All sources 98 Internet sources 77
[12]
https://www.sciencedirect.com/science/article/pii/S0142941815302269 3.9% 20 matches[13]
https://www.researchgate.net/publication...LICA_-_COMING_OF_AGE 2.5% 14 matches[14]
www.sciencedirect.com/science/article/pii/S0142941814002694 2.2% 11 matches[15]
www.polymerjournals.com/pdfdownload/1210168.pdf 2.0% 12 matches[16]
www.tandfonline.com/doi/full/10.1080/14658011.2016.1187477?src=recsys 2.1% 10 matches[17]
web.usm.my/jps/20-2-09/JPS 20_2_ ART 4 _37-59_.pdf 1.6% 10 matches[18]
https://www.sciencedirect.com/science/article/pii/S0142941811001188 1.4% 7 matches[19]
https://link.springer.com/content/pdf/10.1007/s13726-015-0310-y.pdf 1.0% 5 matches
1 documents with identical matches
[22]
www.sciencedirect.com/science/article/pii/S0142941813001475 1.0% 6 matches[23]
https://www.scribd.com/document/334636675/karet-rtf 0.9% 5 matches[24]
download.portalgaruda.org/article.php?ar...NE RUBBER BLENDS 0.9% 5 matches[26]
downloads.hindawi.com/journals/jpol/2013/279529.xml 0.8% 4 matches[28]
https://www.scribd.com/document/52958715...-EPDM-nanocomposites 0.8% 5 matches[29]
https://www.deepdyve.com/lp/elsevier/com...-264-in-a-6pIVoYpcGX 0.7% 3 matches[30]
https://www.researchgate.net/publication...es_of_Natural_Rubber 0.5% 4 matches[31]
https://www.deepdyve.com/lp/elsevier/rol...ca-filled-20wdNOGGBS 0.7% 3 matches[32]
https://www.scribd.com/document/354830512/NR-chitosan-SBR 0.7% 3 matches[33]
https://link.springer.com/content/pdf/10.1007/978-3-319-48806-6_1.pdf 0.6% 2 matches[34]
https://www.researchgate.net/publication...ilane_coupling_agent 0.6% 2 matches[35]
www.tandfonline.com/doi/citedby/10.1080/10236660802190104?scroll=top&needAccess=true 0.6% 1 matches[37]
https://www.hindawi.com/journals/bmri/2013/280512/ 0.4% 4 matches[38]
https://www.sciencedirect.com/science/article/pii/S0142941804000820 0.5% 3 matches18.4%
Results of plagiarism analysis from 2017-12-05 07:52 UTCThe comparison of alkanolamide and silane coupling agent on the properties of silica-filled natural rubber (SMR-L) comp.pdf
0.5% 3 matches
[39]
https://wenku.baidu.com/view/615d5069561252d380eb6ebd.html 0.5% 1 matches[40]
https://www.researchgate.net/publication...ral_rubber_compounds 0.5% 2 matches[41]
https://www.deepdyve.com/lp/wiley/proper...silica-or-qQw0EP0GiY 0.4% 3 matches[42]
iopscience.iop.org/article/10.1088/1757-899X/223/1/012006/pdf 0.4% 3 matches[43]
https://link.springer.com/content/pdf/10.1007/s13726-016-0422-z.pdf 0.4% 2 matches[44]
www.academia.edu/22337397/Comparison_of_...vulcanization_system 0.4% 2 matches[45]
https://patents.google.com/patent/US8211971B2/en 0.4% 2 matches[46]
https://patents.google.com/patent/US6686408B2/en 0.3% 2 matches
1 documents with identical matches
[48]
https://www.thefreelibrary.com/The impac...tread...-a0132468012 0.3% 3 matches[49]
www.google.nl/patents/US6686408 0.3% 2 matches
1 documents with identical matches
[52]
https://www.tandfonline.com/doi/full/10.1080/17458080.2016.1189096 0.3% 2 matches[53]
https://www.sciencedirect.com/science/article/pii/S0032386106006367 0.3% 3 matches[54]
www.tandfonline.com/doi/ref/10.1080/09276440.2017.1241559?scroll=top 0.3% 2 matches[55]
composites.utk.edu/papers in pdf/polymer testing_2017.pdf 0.3% 3 matches
1 documents with identical matches
[57]
engine.scichina.com/publisher/scp/journa...867?slug=full text 0.3% 2 matches[58]
https://www.sciencedirect.com/science/article/pii/B9780081004098000085 0.3% 2 matches[59]
www.tandfonline.com/doi/full/10.1080/00914037.2012.719133?src=recsys 0.3% 2 matches[60]
https://www.scribd.com/document/15694192...of-Fly-Ash-nr-Blends 0.3% 2 matches[61]
journals.sagepub.com/doi/abs/10.1177/0731684417712070?ai=1gvoi&mi=3ricys&af=R 0.3% 2 matches[62]
https://www.hindawi.com/journals/ijbm/2013/639841/ 0.2% 2 matches[63]
https://www.researchgate.net/profile/Sey...yethylene-blends.pdf 0.3% 2 matches[64]
www.tandfonline.com/doi/pdf/10.1080/03602559.2013.769577?needAccess=true 0.2% 1 matches[65]
https://link.springer.com/content/pdf/10.1007/s10853-008-2843-3.pdf 0.2% 2 matches[66]
https://www.deepdyve.com/lp/elsevier/mea...r-polymer-W00r6Gd00c 0.3% 2 matches[67]
https://www.researchgate.net/publication...le-polyurethane_film 0.2% 2 matches[68]
https://www.researchgate.net/publication...lt-Extrusion_Process 0.2% 2 matches[69]
https://www.researchgate.net/profile/Arv...ng-2015-41-17-25.pdf 0.3% 2 matches[70]
repositorio.unesp.br/handle/11449/10102 0.2% 2 matches[71]
https://www.sciencedirect.com/science/article/pii/S0029549301004551 0.2% 2 matches[72]
https://link.springer.com/article/10.1007/s00114-009-0584-z 0.2% 2 matches[73]
https://core.ac.uk/display/37498012 0.2% 2 matches
1 documents with identical matches
[75]
www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2015000300463 0.2% 2 matches[76]
www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=HKGMCJ_2004_v39n4_263 0.2% 2 matches[77]
https://www.sciencedirect.com/science/article/pii/S0142941810000693 0.2% 1 matches[78]
https://www.researchgate.net/publication...nized_Natural_Rubber 0.2% 1 matches[79]
www.tandfonline.com/doi/figure/10.1080/03602559.2010.496388 0.2% 1 matches[80]
www.tandfonline.com/doi/pdf/10.1080/03602550802355206?needAccess=true 0.2% 1 matches[81]
www.tandfonline.com/doi/pdf/10.1080/03602559.2010.496425?needAccess=true 0.2% 1 matches[82]
https://www.sciencedirect.com/science/article/pii/B9781845693961500163 0.2% 1 matches[83]
https://www.sciencedirect.com/science/article/pii/S0014305706003648 0.1% 1 matches[84]
https://link.springer.com/article/10.1007/s13361-015-1134-x 0.2% 2 matches[85]
https://www.deepdyve.com/lp/elsevier/a-d...amability-Vxis3dHEfY 0.2% 1 matches[86]
https://www.sciencedirect.com/science/article/pii/S0142941809000373 0.2% 1 matches[87]
https://www.scribd.com/document/121686466/Polymer-Testing 0.2% 1 matches[88]
https://www.sciencedirect.com/science/article/pii/S2351978915005600 0.1% 1 matches[89]
www.me.sc.edu/Research/DBMML/pdfs/PT2017_1.pdf 0.2% 1 matches
1 documents with identical matches
[93]
https://www.researchgate.net/publication...urface_active_agents 0.1% 1 matches[94]
0.1% 2 matches
[95]
www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=HKGMCJ_2012_v47n1_18 0.1% 1 matches[98]
https://www.sciencedirect.com/science/article/pii/S0142941800000179 0.1% 1 matches[99]
https://www.tandfonline.com/doi/full/10.1080/09276440.2017.1241559 0.1% 1 matches[100]
www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=HKGMCJ_2006_v41n1_10 0.1% 1 matches
1 documents with identical matches
[102]
biblehub.com/proverbs/1-17.htm 0.1% 1 matches[103]
https://www.sciencedirect.com/science/article/pii/S0378874106001280 0.1% 1 matches[104]
biblehub.com/commentaries/proverbs/1-17.htm 0.1% 1 matches9 pages, 6010 words
PlagLevel: selected / overall
274 matches from 105 sources, of which 90 are online sources.
Settings
Data policy: Compare with web sources, Check against my documents, Check against my documents in the organization repository, Check against organization repository, Check against the Plagiarism Prevention Pool
Sensitivity: Medium Bibliography: Consider text
--Material properties
[41]The comparison of alkanolamide and
silane coupling agent
on the properties of silica-
fi
lled natural rubber
(SMR-L)
compounds
Indra Surya
a, H.
Ismail
b,* [16], A.R.
Azura
baDepartment of Chemical Engineering, Engineering Faculty, University of Sumatera Utara, Medan, 20155, Sumatera Utara, Indonesia bSchool of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Engineering Campus, Nibong Tebal, 14300, Penang, Malaysia
a
e
i
r
n
t
f
i
o
c
l
Article history: Received 20 May 2014 Accepted 6 August 2014 Available online 27 August 2014
Keywords: Alkanolamide [40]
Reinforcing ef ciencyfi
APTES
Silica-reinforcement Plasticiser
a
b
s
t
r
a
c
t
Alkanolamide (ALK) and Aminopropyltriethoxy Silane (APTES) wereincorporated separately
[14]
intosilica- lledfi SMR-L compounds at 1.0, 3.0, 5.0 and 7.0 phr.It was found thatcompounds withboth ALK and APTES exhibitedcure enhancement, betterfillerdispersion and greater
[34]
rubber- ller interaction.fi Both additivesalso produced modulus and tensile enhancements in thesilica- lledfi SMR-L compounds, especially up to a 5.0 phr loading. At a similar loading, ALK exhibited higher reinforcing ef ciency of silica than APTES.fi
©2014Published by Elsevier Ltd. [28]
1. Introduction
The mechanical properties such as tensile modulus, tensile strength, tear and abrasion resistances of a rubber vulcanisate are enhanced as a result of the incorporation of reinforcing ller into the rubber compound. Carbon blackfi and silica are the best known reinforcing llers, and havefi been widely utilised in the rubber industry. Each type of reinforcing filler produces useful mechanical properties due to their speci c surface chemistry. Carbon black andfi silica have apparently a dissimilar surface chemistry. The surface of carbon black is saturated with hydrocarbon functional groups that react with sulphur during vulcani-sation. They form sulphur bonds that link the rubber
chains, and also tie the carbon black to the rubber [1e2].[In 1 3 ]
marked contrast to the hydrocarbon functionality of carbon black, silica does not react with sulphur.The surface of silica is highly polar and hydrophilic due to the presence of
[13]
numerous silanol groups.The silanol groupsare relatively incompatible with hydrocarbon rubbers, such as natural
rubberand styrene butadiene rubber, therefore coupling
bonds are not formed.On the other hand, the silica parti-cles have a strong tendency to interact with each other to form aggregates. Since the silica-hydrocarbon rubber interaction is weaker than the silica-silica interaction, the results are the formation of large agglomerates, poor dispersion of silica and lower reinforcing ef ciency.fi
Modulus is a well-recognised criterion of filler rein-forcement[3]. Due to the nature of its surface chemistry, silica has a low level of surface activity for rubber bonding, resulting in small amounts of effectively immobilised rub-ber. Therefore, silica reinforced rubbers have lower modulus than carbon black reinforced rubbers.
In order to overcome the de ciencies of silica, couplingfi agents are used for the reinforcement of hydrocarbon rubbers. The most ef cient and presently known couplingfi agent is organosilane[4]. Organosilanes are reactive addi-tives. They are utilised to improve the silica-rubber inter-action of silica- lled rubbers and, consequently, enhancefi
*Corresponding author.
E-mail addresses:ihana @usm.myfi ,profhana @gmail.comfi (H. Ismail).
Contents lists available at ScienceDirect [55]
Polymer Testing
j o uh ro nm :awe l pw aw g.[ceem6ol6/ s]l eo vc ia et re . / p o l y t e s t
http://dx.doi.org/10.1016/j.polymertesting.2014.08.007 0142-9418/©2014 Published by Elsevier Ltd.
the reinforcing ef ciencyfi [5 6]e . The organosilanes modify the surface of silica[7 8]e . The modi ed silica provides afi chemically active surface that can participate in vulcani-sation; providing coupling bonds between organosilane and both silica and rubber phases. There is much evidence con rming the existence of such bondsfi [9e10 ]. The coupling bonds were noted to mark improvements in the mechanical properties of the rubber- lled vulcanisatesfi [11e16 ].
In our previous work[17],the preparation and
appli-[12] [12]
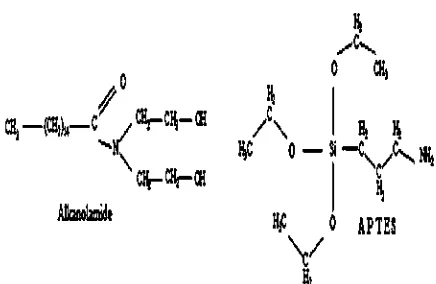
cationof Alkanolamide (ALK) in silica- lledfi natural rubber compounds were reported. The incorporation of ALK within silica- lledfi natural rubber compounds gave enhanced mechanical properties viz. tensile strength, ten-sile modulus and hardness. The enhancement of these properties was attributed to the improvement of silica dispersion in the rubber compounds, and higher crosslink density that stemmed from the incorporation of ALK. The results also indicated that ALK may function as an accel-erator and a plasticiser. In this study, the properties of silica- lled natural rubber compounds with ALK werefi compared with those of silica- lled natural rubber com-fi pounds with Aminopropyltriethoxy Silane (APTES). APTES is a type of organosilane. Similar to ALK, APTES also con-tains amine within its molecule, and its molecular structure is shown in Fig. 1.
2. Experimental
[12]
2.1. Materials
Natural rubbergrade SMR-L was obtained from Guthrie
[23]
(M) Sdn.Bhd., Seremban, Malaysia. Other compounding
ingredientssuch as sulphur, zinc oxide, stearic acid, N-isopropyl-N'-phenyl-p-phenylenediamine (IPPD), benzo-thiazolyl disul de (MBTS) and precipitated silica (gradefi Vulcasil S) were supplied by Bayer Co.e (M) Sdn. Bhd.[42],
Petaling Jaya, Selangor, Malaysia.The APTES (C9H23NO3Si) [16]
was supplied by Sigma-Aldrich.The ALK was synthesised in
our laboratory using Re ned Bleached Deodorizedfi Palm
[16]
Stearin (RBDPS) and diethanolamine. The reaction
pro-cedures and molecular characterisationsof the ALK were given in our previous report[17].
2.2. Compounding
A semi-ef cient vulcanisation system was applied forfi the compounding. The compounding procedure was per-formed on a two-roll mill (Model XK-160). Table 1 dis-played the compound designation and formulation of silica- lled SMR-L compounds with ALK and APTES.fi
[17]
2.3. Cure characteristics
The cure characteristics of thesilica- lled SMR-L com-fi pounds were obtained using a Monsanto Moving Die
Rheometer (MDR 2000), which was employed to
deter-minethe scorch time(ts2), cure time (t90) and torque
dif-ference (MHdML),according to ISO 3417[24].Samples of the respective compounds were tested at 150C. The com-[12] poundswere subsequently compression-moulded using a stainless steel mould at 150C, with a pressure of 10 MPa, based on respective curing times.
[12]
2.4. Tensile, hardness and resilience properties
Dumbbell-shaped samples were cut from the moulded
[12]
sheets.Tensile tests were performed at a cross-head speed of 50 0mm/min using an Instron 3366 universal tensile
[17]
machine, according to ISO 37. The tensile strength (TS),
stress at 100% elongation (M100),stress at 300% elongation
(M300) andelongation at break(EB) were determined.The
hardness of the samples was measured according to ISO
[12]
7691-I, using a Shore A type manual durometer.The resil-ience was studied by utilisinga Wallace Dunlop
Trips-[12]
ometer,according to BS 903 Part A8.The rebound resilience was calculated according to Equation(1).
% Resilience¼ ð1cos½q
[14]
2Þ ð =1 cosq1Þ 100 (1)
where
q
1is the initial angle of displacement (45), andq
2isthe maximum rebound angle.
[14]
2.5. Scanning electron microscopy (SEM)
The tensile fractured surfaces of the natural rubber
compounds were examinedbyusing a Zeiss Supra-35VP
scanning electron microscope (SEM) to obtain
informa-tion regarding the filler dispersion, and to detect the possible presence of micro-defects.The fractured pieces were coated with a layer of gold to eliminate electrostatic charge build-up during examination.
[32]
2.6. Measurement of rubberefiller interaction
The rubber- llerfi interaction was determined by
swelling the cured silica- lled SMR-Lfi compounds in
[15]
toluene, according to ISO1817.Test pieces with dimensions
of30mm5mm2mmwere prepared from the moulded
[18]
sheets.The initial weights were recorded prior to testing.
The test pieces were then immersed in toluene and
[15]
conditioned at room temperature in a dark environment
[12]
for 72 hours.After the conditioning period, the weights of
[15]
the swollen test pieces were recorded. The swollentest
pieces were then dried in an oven at 70C for 15 minutes
Fig.1.Molecular structure of Alkanolamide and APTES.
[15]
and allowed tocool at room temperature for another 15
[12]
minutes before the nal weights were recorded. Thefi Lorenz
[12]
and Park's equation[18 20]e was applied in this study.The swelling indexwas calculated according to Equation(2).
Qf Qg= ¼ae
[17] zþb (2)
where, thesubscripts f and g referred tofilledand gum
[17]
vulcanisates, respectively; z wasthe ratio by weight offiller to hydrocarbon rubberin the vulcanisate;while a and b
[17]
were constants.The higher the Qf/Qgvalue, the weaker the rubber- ller interaction became.fi
In this study, the weight of the toluene uptake per gram
[12]
of hydrocarbon rubber (Q) was calculated based on
Equa-tion(3).
Q¼ Swollen½Dried weight ½=Initial weight
100=Formula weight (3)
[28]
3. Results and discussion
3.1. Effects of ALK and APTES on cure characteristics of
silica-filled SMR-L compounds
The effects of ALK and APTES on the scorch and cure times of silica- lled SMR-L compounds are shown infi Fig. 2. It is well-known that the addition of a silicafiller into a SMR-L compound (de ned as the control compound) cau-fi ses cure retardation. Due to its high polarity, silica inter-acted with zinc oxide during curing and formed silica bound zinc, which was unable to activate the accelerator.
Consequently, zinc activity was reduced and the curing was
[78]
retarded[2,21]. Compared to the control compound, the
addition of ALK and APTES decreased the cure and scorch times of silica- lled SMR-L compounds. Both additives mayfi be considered as co-curing agents since the polar parts of these additives reacted with the silanol groups of silica to transform the hydrophilic silica into hydrophobic silica; which interacted relatively less with zinc oxide. In this manner, the performance of zinc oxide in activating the accelerator was maximised. It was seen that the higher the loading of these additives, the lower the scorch and cure times. This was attributed to the amine-content of both additives. As presented in Fig. 1, both chemicals contained amine within their molecules. Amine, being one of the
accelerator activators, is an alkaline substance that
in-[58]
creases the cure rate[22].Amine may also beutilisedto overcome cure retardation problems in silica reinforcement
[2,23].
The scorch and cure times of ALK were longer than those of APTES. This was attributed to the concentration of amine in each of the additive molecules. From the molec-ular structures of the additives, as presented in Fig. 1, t as i w seen that the mass fraction of amine in ALK was lower than that of APTES. Therefore, at a similar loading, ALK provided a lower concentration of amine than APTES.
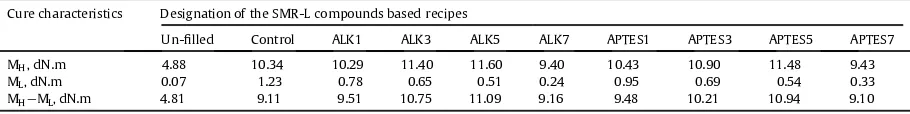
Table 2 presents the torque difference (MHdML) of the
silica- lled SMR-L compounds at various ALK and APTESfi
[14]
loadings.The incorporation of 1.[14]0 phr ofthese additives into silica- lled SMR-Lfi compounds produced compounds
withahigher torque difference, compared to the control
compound.The addition of up to 5.0 phr of these additives increased the torque difference; however, it was decreased with further increase of these additives. It was also evident that the minimum torque (ML) decreased with increase of
the loading of these additives. The minimum torque rep-resented thefiller- ller inter agglomerationfi [13], and the value was used to measure the relative viscosity of a rubber compound[24]. The lower the value, the weaker the ller-fi
[48]
filler interaction became;resulting in lowerviscosity of the compound.The incorporation of these additives into silica-filled SMR-L compounds reduced the filler- ller interac-fi tion, which led to a lower viscosity that enhanced the processability of the silica- lled SMR-L compounds.fi Table 1
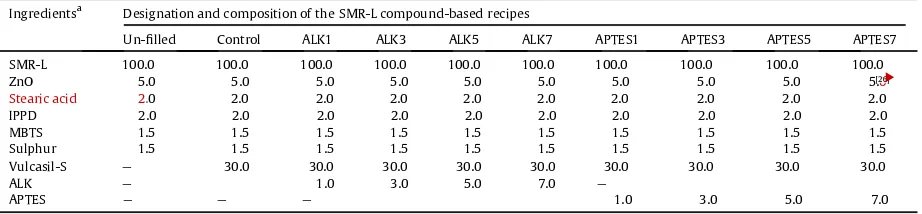
The compound designation and formulation of silica- lled SMR-L compounds with ALK and APTES.fi
Ingredientsa Designation and composition of the SMR-L compound-based recipes
Un- lledfi Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
SMR-L 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
ZnO 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.[26]0
Stearic acid 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0
IPPD 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0
MBTS 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5
Sulphur 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5
Vulcasil-S e 30.0 30.0 30.0 30.0 30.0 30.0 30.0 30.0 30.0
ALK e 1.0 3.0 5.0 7.0 e e e e e
APTES e e e 1.0 3.0 5.0 7.0 e e e
aParts per hundred parts of rubber.
Fig. 2.Scorch times (ts2) and cure times (t90) of the silica- lled SMR-Lfi
compounds at various ALK and APTES loadings.
[83]
I. Surya et al./ Polymer Testing 40 (2014) 24 32e
In theory, the torque difference may be used as an
indication of the crosslink density ofa rubber compound
[25 28]e . The greater the torque difference value, the higher the crosslink density. The total crosslink density was contributed by sulphide crosslinks and physical crosslinks [29 30]e . The addition of up to 5.0 phr of these additives into the silica- lled SMR-L compounds increased the tor-fi que difference of the SMR-L compounds. This was clearly attributed to the actions of these additives. As previously mentioned, the hydrophobic silica (due to the incorpora-tion of ALK and APTES) maintained the performance of zinc oxide in activating the MBTS, which accelerated the sulphur reaction and enhanced the state of the sulphide crosslink. The more hydrophobic silica became, the more compatible it was to SMR-L. As a consequence, the addition of these additives not only reduced the ller- ller interac-fi fi
[13]
tion, but also enhanced the rubber- ller interaction;fi
lead-ing to the formation of coupling bonds between the
[13]
additiveswithboth silica and SMR-L. Thesecoupling bonds wereconsidered to be another type of crosslink, which contributed to the total crosslinks of silica- lled SMR-Lfi compounds.
The reduction of the torque difference after the 5.0 phr of ALK and APTES loading was most probably attributed to the dilution effect of excessive amounts of additives, which lowered the crosslink density.
[30]
3.2. Effects of ALK and APTES on the silica dispersion
The degree of silica dispersion, with or without the
addition of ALK and APTES in the SMR-L phase, was
determined quantitatively by Equation (4) [17,31e32].
L¼hrmr (4)
where:
h
r¼[MLf/MLg], and mr¼[MHf/MHg];[30]where MLfandMHfwerethe minimum and the maximum torques of the
[30]
filled compounds; andMLgand MHg werethe minimum
and the maximum torques of the un lled/gum SMR-Lfi
compound.From Table 2, Lg¼0.07 and MM Hg¼4.88.[22]The
lower the value of L at a particular silica loading, the better the silica dispersion became in the SMR-L phase.
The value of L for the silica dispersion in the SMR-L phase is presented in Table 3andFig. 3. It is seen that the value of L of the silica dispersion in the rubber phase of the control compound was the highest. This was attributed to the surface of silica, which was saturated with hydrophilic silanol groups, and its relatively weak interaction with SMR-L. The silica particles also had a strong tendency to interact with each other and form large agglomerates[33]. Consequently, the dispersion of silica in the SMR-L com-pounds became poor. The addition of ALK and APTES at 1.0 phr into the silica- lled SMR-L compounds lowered thefi value of L. This was clearly due to the polar parts of these additive molecules that had a strong interaction with silica, which transformed the filler into a hydrophobic one. Consequently, silica became more compatible with SMR-L; hence improving the dispersion. The higher the loading of the additives, the lower the value of L; which meant enhanced silica dispersion.
The comparison of the L value of ALK with that of APTES is presented in Table 3andFig. 3. It is seen that at a similar loading, ALK caused a lower L value of silica in the SMR-L
[12]
compound.Thiswas attributed to the additional function of ALK as an internal plasticiser, which caused the reduc-tion of thefiller- ller interaction to lead to a betterfi filler dispersion compared to APTES.This explanation was in line with the data in Table 2. At a similar loading, the minimum torque (ML) of ALK was lower than that of APTES.
Table 2
Torque differences of silica- lled SMR-L compounds at various ALK and APTES loadings.fi
Cure characteristics Designation of the SMR-L compounds based recipes
Un- lledfi Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
MH, dN.m 4.88 10.34 10.29 11.40 11.60 9.40 10.43 10.90 11.48 9.43
ML, dN.m 0.07 1.23 0.78 0.65 0.51 0.24 0.95 0.69 0.54 0.33
MHeML, dN.m 4.81 9.11 9.51 10.75 11.09 9.16 9.48 10.21 10.94 9.10
Table 3
The value of L for silica dispersion in SMR-L compounds.
Dispersion parameter Designation of the SMR-L compounds based recipes
Control ALK1 ALK3 ALK5 ALK7 APTES1 APTES3 APTES5 APTES7
hr 17.57 11.14 9.29 7.29 3.43 13.57 9.86 7.71 4.71
mr 2.12 2.11 2.34 2.38 1.93 2.14 2.33 2.35 1.93
L¼hremr 15.45 9.03 6.95 4.91 1.50 11.43 7.62 5.36 2.78
Fig. 3.The L values of silica with ALK and APTES.
3.3. Effects of ALK and APTES on rubberefiller interactions
The rubber- ller interaction depends on the degree offi the filler dispersion in the rubber phase. Better filler dispersion results in stronger rubber- ller interactions. Thefi rubber- ller interaction, with the addition of ALK andfi APTES to the silica- lled compound (based on the Lorenzfi and Park's equation), is presented inFig. 4.
It is shown that Qf/Qg decreased with increase of the ALK and APTES loading, up to 5.0 phr, and then increased with further increase to the loading. The decrease in Qf/Qg indicated that the SMR-L silica interactions becamee stronger with the addition of ALK and APTES. This was attributed to the ability of these additives to chemically modify the surface of silica, which was then more compatible with SMR-L; hence, improving the wetting/ dispersion of silica and, consequently, improving the
SMR-[53]
L silica interaction.e This explanation is in agreement with
Fig.3.
The increase of Qf/Qg after 5.0 phr loading was probably largely due to the excessive loading forming a layer in the silica- lled SMR-L system. The layer absorbed, coated andfi trapped the silica reducing the rubber- ller interaction.fi
At a similar loading, the Qf/Qg values of ALK were lower than those of APTES. Again, this was attributed to the additional function of ALK as an internal plasticiser, which caused better ller dispersion and led to a stronger rubber-fi filler interaction.
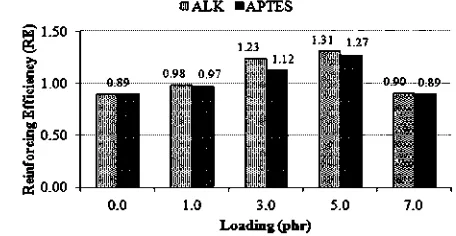
3.4. Effects of ALK and APTES on reinforcing ef ciency of silicafi
The degree of reinforcement provided by the ller wasfi calculated through its reinforcing ef ciency (RE), which infi its simplest form is given by Equation 5 31]. [
RE¼ MHMLð
High RE meant a high rubber- ller interaction, whichfi was influenced by the degree of ller dispersion. Enhancedfi filler dispersion provided a greater surface area for
rubber-[14]
filler interactions.RE of silica on SMR-L,due to the addition of ALKand APTES into the silica- lled SMR-L compounds, isfi shown inFig.5.
As presented in Fig. 5, ALK and APTES, with various loading, increased the RE of silica on the SMR-L. This is associated with the function of the additives as surface modi ers of silica that improved its compatibility withfi SMR-L; hence, improving the dispersion and rubber- llerfi interaction.
At a similar loading, ALK caused a higher RE of silica.
This was due to better dispersion of silica and a stronger [37]
interaction of SMR-L silicae in the presence of ALK as
[53]
compared toAPTES.This explanation is in agreement with
theresults inFigs.3 and 4.
3.5. Effects of ALK and APTES on mechanical properties
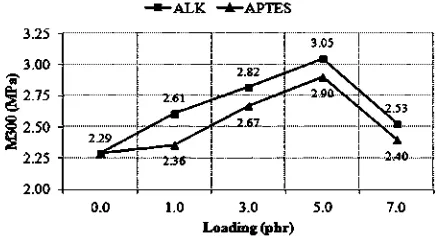
The effects of ALK and APTES on the M100, M300, hardness, EB, resilience and TS of silica- lled SMR-L com-fi pounds are shown in Figs. 6, 7, 8, 9, 10 and 11, respectively.
The incorporation of up to5.0 phr of ALK and APTES into
[16] [18]
the silica- lled SMR-L compounds increasedfi the tensile
modulus (M100 and M300), but modulus decreased with
further increase in the loadings.The results of hardness, tensile strength and resilience also exhibited a similar trend.
Since the tensile modulus of a rubber vulcanisate is only
[13]
dependent on the degree of crosslinking [34 35]e [13], the enhancement of tensile modulus to 5[13].0 phr was attributed to a higher crosslink density due to the formation of coupling bonds between the additives and both silica and
[13]
SMR-L.The coupling bonds were another type of crosslink
[2][13],which contributed to the total crosslink density of the
[13]
SMR-L compounds. Therefore, with the addition of the
additives, their effects were the same as an increase in
crosslink density.This explanation is in line with the results in Table 2. The silica- lled SMR-L compounds with addi-fi tives had a higher value of (MHML) than the silica- lledfi
vulcanisate without additives (control compound). The deterioration of the tensile modulus beyond 5.0 phr was attributed to the excessive loading of these additives, which caused a lower crosslink density. Presumably, the excessive amounts of these additives formed boundary layers which dissolved and coated part of the elemental curatives and silica particles, leading to decreased
Fig. 4.The Qf/Qg values of silica with ALK and APTES. Fig. 5.Reinforcing ef ciency of silica with ALK and APTES.fi I. Surya et al. / Polymer Testing 40 (2014) 24 32e
formation of both sulphide and coupling bond crosslinks.
Again,this explanation is in line with thedata inTable 2. [37]
As presented in Figs. 6 and 7, at a similar loading, ALK caused a decrease in M100 and an increase in M300, compared to the silica- lled SMR-L compounds withfi APTES. A decrease in M100 was attributed to the plasti-cising effect of ALK, which modi ed the modulus/stiffnessfi property. A plasticiser may be used, not only to improve the rubber compound processing, but also to modify physical properties (such as stiffness andflexibility) of a rubber vulcanisate[36] .
An increase in M300 was attributed to a stronger rubber- ller interaction due to the plasticising effect offi
ALK. The ALK lowered the viscosity of the silica- lled SMR-fi L compound, which led to better filler dispersion and greater rubber- ller interaction. M300 displayed the de-fi gree of rubber- ller interactionsfi [37e38]. This explanation is in line with the data inFig. 4, which demonstrated that the Qf/Qg values of ALK were lower than those of APTES.
As presented in Fig. 8, hardness showed a similar behaviour as M100, which displayed the stiffness of a rubber vulcanisate[39]. Like tensile modulus, hardness also depends solely on the degree of crosslinks[34 35]e . The enhancement of hardness up to 5.[13]0 phr was attributed to a higher crosslink density, and the deterioration of hardness beyond 5.[13]0 phr was attributed to alower crosslink density.
At a similar loading, the hardness of ALK was lower than that of APTES. This was due to the plasticising effect of ALK, which softened the silica- lled SMR-L vulcanisates.fi
Fig. 9 represented the effects of ALK and APTES on the elongation at break (EB) of the silica- lled SMR-L com-fi pounds. As seen, APTES reduced the EB of silica- lled SMR-fi L vulcanisate up to 5.0 phr of loading, and then increased slightly it as the loading further increased. EB depends mostly on the degree of crosslink density[35]. The reduc-tion of the EB up to 5.[13]0 phr wassimply attributed to a
higher crosslink density, which immobilised the SMR-L
segments from the silica surface. The increase of EB beyond 5.0 phr was attributed to a lower crosslink density. A contrary result was obtained when ALK was utilised.
[12]
The EB increased with the increasing of ALK loadings.This
was attributed to the function of ALK as an internal
Fig.6.Modulus at 100% of the silica- lled SMR-L compounds at various ALKfi and APTES loadings.
Fig. 7.Modulus at 300% of the silica- lled SMR-L compounds at various ALKfi and APTES loadings.
Fig. 8.Hardness of the silica- lled SMR-L compounds at various ALK andfi APTES loadings.
Fig. 9.Elongation at break of the silica- lled SMR-L compounds at variousfi ALK and APTES loadings.
Fig. 10.Resilience of the silica- lled SMR-L compounds at various ALK andfi APTES loadings.
plasticiser, which modi ed the exibility of the silica- lledfi fl fi SMR-L compounds. As an internal plasticiser, ALK provided a free volume that allowed increased mobility/ exibility forfl the rubber chains to move.
Fig. 10 displays the effects of ALK and APTES on the resilience of the silica- lled SMR-L compounds. It showsfi that APTES increased the resilience of silica- lled SMR-Lfi compounds up to 5.0 phr of loading, and then decreased it as the loading further increased.
Like tensile modulus and hardness, resilience also de-pends on the number of crosslinks[36,39]. The enhance-ment of resilience up to 5.0 phr was attributed to a higher crosslink density, and the deterioration of resilience beyond 5.0 phr was attributed to a lower crosslink density. ALK presented a similar trend as APTES with loading up to 5.0 phr; however, beyond 5.0 phr, the ALK displayed a
[65]
further increase in resilience.Again, this wasattributed to
the plasticising effect of the ALK, which improved the
flexibility of the silica- lled SMR compounds.fi According to Hofmann [39] and Ignatz-Hoover & To [36], rebound resilience not only depends on the degree of crosslinks, but also on theflexibility of the rubber chains. The morefl ex-ible the rubber chains, the higher the resilience. The excessive amount of ALK (7.0 phr) caused a moreflexible silica- lled SMR chains.fi
At a similar loading, the resiliencies of ALK were higher
[65]
than those of APTES. Again, this was attributed to the plasticising effect ofALK, which caused a higher crosslink density and a higher EB (more exibility).fl
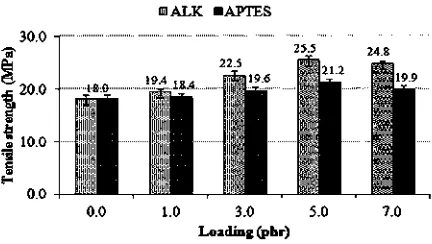
Fig. 11 illustrates the tensile strength of silica- lledfi SMR-L compounds at various ALK and APTES loadings. The tensile strength enhancement was attributed to the ability of ALK and APTES to improve the silica SMR-L in-e teractions. The silica surface was saturated with hydro-philic silanol groups, which were relatively incompatible with SMR-L, and its interaction with SMR-L was relatively
[41]
low.Boththe silane coupling agent[40]and ALK[17] may chemically modify the surface of silica, and transform it into hydrophobic silica. This additive-modi ed silica wasfi more compatible with SMR-L; as a result, the improved wetting/dispersion and the improved silica SMR-L inter-e
[13]
action led to the formation of physical crosslinks.These physical crosslinks furthercontributed to the total crosslink density[29 30]e . The greater the physical crosslink s, the higher the reinforcing ef ciency of the ller, and a higherfi fi tensile strength is produced.
At a similar loading, ALK produced a higher tensile
[14]
[26]
strength than APTES.This may be attributed to a higher
reinforcing ef ciency of ALK due, for example, to betterfi filler dispersion and greater rubber- ller interaction.fi Ac-cording to Cohan [41], higher breaking elongation tends to give higher tensile strength. A higher EB meant a higher strain at break of the silica- lled SMR-L vulcanisate; whichfi was delayed until a larger strain.
3.6. Scanning electron microscopy (SEM) study
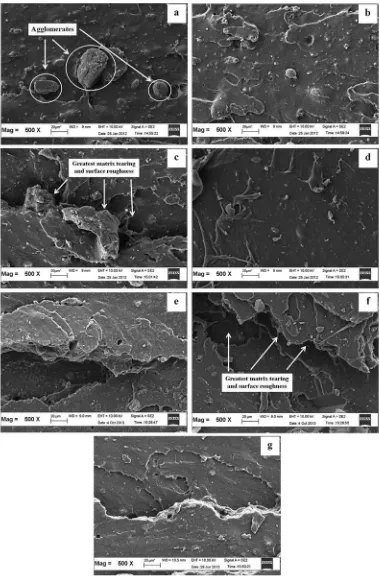
The SEM micrographs inFig. 12demonstrate the frac-tured surfaces of silica- lled SMR-L compounds with ALKfi and APTES at a magni cation of 200X. The micrographsfi showed the improvement of the silica dispersion due to the addition of ALK and APTES. The dispersion of the llerfi was the least homogeneous in (a), where large silica ag-glomerates were observed (indicated by arrows in Fig. 12a). The SEM micrograph of (a) also seemed relatively smooth compared to the others, which indicated that (a)
[12]
was less ductile than the others. However, the SEM micrograph for (c) exhibited a comparablematrix tearing
[14]
line and surface roughnesswith (f). Both (c) and (f) pre-sented the greatest matrix tearing line and surface roughnesscompared to the others (b, d, e and g).A greater rubber- ller interaction in both (c) and (f) altered thefi crack path, which led to increased resistance to crack propagation that caused an increase in tensile modulus,
[63]
tensile strength and hardness. The micrographs of the
tensile fractured surfaces werein mutual agreement with
the results inFig.4, which illustrated that the Qf/Qg values of ALK-5 and APTES-5 were the lowest ones. An enhancement in rupture energy, due to a greater rubber-filler interaction, was responsible for the roughness and
[63]
matrix tearing line of the fractured surface. The micro-graphsof the tensile fractured surfaces werein mutual agreement with the results obtained by other researchers [42 43]e who reported that an increase in rupture energy was responsible for the roughness and matrix tearing line of the fractured surface.
However, the matrix tearing lines and surfaces rough-ness of (d) and (g) were smoother than those of (c) and (f), which indicated lower crosslink densities.
4. Conclusions
From this study, the following conclusions were drawn:
1. Alkanolamide and aminopropyltriethoxy silane (APTES) acted as co-curing additives in silica- lled natural rub-fi ber compounds. Both of the additives increased the cure rate and torque difference.
2. A 5.0 phr loading of alkanolamide and amino-propyltriethoxy silane (APTES) was the optimum loading to improve the properties of silica- lled natural rubberfi compounds.
3. At a similar loading, alkanolamide indicated a higher degree of silica dispersion, greater silica natural rubbere interaction and higher reinforcing ef ciency than ami-fi nopropyltriethoxy silane (APTES).
Fig. 11.Tensile strength of silica- lled SMR-L compounds at various ALK andfi APTES loadings.
I. Surya et al. / Polymer Testing 40 (2014) 24 32e
Fig. 12.SEM micrographs of the failed fracture of silica- lled vulcanisate at a magni cation of 500x: (a) Control, (b) ALK3, (c) ALK5, (d) ALK7, (e) APTES3, (f)fi fi APTES5, and (g) APTES7.
[60]
Acknowledgements
The authors would like to thank Universiti Sains
Malaysia for providing the research facilities for carrying out the experiment and for making this research work
[88]
possible. One of the authors (Indra Surya)is grateful to the
Directorate Generalof Higher Education (DIKTI), Ministry
of Education and Culture (Kemdikbud) of the Republicof
Indonesia, forthe award of a scholarship under thefifth
batch of the Overseas Postgraduate Scholarship Program.
References
[1] M.Q. Fetterman, The unique properties of precipitated silica in the design of high performance rubber, Elastomerics 116 (1984) 18 31e .
[2] M. Fetterman, Precipitated silica coming of age, Rubber World 194e
(1986) 38 40e .
[3] P.E. Pinter, C.R. Mc. Gill, Comparing rubberfillers in an energy conscious economy, Rubber World International 177 (1978) 30 37e .
[4] E.P. Plueddemann, in: E.P. Plueddemann (Ed.[76]), Silane Coupling Agents,Plenum Press, New York, 1982, p.4. Chapter 1.
[5] I. Gelling, M. Porter, A. Roberts, in: F.R. Eirich (Ed.[76]),Natural Rubber Science andTechnology, 1988, p.367.
[6] F. Lautenschlaeger, K. Edwards, Model compound vulcanization-part V. The effect of chemical additives andfillers, Rubber Chemis-try and Technology 53 (1980) 27 47e .
[7] M. Ranney, C. Pagano, Silane coupling agent effects in ethylene propylene diene terpolymers, Rubber Chemistry and Technology 44 (1971) 1080 1092e .
[8] M. Wagner, Heat generation and rubber- ller coupling bonds,fi Rubber Chemistry and Technology 47 (1974) 697 716e .
[9] A. Gent, E. Hsu, Coupling reactions of vinylsilanes with silica and poly (ethylene-co-propylene), Macromolecules 7 (1974) 933 936e .
[10] U. Goerl, A. Hunsche, A. Mueller, H. Koban, Investigations into the silica/silane reaction system, Rubber Chemistry and Technology 70 (1997) 608 623e .
[11] M. Fetterman, Filler effect on the heat stability of vulcanized elas-tomeric compositions, Rubber Chemistry and Technology 46 (1973) 927 937e .
[12] M. Wagner, Reinforcing silicas and silicates, Rubber Chemistry and Technology 49 (1976) 703 774e .
[34]
[13] A.K. Manna, P. De, D. Tripathy, S. De, D.G.Peiffer,Bonding between precipitated silica and epoxidized natural rubber in the presence of silane coupling agent, Journal of Applied Polymer Science 74 (1999) 389 398e .
[29]
[14] P. Sae-Oui, C. Sirisinha, U. Thepsuwan, K.Hatthapanit,Comparison of reinforcingef ciencyfi between Si-69 and Si-264 in a conventional vulcanization system, Polymer Testing 23 (2004) 871 879e .
[29]
[15] P. Sae-Oui, C. Sirisinha, K. Hatthapanit, U.Thepsuwan,Comparison of reinforcingefficiencybetween Si-69 and Si-264 inan ef cientfi
vulcanization system, Polymer Testing24 (2005) 439 446e .
[31]
[16] P. Sae-oui, C. Sirisinha, U. Thepsuwan, K.Hatthapanit,Roles of silane coupling agents on properties of silica- lled polychloroprene, Eu-fi ropeanPolymer Journal 42 (2006) 479 486e .
[22]
[17] I. Surya, H. Ismail, A.Azura,Alkanolamide as an accelerator,fi ller-dispersant and a plasticizer insilica- lled natural rubber com-fi
pounds, Polymer Testing 32 (2013) 1313 1321e .
[54]
[18] O. Lorenz, C.Parks, The crosslinking ef ciencyfi of some vulcanizing agents in natural rubber, Journal of Polymer Science50 (1961) 299 312e .
[19] H. Ismail, M. Nasaruddin, U. Ishiaku, White rice husk ashfilled natural rubber compounds: the effect of multifunctional additive and silane coupling agents, Polymer Testing 18 (1999) 287 298e .
[35]
[20] H. Ismail, S. Shaari, N.Othman,The effect of chitosan loading on the curing characteristics, mechanical and morphological properties of chitosan- lledfi natural rubber (NR), epoxidised natural rubber (ENR)
and styrene-butadiene rubber (SBR) compounds,Polymer Testing 30(2011) 784 790e .
[21] R. Mukhopadyay, S. De, Effect of vulcanization temperature and differentfillers on the properties of ef ciently vulcanized naturalfi rubber, Rubber Chemistry and Technology 52 (1979) 263 277e .
[22] H. Long (Ed.), Basic Compounding and Processing of Rubber, Rubber Division, American Chemical Society Inc. The University of Akron, Ohio, USA, 1985.
[23] H.L. Stephens, The compounding and vulcanization of rubber, in: M. Morton (Ed.), Rubber Technology, Van Nostrand Reinhold, New York, 1987, pp. 20 58e .
[15]
[24] H. Ismail, R. Nordin, A.Noor,Cure characteristics, tensile properties and swelling behaviour of recycled rubberpowder- lledfi natural rubber compounds,Polymer Testing 21 (2002) 565 569e .
[23]
[25] B. Boonstra, H. Cochrane, E.Dannenberg,Reinforcement of silicone rubber by particulate silica, Rubber Chemistry and Technology 48 (1975) 558 576e .
[26] H. Cochrane, C. Lin, The in uence of fumed silica properties on thefl processing, curing, and reinforcement properties of silicone rubber, Rubber Chemistry and Technology 66 (1993) 48 60e .
[27] H. Ismail, C. Ng, Palm oil fatty acid additives (POFA's): preparation and application, Journal of Elastomers and Plastics 30 (1998) 308 327e .
[19]
[28] P. Teh, Z. Mohd Ishak, A. Hashim, J. Karger-Kocsis, U.Ishiaku,Effects of epoxidized natural rubber as a compatibilizerin melt com-pounded natural rubber organoclay nanocomposites, Europeane
Polymer Journal 40 (2004) 2513 2521e .
[29] G. Kraus, Interactions of elastomers and reinforcingfillers, Rubber Chemistry and Technology 38 (1965) 1070 1114e .
[16]
[30] K. Polmanteer, C. Lentz, Reinforcement studies-effect of silica structure on properties and crosslink density, RubberChemistry and Technology 48 (1975) 795 809e .
[39]
[31] B.Lee,Reinforcement of uncured and cured rubber composites and its relationship to dispersive mixing-an interpretation of cure meter rheographs of carbon black loaded SBR and cis-polybutadiene compounds, Rubber Chemistry and Technology 52 (1979) 1019 1029e .
[32] P. Pal, S. De, Effect of reinforcing silica on vulcanization, network structure, and technical properties of natural rubber, Rubber Chemistry and Technology 55 (1982) 1370 1388e .
[33] N. Hewitt, Processing technology of silica reinforced SBR, Elasto-merics, (March, 1981) 16 (1981).
[34] D.L. Hertz Jr., S.E. Inc, theory&practice of vulcanization, Elasto-merics (November, 1984).
[28]
[35] H. Ismail, H.Chia, The effects of multifunctional additive and epoxidation in silica filled natural rubber compounds, Polymer Testing 17 (1998) 199 210e .
[36] B. Rodgers, Rubber Compounding: Chemistry and Applications, CRC, 2004.
[37] G. Kraus, Reinforcement of elastomers by particulate llers, Sciencefi and Technology of Rubber (1978) 387 416e .
[16] [57]
[38] S.Wolff,Optimization of silane-silica OTR compounds.Part 1: var-iationsof mixing temperature and time during themodi cationfi of silica with bis-(3-triethoxisilylpropyl)-tetrasul de, Rubber Chemis-fi
try and Technology 55 (1982) 967 989e .
[39] W. Hofmann, F. Bayer, Vulcanization and Vulcanizing Agents, Maclaren, 1967.
[40] M.A. Lutz, K.E. Polmanteer, H.L. Chapman, Novel wet-process silica prepared from alkyl silicates. Part I: synthesis, Rubber Chemistry and Technology 58 (1985) 939 952e .
[41] L.H. Cohan, The mechanism of Re€enforcement of elastomers by pigments, Rubber Chemistry and Technology 21 (1948) 667 681e .
[42] H. Ismail, M. Mathialagan, Comparative study on the effect of partial replacement of silica or calcium carbonate by bentonite on the properties of EPDM composites, Polymer Testing 31 (2012) 199 208e .
[43] H. Nabil, H. Ismail, A. Azura, Compounding, mechanical and morphological properties of carbon-black- lled natural rubber/fi recycled ethylene-propylene-diene-monomer (NR/R-EPDM) blends, Polymer Testing 32 (2013) 385 393e .
I. Surya et al. / Polymer Testing 40 (2014) 24 32e