding of zooplankton in diluted sea-water. Nature 4914, 100–101.
Lance, J., 1965. Respiration and osmotic behaviour of the copepod Acartia tonsa in diluted sea water. Comp. Biochem. Physiol. 14, 155–165.
Lee, W.Y., McAlice, B.J., 1979. Seasonal succession and breeding cycles of three species of Acartia (Copepoda: Calanoida) in a Maine estuary. Estuaries 2, 228–235.
Le Borgne, R., 1986. The release of soluble end products of metabolism. In: Corner, E.D.S., O’Hara, S.C. (Eds.), The Biological Chemistry of Marine Copepods, Oxford University Press, Oxford, pp. 109–164. Lonsdale, D.J., Heinle, D.R., Siegfried, C., 1979. Carnivorous feeding behavior of the adult calanoid copepod
Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 36, 235–248.
Lutz, R.V., Marcus, N.H., Chanton, J.P., 1992. Effects of low oxygen concentrations on the hatching and viability of eggs of marine calanoid copepods. Mar. Biol. 114, 241–247.
¨
Paffenhofer, G.A., Stearns, D.E., 1988. Why is Acartia tonsa (Copepoda, Calanoida) restricted to nearshore environments? Mar. Ecol. Prog. Ser. 42, 33–38.
´ ´ ´
Pagano, M., Gaudy, R., 1986. Biologie d’un copepode des mares temporaires du littoral mediterraneen ´
franc¸ais, Eurytemora velox. II. Respiration et excretion. Mar. Biol. 90, 551–564.
Precht, H., 1958. Concepts of temperature adaptation of unchanging reaction systems of cold blooded animals. In: Prosser, L. (Ed.), Physiological Adaptation, Physiological Society, Washington, pp. 50–78.
Rao, K.P., Bullock, Th., 1954. Q10as a function of size and habitat temperature in poikilotherms. Am. Nat. 88, 33–44.
Reeve, M.R., Walter, M.A., 1977. Observations on the existence of lower threshold and upper critical food concentration for the copepod Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 29, 211–221.
Robertson, J.R., 1983. Predation by estuarine zooplankton on tintinnid ciliates. Estuar. Coast. Shelf Sci. 16, 27–36.
Roddie, B.D., Leakey, R.J.G., Berry, J., 1984. Salinity–temperature tolerance and osmoregulation in
Eurytemora affinis (Poppe)(Copepoda, Calanoida) in relation to its distribution in the zooplankton of the
upper reaches of the Forth Estuary. J. Exp. Mar. Biol. Ecol. 79, 191–211.
Sheldon, R.W., Nival, P., Rassoulzadegan, F., 1986. An experimental investigation of a flagellate–ciliate– copepod food chain with some observations relevant to the linear biomass hypothesis. Limnol. Oceanogr. 31, 184–188.
Spaargaren, D.H., 1982. The ammonium excretion of the shore crab, Carcinus maenas, in relation to environmental osmotic conditions. Neth. J. Sea Res. 15, 273–283.
Stoecker, D.K., Egloff, D.A., 1978. Predation by Acartia tonsa Dana on planktonic ciliates and rotifers. J. Exp. Mar. Biol. 110, 53–68.
Tester, P., Turner, T., 1991. Why is Acartia tonsa restricted to estuarine habitats. In: Proc. 4th Int. Conf. on Copepoda, Vol. 1 (special issue), Bull. Plankton Soc. Jpn, pp. 603–611.
UNESCO, 1968. Zooplankton sampling. In: Monogr. Oceanogr. Methodol, Vol. 4, Unesco Press, New York, p. 174.
Vernberg, W.B., Vernberg, F.J., 1972. Environmental Physiology of Marine Animals, Springer, New York. White, J.R., Roman, M.R., 1992. Egg production by the calanoid copepod Acartia tonsa in the mesohaline
Chesapeake Bay, the importance of food resource and temperature. Mar. Ecol. Prog. Ser. 86, 239–249. Wiadnyana, N.N., Rassoulzadegan, F., 1989. Selective feeding of Acartia clausi and Centropages typicus on